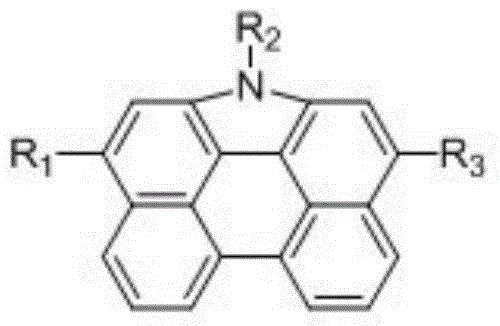
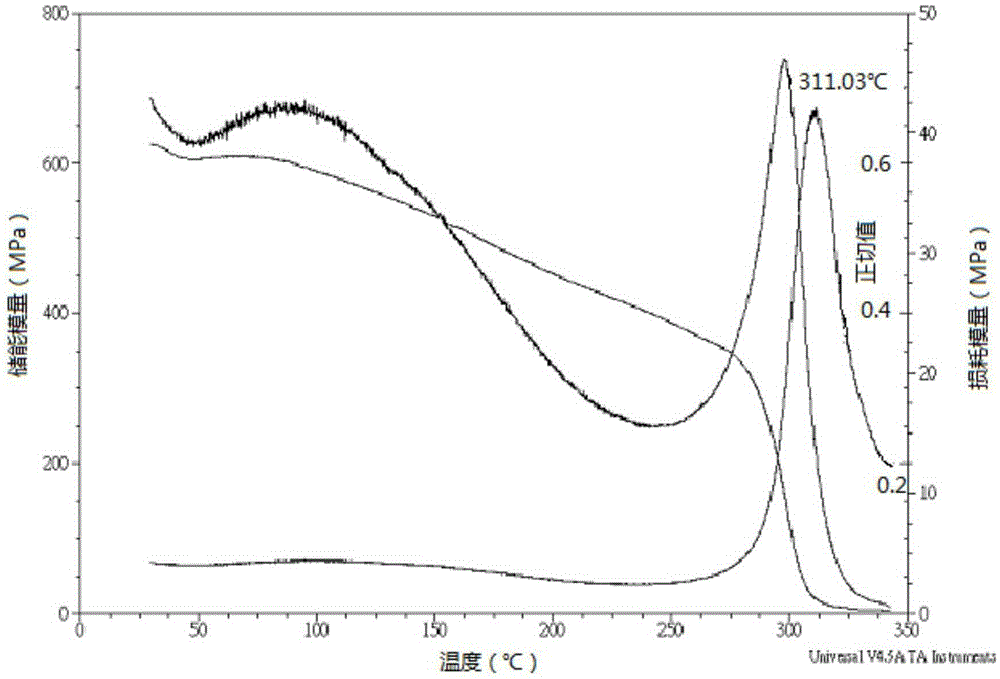
