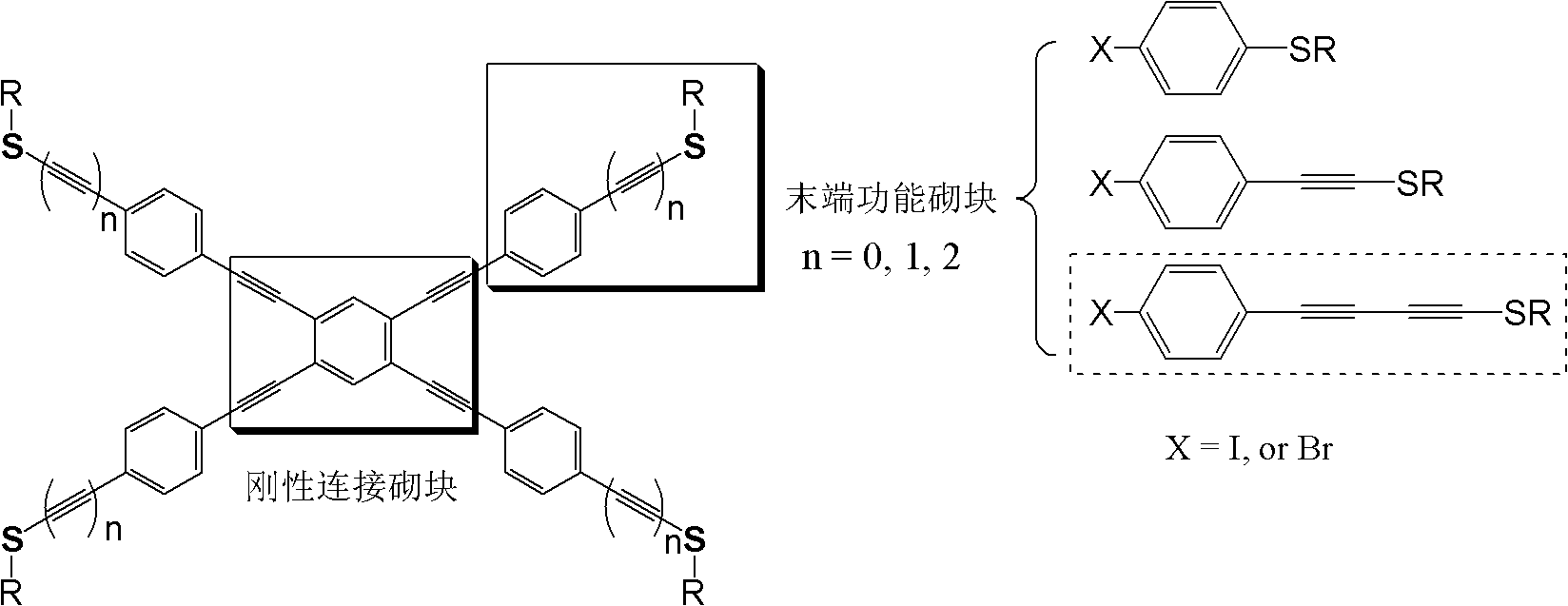
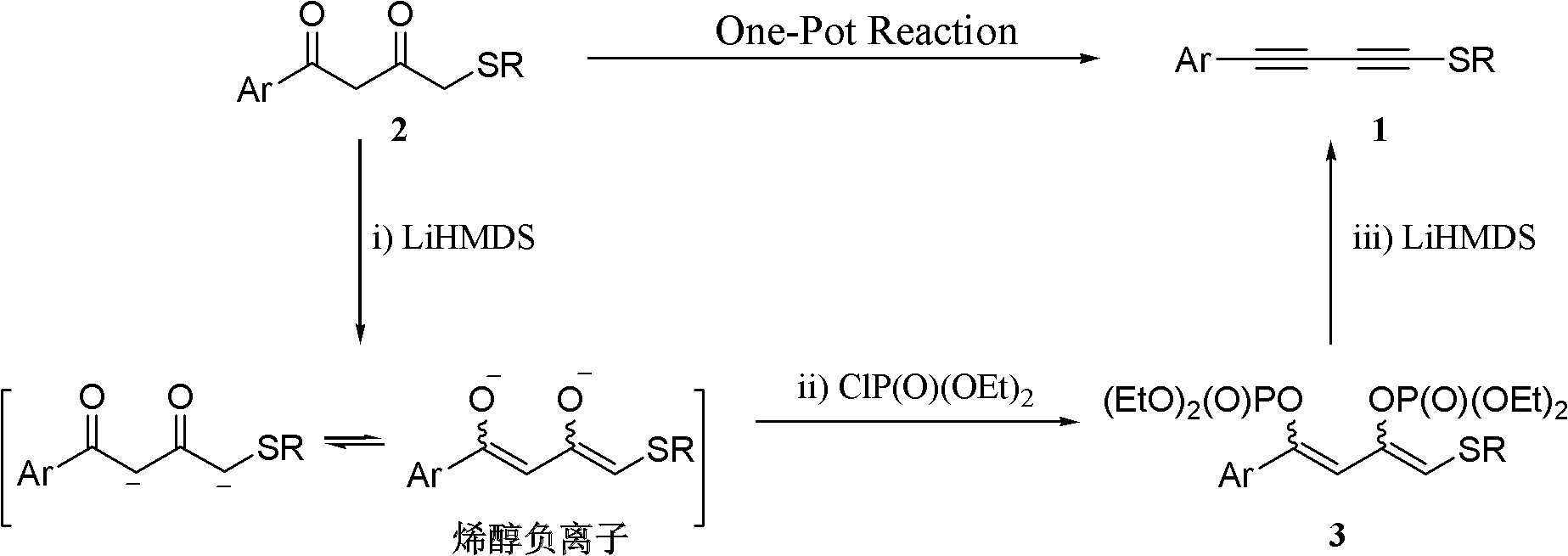
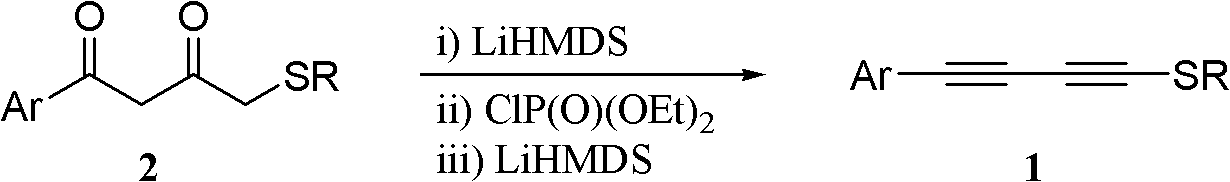One-pot synthesis method of methylthio aryl alkynye
A technique for diacetylenic sulfides and synthesis methods, which is applied in the fields of thioether preparation and organic chemistry, and can solve the problems of obtaining aryldiyne sulfides, which have not been found in literature reports, etc., and achieve simple operation, good versatility, and easy separation and the effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] R=CH 3 ;Ar=Ph
[0031] At room temperature, add sodium methyl mercaptide (421 mg, CH 3 SNa content 50%) and methanol (15mL), then slowly add 4-bromo-1-phenyl-1,3-butanedione (603mg, 2.5mmol) in the reaction system, after adding, TLC tracking reaction, The reaction was completed in about 15 minutes, and 100 mL of water was added to the reaction solution, extracted with dichloromethane (30 mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure, and the residue was passed through silica gel Column chromatography separation [V (petroleum ether): V (CH 2 Cl 2 )=5:1] to obtain 364 mg of substrate 2a with a yield of 70%.
[0032] 2a Pale yellow oil
[0033] (keto form: enol form = 0.08: 1.00) (enol form) 1 H NMR (400MHz, CDCl 3 ): δ=2.19(s, 3H), 3.30(s, 2H), 6.40(s, 1H), 7.46(t, J=7.60Hz, 2H), 7.54(t, J=7.60Hz, 1H), 7.90 (d, J=7.60Hz, 2H), 15.77(s, 1H). (keto form)1 ...
Embodiment 2
[0036] R=CH 3 ;Ar=Ph
