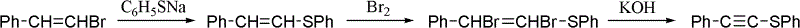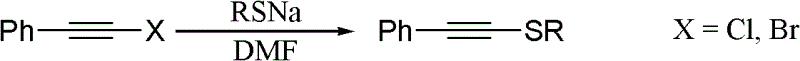One-pot synthesis method of alkyne thioether
A technology of arylalkyne sulfide and synthesis method, which is applied in sulfide preparation, organic chemistry and other directions, can solve the problems of long synthetic route separation of intermediates, limited application, high price, etc., and achieves easy separation and purification, good versatility, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] R=CH 3 ;Ar=Ph
[0035] At room temperature, sodium methyl mercaptide (1.05 g, CH 3 SNa content 20%) and methanol (15mL), then slowly add α-bromoacetophenone (498mg, 2.5mmol) in the reaction system, after adding, TLC tracks the reaction, and about 15min has reacted and is added to the reaction solution Add 100mL of water, extract with dichloromethane (30mL×3), wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, filter and evaporate the solvent under reduced pressure, and the residue is separated by silica gel column chromatography [V (petroleum ether ): V(CH 2 Cl 2 )=5:1] to obtain 316 mg of substrate 2a with a yield of 76%.
[0036] 2a colorless oil
[0037] 1 H NMR (400MHz, CDCl 3 ): δ=2.14(s, 3H), 3.77(s, 2H), 7.47(t, J=7.60Hz, 2H), 7.58(t, J=7.60Hz, 1H), 7.98(d, J=7.60Hz , 2H).
[0038] MS (EI) m / z (%): 166 (M + , 77).
Embodiment 2
[0040] R=CH 3 ;Ar=Ph
[0041] Under the protection of nitrogen at -78°C, LiHMDS (1.8 mL of 1.0 mol / L THF solution) was added dropwise to a THF (15 mL) solution of substrate 2a (300 mg, 1.8 mmol) and stirred for 30 minutes. ClP(O)(OEt) 2 (0.3mL, 2.2mmol) was added dropwise into the above reaction system liquid. After the dropwise addition was completed, the cooling device was removed, and the mixture was naturally raised to room temperature and stirred for 30 minutes. The reaction was cooled to -78°C again, and LiHMDS (4.5 mL of tetrahydrofuran solution with a concentration of 1.0 mol / L) was added dropwise to the above reaction system, and stirring was continued at this temperature for 1 hour. The reaction was quenched with saturated ammonium chloride solution, the reaction mixture was poured into water, extracted with ethyl acetate (30mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under r...
Embodiment 3
[0048] Step is with embodiment 1. Substrate 2b was prepared from α-bromo-4-methoxyacetophenone (R=CH 3 ; Ar=4-CH 3 OC 6 h 4 ), yield 64%.
[0049] 2b colorless oil
[0050] 1 H NMR (400MHz, CDCl 3 ): δ=2.15(s, 3H), 3.73(s, 2H), 3.88(s, 3H), 6.95(d, J=8.80Hz, 2H), 7.97(d, J=8.80Hz, 2H).
[0051] MS (EI) m / z (%): 196 (M + , 88).
[0052] Step is with embodiment 2. The substrate is 2b (R=CH 3 ; Ar=4-CH 3 OC 6 h 4 ), the product is 1b, and the yield is 69%.
[0053] 1b colorless oil
[0054] 1 H NMR (400MHz, CDCl 3 ): δ=2.46(s, 3H), 3.81(s, 3H), 6.82(d, J=8.40Hz, 2H), 7.37(d, J=8.80Hz, 2H).
[0055] 13 C NMR (100MHz, CDCl 3 ): δ=19.50 (SCH 3 ), 55.28 (OCH 3 ), 78.94(C≡), 91.62(C≡), 113.91(CH), 115.50(C), 133.39(CH), 159.63(C).
[0056] FT-IR (KBr): 2927, 2837, 2167 (C≡C), 1605, 1569, 1506, 1463, 1441, 1374, 1290, 1248, 1172, 1106, 1032, 977, 832, 777, 956, 832, 777, 644, 588, 537cm -1 .
[0057] MS (EI) m / z (%): 178 (M + , 85).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com