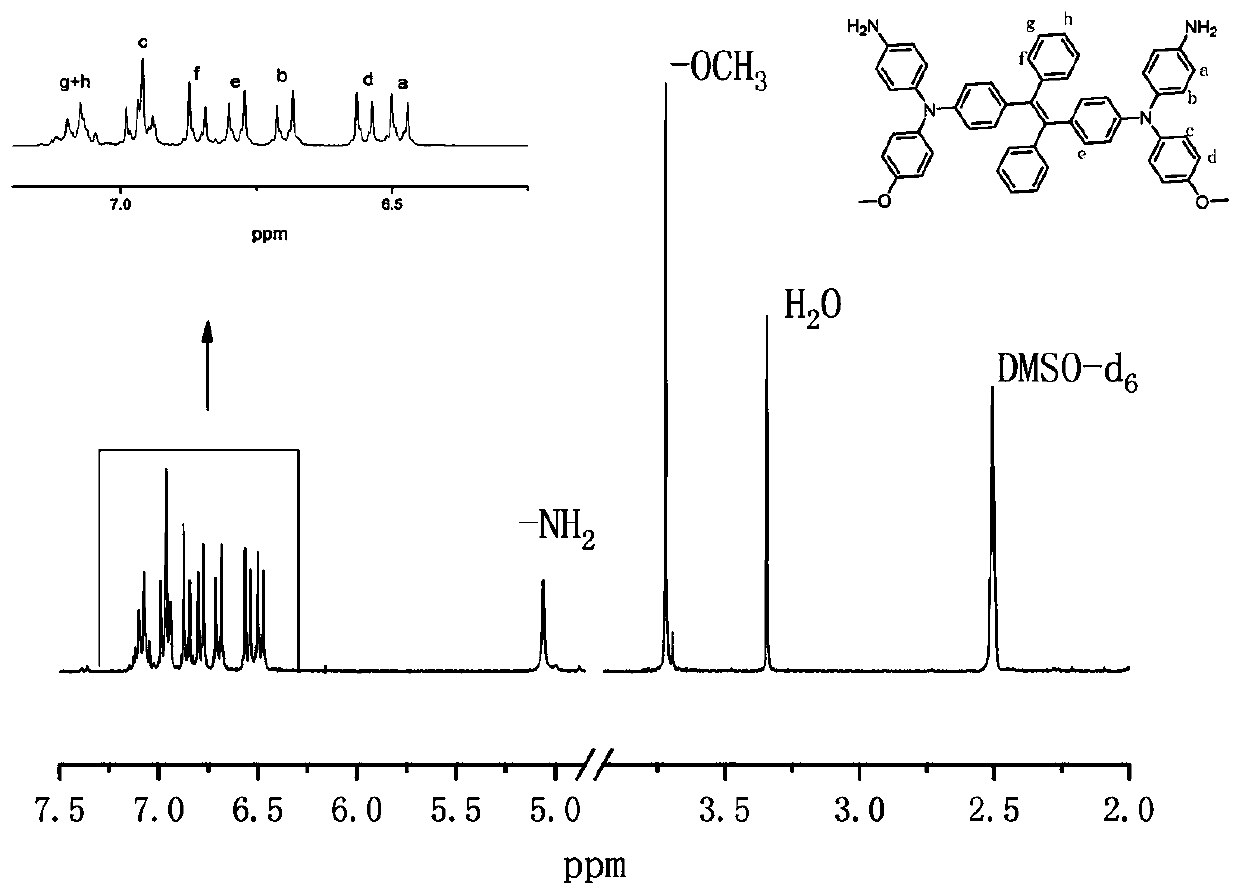
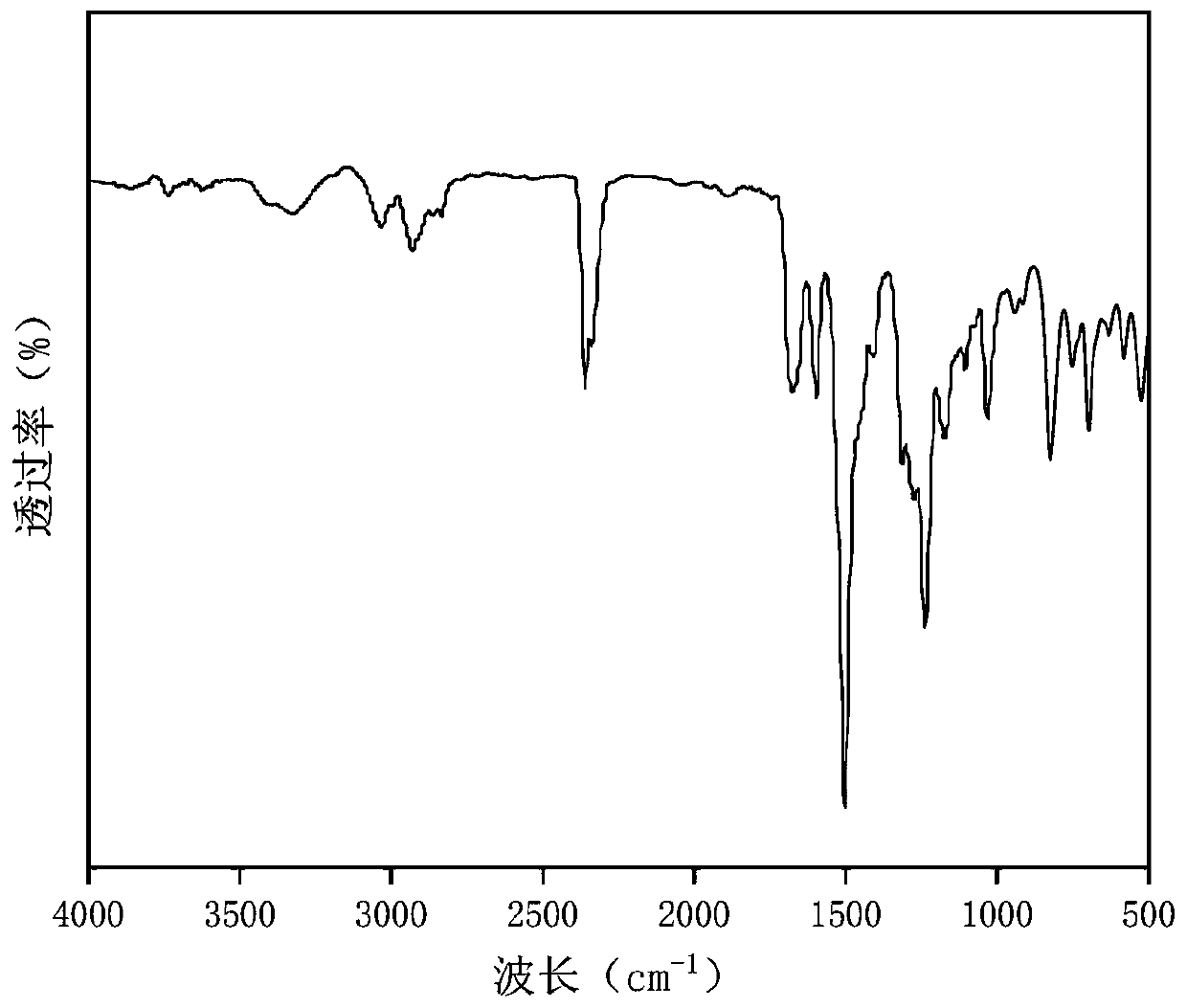
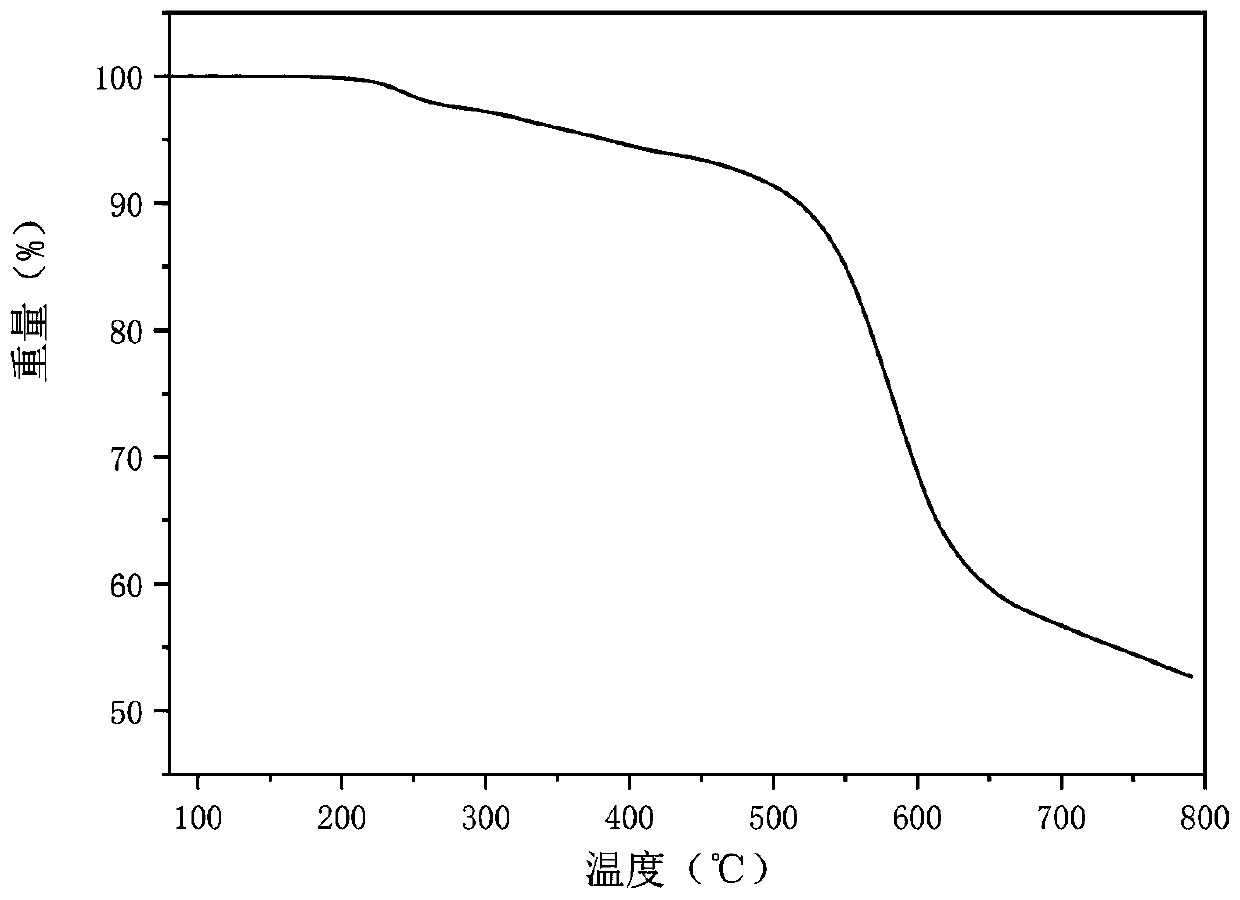