[0037] Under nitrogen protection at -78°C, LiHMDS (1.0 mol / L THF solution, 2.8 mL) was added dropwise to substrate 2a (300 mg, 1.4 mmol) in THF (15 mL), and stirred for 30 minutes. ClP(O)(OEt) 2 (0.4mL, 2.9mmol) was added dropwise to the above reaction system. After the dropwise addition was completed, the cooling device was removed, and the mixture was naturally raised to room temperature and stirred for 30 minutes. The reaction system was cooled to -78°C again, and then LiHMDS (7.0 mL of tetrahydrofuran solution with a concentration of 1.0 mol / L) was added dropwise to the above reaction system, and stirring was continued at this temperature for 1 hour. The reaction was quenched with saturated ammonium chloride solution, the reaction mixture was poured into water, extracted with ethyl acetate (30 mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressu...
Embodiment 3
[0044] Step is with embodiment 1. Substrate 2b was prepared starting from 4-bromo-1-(4-methyl)phenyl-1,3-butanedione (R=CH 3 ; Ar=4-CH 3 C 6 h 4 ), yield 63%.
[0045] 2b Light yellow solid, melting point: 51~55℃
[0046] (keto form: enol form = 0.10: 1.00) (enol form) 1 H NMR (400MHz, CDCl 3 ): δ=2.19(s, 3H), 2.42(s, 3H), 3.29(s, 2H), 6.37(s, 1H), 7.26(d, J=8.40Hz, 2H), 7.80(d, J= 8.00Hz, 2H), 15.86(s, 1H). (keto form) 1 H NMR (400MHz, CDCl 3 ): δ=2.07(s, 3H), 2.42(s, 3H), 3.36(s, 2H), 4.30(s, 2H).
[0047] MS (EI) m / z (%): 222 (M + , 100).
[0048] Step is with embodiment 2. The substrate is 2b (R=CH 3 ; Ar=4-CH 3 C 6 h 4 ), the product is 1b, and the yield is 59%.
[0049] 1b yellow oil
[0050] 1 H NMR (400MHz, CDCl 3 ): δ=2.34(s, 3H), 2.44(s, 3H), 7.11(d, J=8.00Hz, 2H), 7.36(d, J=8.40Hz, 2H).
[0051] 13 C NMR (100MHz, CDCl 3 ): δ=18.99 (SCH 3 ), 21.55 (CH 3 ), 73.69(C≡), 75.00(C≡), 77.96(C≡), 79.40(C≡), 118.50(C), 129.14(CH), 132.43(CH), 139.49(C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com