Method for synthesizing flavone alkyl phosphate compound and application thereof in cholesterol esterase inhibitor medicine
A technology for flavonoid phosphate and compound, which is applied to the synthesis of flavonoid phosphate alkyl ester compounds and the application field of cholesterol esterase inhibitor drugs, can solve the problems of low solubility, difficult phosphorylation reaction and the like, and achieves conversion efficiency High, good economic and social benefits, obvious catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
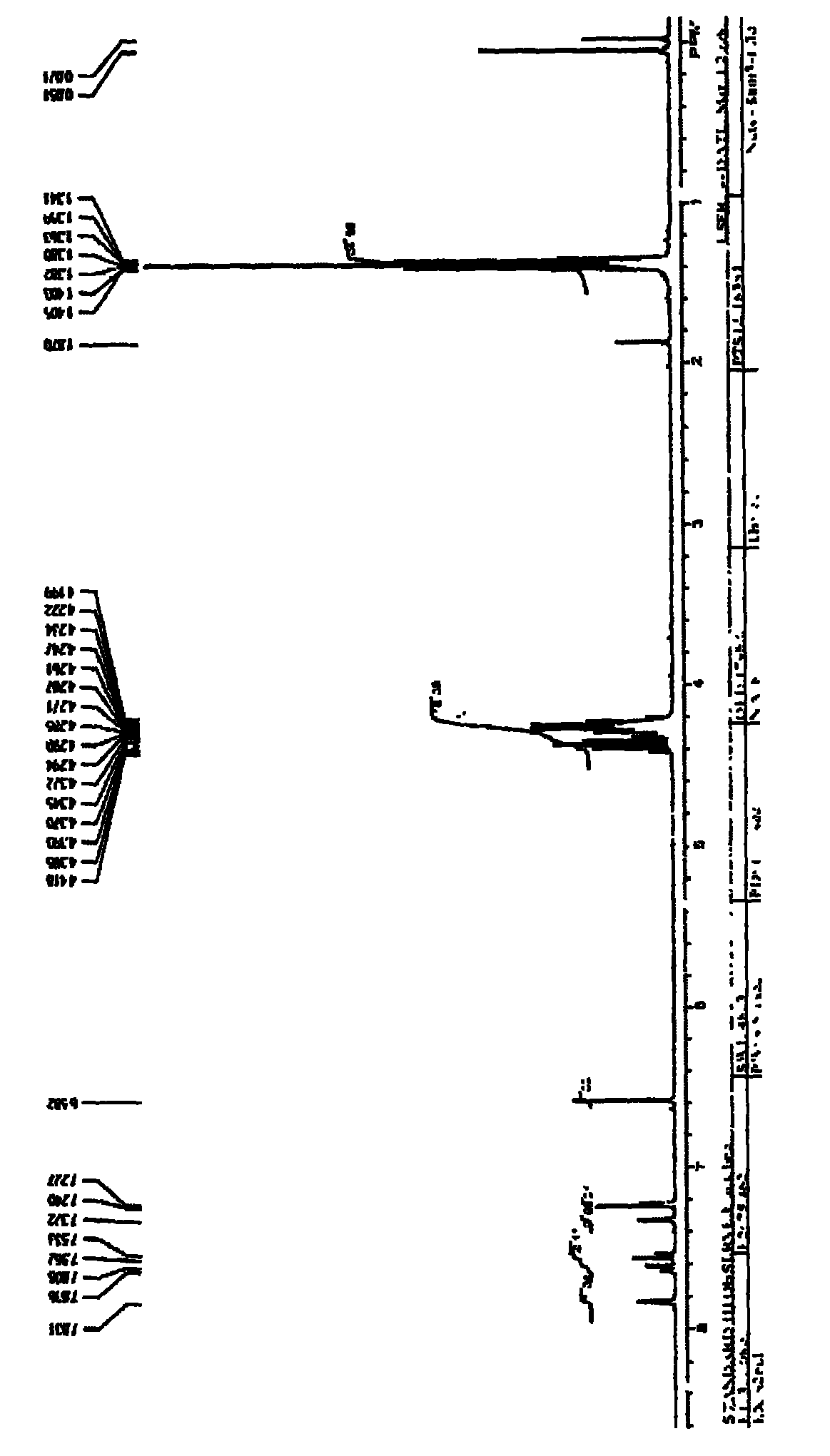
[0039] Weigh 1a (715.6mg, 2.5mmol), DMAP (1.8326g, 15mmol) in a dry 150mL round bottom flask, add THF (30.0mL) under stirring, and slowly add Et 3 N (2.80 mL, 20 mmol). Measure CIP(O)(OEt) 2 (2.5883g, 15mmol), diluted with THF (20.0mL), reacted at room temperature for 24h. Add about 1 times the total volume of the reaction system with ethyl acetate to dilute, then wash with 0.5 mol / L HCl, 5% (w / v) potassium carbonate solution, saturated sodium chloride solution, and water respectively. The ethyl acetate layer (product layer) was dried with anhydrous sodium sulfate, and then concentrated and dried under reduced pressure to obtain 1.8753 g of a yellow liquid with a yield of 90.3%. According to NMR, MS, IR, and UV spectroscopic analysis, the product was 2a.