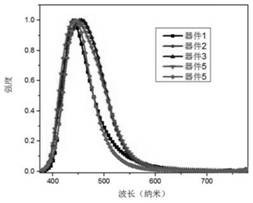
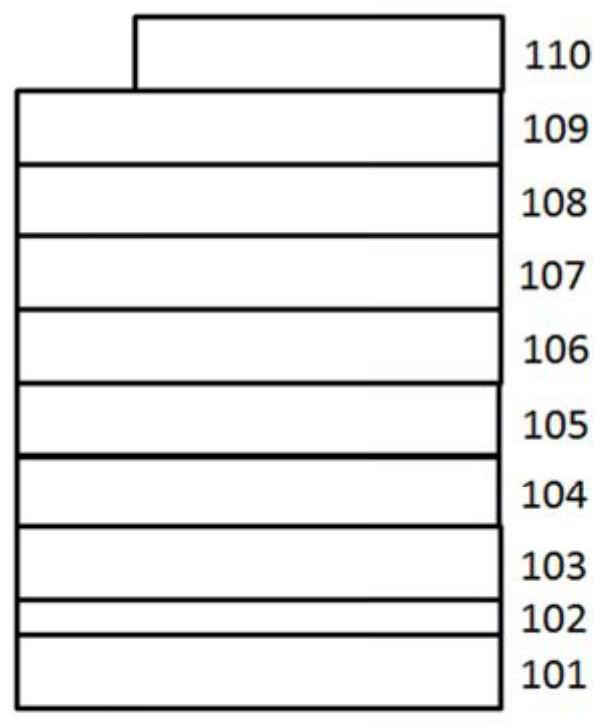
