Fluorescent probe for detecting sarin and analogue thereof as well as synthesis method and application of fluorescent probe
A technology of fluorescent probe and synthesis method, which is applied in the field of fluorescent sensing, can solve the problem of inability to detect sarin poison and its simulants, and achieve excellent sensing performance, easy mass preparation, and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of sensing material HBP-PFTP and preparation of fluorescent probes
[0037] Synthesis of pyrene-fluorene copolymerized hyperbranched polymer HBP-PFTP with terpyridine as terminal group and preparation of sensing film:
[0038]
[0039] Under nitrogen protection, compound 1 (156mg, 0.3mmol), compound 2 (773mg, 1.2mmol), Pd(PPh 3 ) 4 (76.023mg, 0.06mmol), K 2 CO 3 Aqueous solution (10mL, 2M), dioxane 30mL, heated to reflux for 48 hours. Add compound 3 (200mg) and Pd(PPh 3 ) 4 (50 mg), refluxed for 24 hours and cooled to room temperature. Extract with saturated brine / dichloromethane, combine the organic phases and dry over anhydrous magnesium sulfate, spin off the solvent to obtain a crude product. The resulting crude product was dissolved in a small amount of CH 2 Cl 2 in, dropwise added to CH 3 Precipitate in OH, repeat the steps of dissolution and separation three times, and then reflux for three days with a Soxhlet extractor to wash awa...
Embodiment 2
[0046] Example 2 Synthesis of sensing material TPF and preparation of fluorescent probes
[0047]
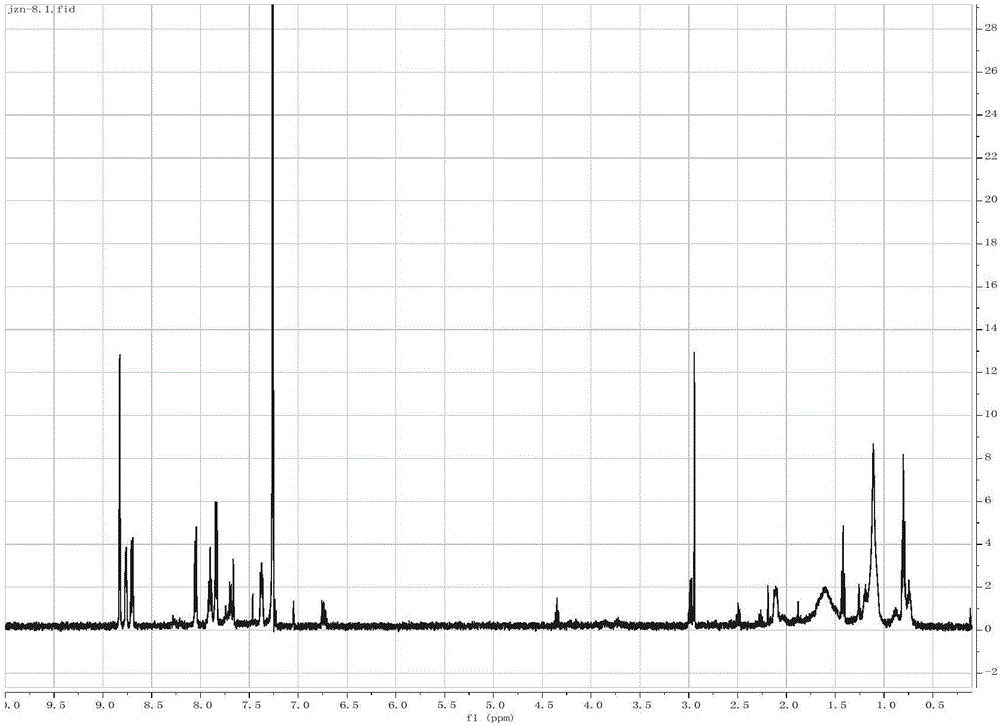