Embodiment 2
[0041]
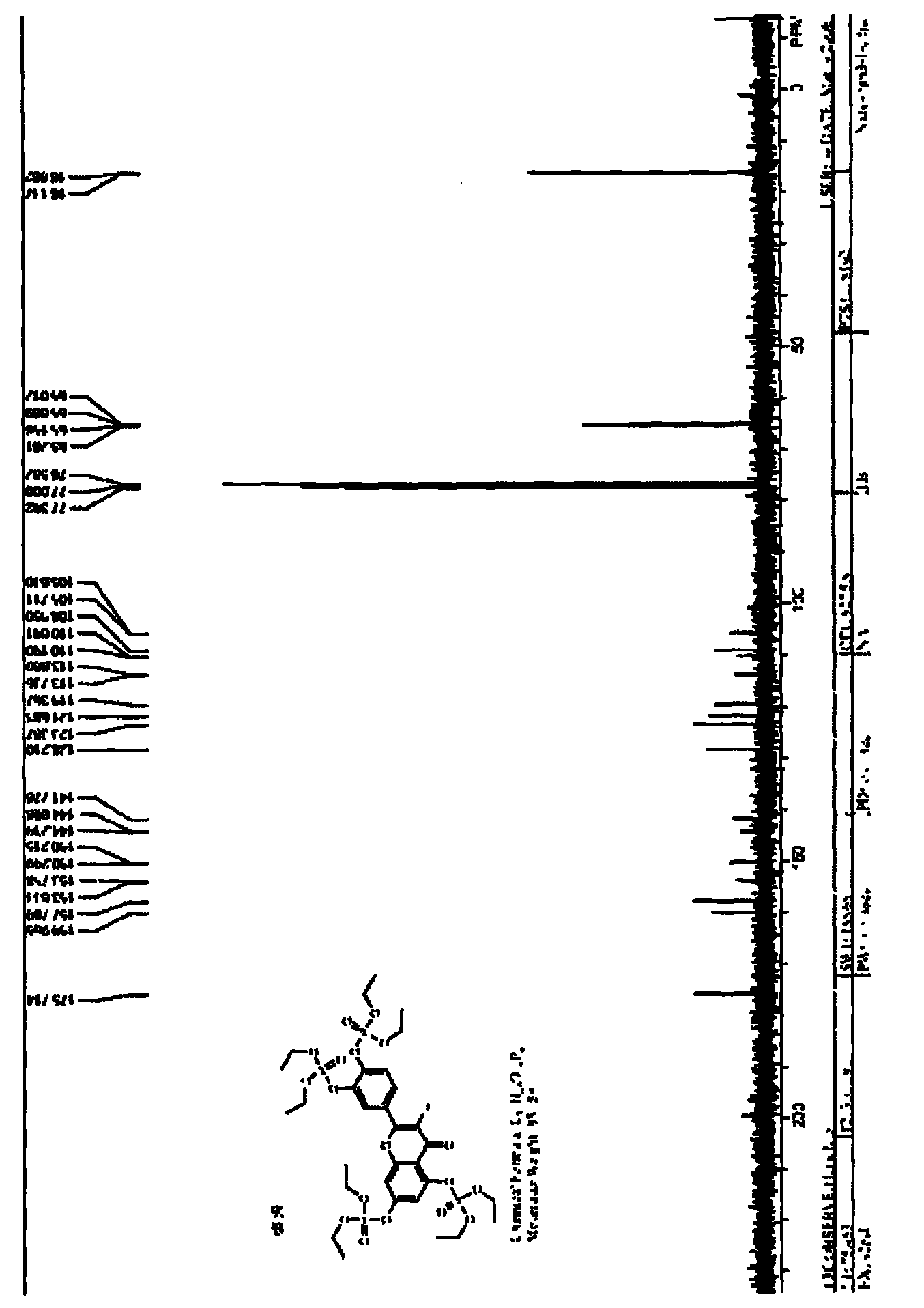
[0042] Under nitrogen protection, 1b (302.1 mg, 1.0 mmol), DMAP (1.2217 g, 10.0 mmol) were added to a dry 50 mL round bottom flask. Add HMPA (10.0mL) under stirring, and slowly add Et 2 NH (1.1mL, 10.7mmol), measure ClP(O)(OEt) 2 (1.7255g, 10.0mmol), diluted with 5.0mL HMPA, reacted at 0°C for more than 40h, diluted with ethyl acetate, passed through 0.5mol / L HCl solution, 5% (w / v) K 2 CO 3 After washing with aqueous solution, saturated NaCl aqueous solution and water, add anhydrous NaCl 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain 817.3 mg of yellow viscous liquid with a yield of 83.2%. According to NMR, MS, IR, and UV spectroscopic analysis, the product is 2b.