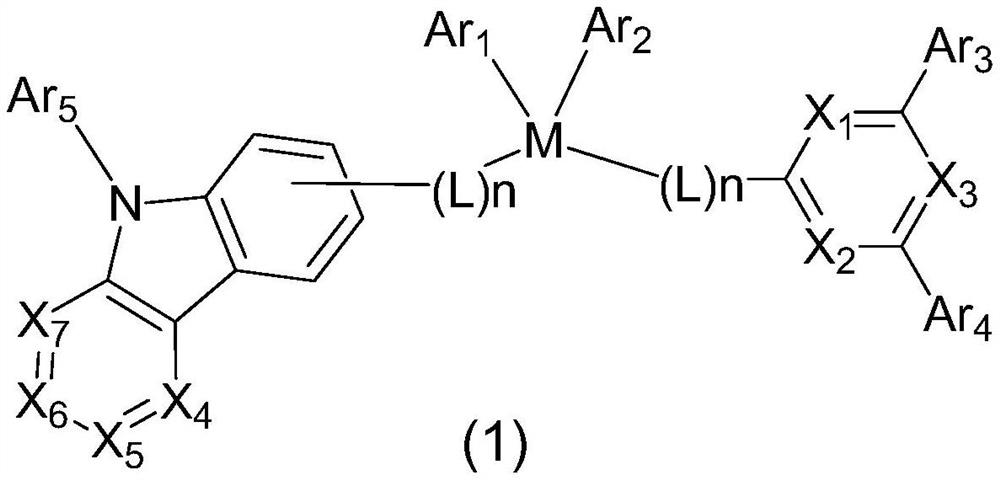
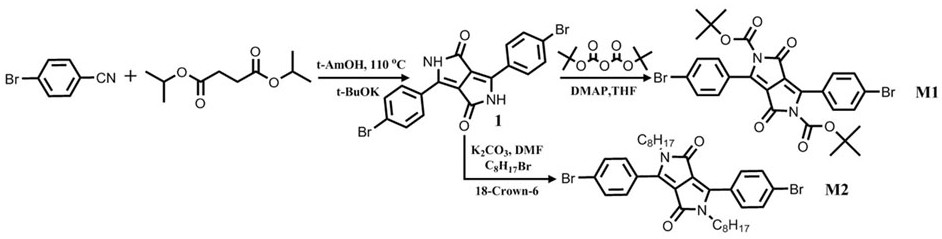
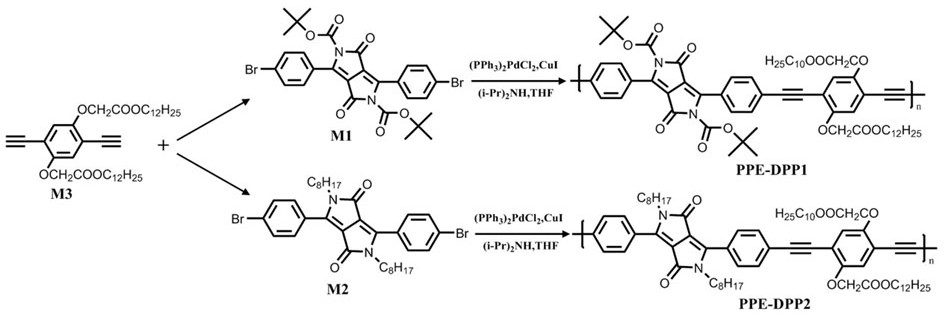
