Anilino-containing amphiphilic 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene derivative as well as preparation method and application thereof
A technology of fluoroboron dipyrrole and derivatives is applied in the field of supramolecular fluorescence sensing thin film materials, which can solve the problems of complicated operation, high instrument cost, slow detection speed, etc., and achieves simple preparation method, low detection limit, high detection rate and so on. selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of the compound of formula III-1
[0031] Under nitrogen protection conditions, 620.97mg (1.0mmol) formula II-1 compound, 990.70mg (2.5mmol) formula I-1 compound, 460.70mg (2.0mmol) potassium carbonate and 220.12mg (0.3mmol) palladium dichloride Add it to a mixture of 30mL dioxane and 1.5mL distilled water, heat and reflux at 90°C and stir for 12 hours, distill off dioxane and distilled water under reduced pressure, add 60mL dichloromethane to the residue to dissolve, and then Wash with ultrapure water (20mL×2 times) and saturated NaCl aqueous solution (20mL×2 times), combine the organic phases and filter and dry with anhydrous sodium sulfate, dichloromethane is distilled off under reduced pressure, and the obtained oily substance is washed with ethyl acetate and petroleum A mixed solvent with an ether volume ratio of 7:3 was used as the mobile phase and silica gel as the stationary phase for column chromatography separation and purification, and vacuum-d...
Embodiment 2
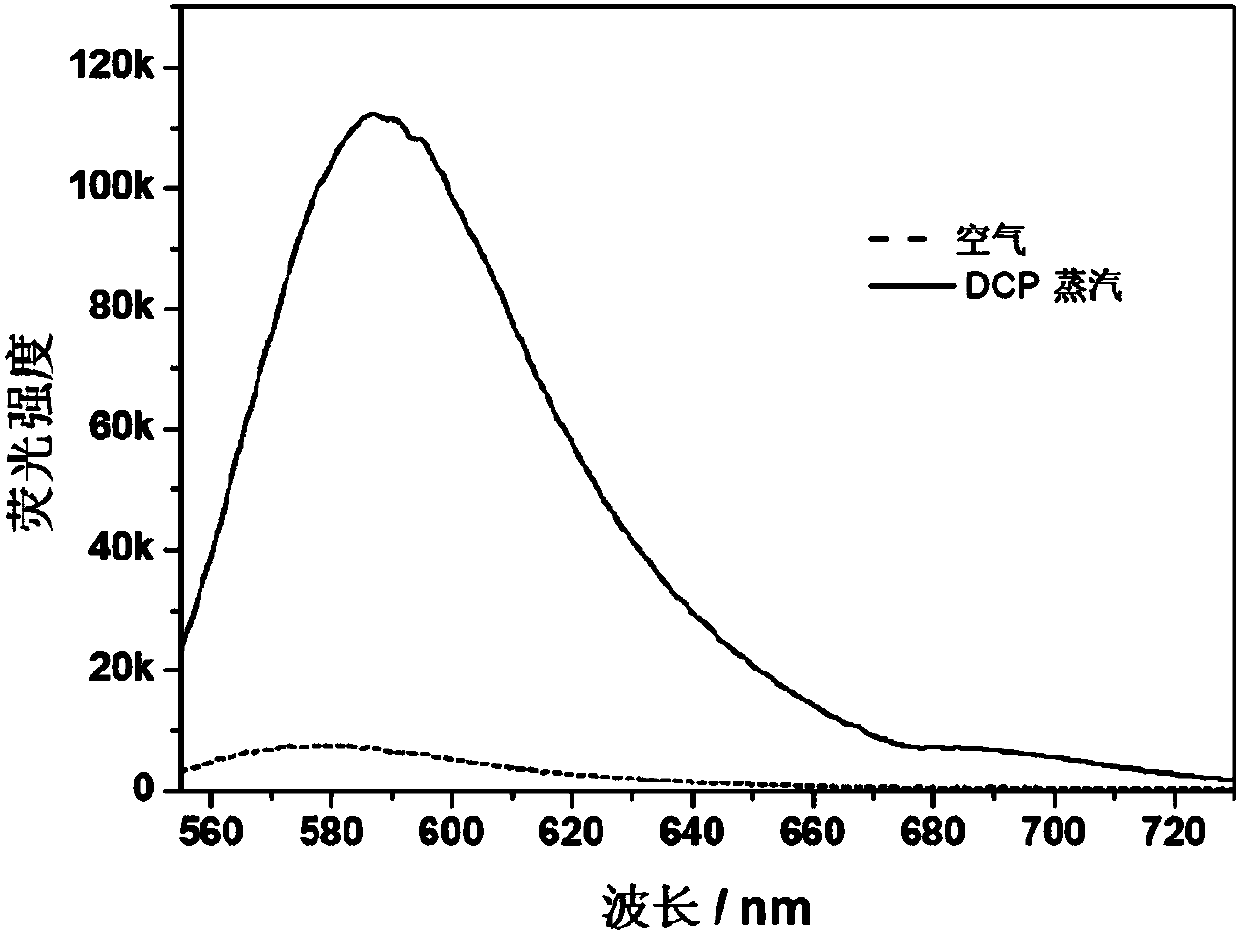
[0039] The use of the aniline-containing amphiphilic fluoroborate dipyrrole derivatives prepared in Example 1 in the detection of DCP gas, the specific detection method is as follows:
[0040] Aniline-containing amphiphilic fluoroboron dipyrrole derivatives were dissolved in PEG200 to prepare a stock solution of 18 μmol / L aniline-containing amphiphilic fluoroborate derivatives; The dipyrrole derivative stock solution is in contact with the gold substrate with hydrophilic and hydrophobic micro-regions, and an ordered pattern with a droplet diameter of 50 μm is formed in the hydrophilic mercaptoundecanoic acid monolayer region, and an aniline-containing amphiphile is prepared. The PEG200 microarray monolayer fluorescent sensing film of the active fluoroboron dipyrrole derivative (see figure 1 and figure 2 ).
[0041] Place the above-mentioned PEG200 microarray monolayer fluorescent sensing film containing aniline-containing amphiphilic fluoroborate dipyrrole derivatives in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com