Embodiment 3
[0044]
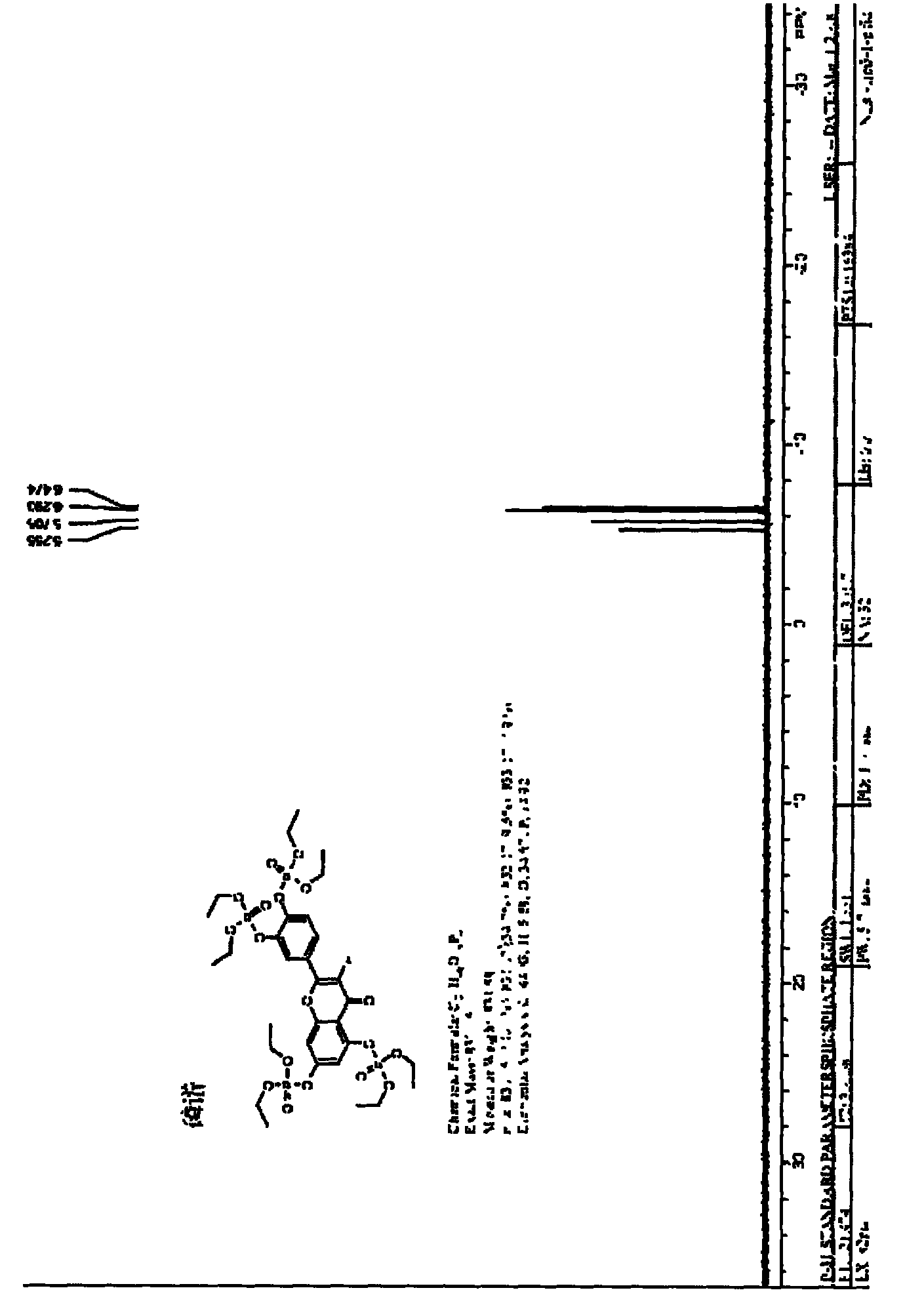
[0045] Under nitrogen protection, 1c (635.6mg, 2.5mmol), DMAP (0.9163g, 7.5mmol) were added to a dry 150mL round bottom flask, DMSO (30.0mL) was added under stirring, and pyridine (0.61 mL, 7.5mmol), measure ClP(O)(OEt) 2 (1.2941g, 7.5mmol), diluted with DMSO (20.0mL), reacted at 120°C for 2h, diluted with ethyl acetate, passed through 0.5mol / L HCl solution, 5% (w / v) K 2 CO 3 After washing with aqueous solution, saturated NaCl aqueous solution and water, add anhydrous NaCl 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain a yellow radial solid. This product is treated with anhydrous ether / n-hexane to obtain 1.1344 g of a light yellow (near white) solid, with a yield of 86.2%. NMR, MS, IR, and UV spectra Analysis, the product was 2c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com