[0048] According to the Suzuki-Miyaura method in Example 1, TPF was synthesized and corresponding fluorescent probes were prepared. TFP 1 HNMR and 13 C NMR, elemental analysis results are as follows:
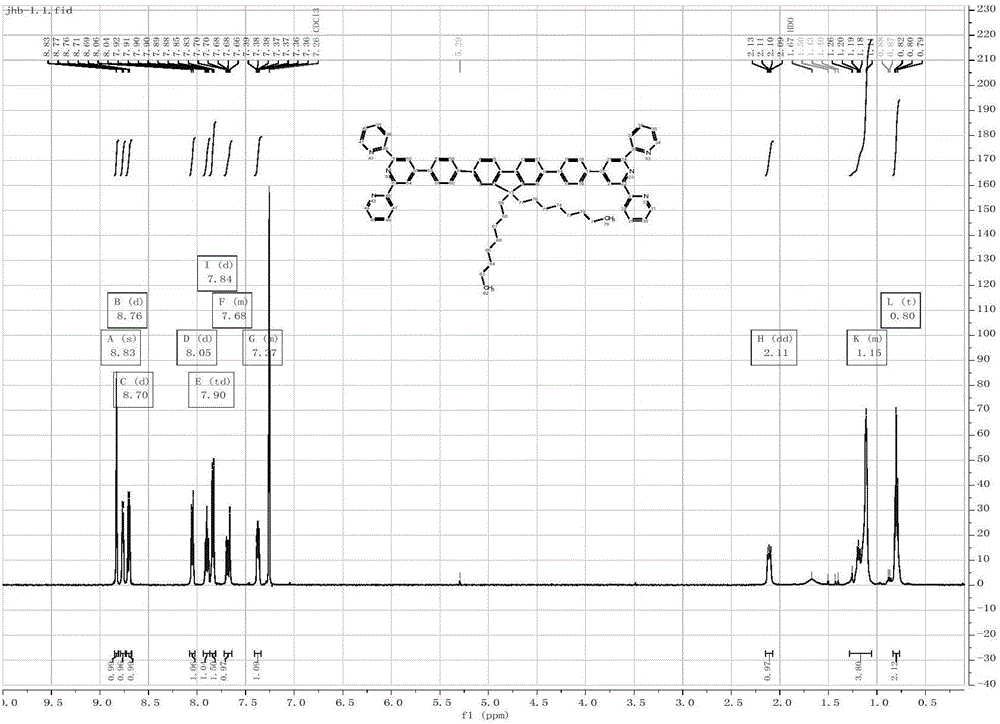
[0049] TPF 1 H NMR spectrum see image 3 . 1 H NMR (500MHz, Chloroform) δ8.82(s, 4H), 8.76, (d, J=4.7Hz, 4H), 8.69(d, J=8.0Hz, 4H), 8.08-8.01(d, J=8.0 Hz, 4H), 7.88(td, J=7.7, 1.8Hz, 4H), 7.83(d, J=8.0Hz, 8H), 7.71-7.64(m, 4H), 7.36(m, 4H), 2.21-2.00 (dd, J=10.8, 5.7Hz, 4H), 1.15(s, 20H), 0.80(t, J=7.0Hz, 10H).TPF 13 C NMR spectrum see Figure 4 . 13 CNMR(126MHz,CDCl3)δ156.34,156.02,151.91,149.79,149.15,142.32,140.39,139.33,137.14,136.85,127.72,127.64,126.12,123.81,121.48,121.40,120.19,118.68,77.26,77.21,77.01, 76.76, 55.42, 40.39, 31.77, 30.02, 29.20, 23.89, 22.59, 14.05. High Resolution MS: search on mass 1005.56; found 1005.5574. Anal. Calcd. for C 71 h 68 N 6 :...
Embodiment 3
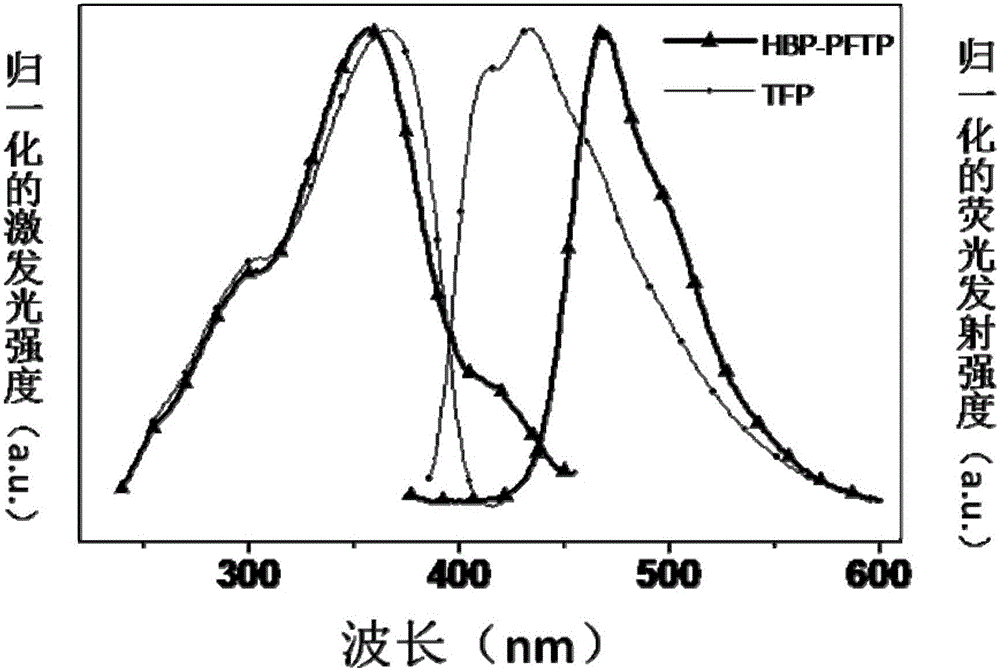
[0052] The stability of HBP-PFTP and TPF and the response curves to DCP were tested at their respective maximum excitation wavelength and emission wavelength ( Figure 5 ). As shown in the figure, the stability of HBP-PFTP is better than that of TPF, and the fluorescence intensity remains basically unchanged within 300s. Both HBP-PFTP and TPF respond quickly to DCP saturated steam, and the fluorescence intensity quenching rate reaches 99% within 3s, indicating that both HBP-PFTP and TPF respond well to DCP and are very sensitive. The effect is much better than the results obtained by others, quenching 95% above 10s.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com