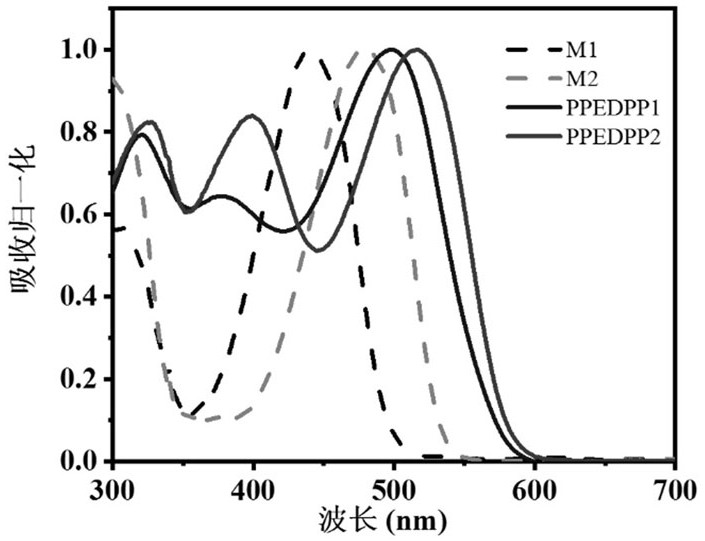
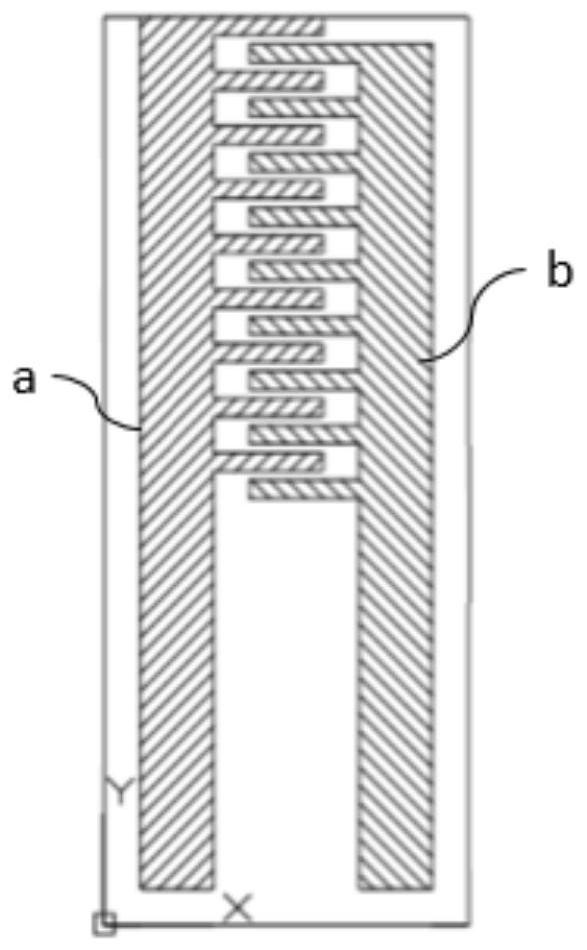
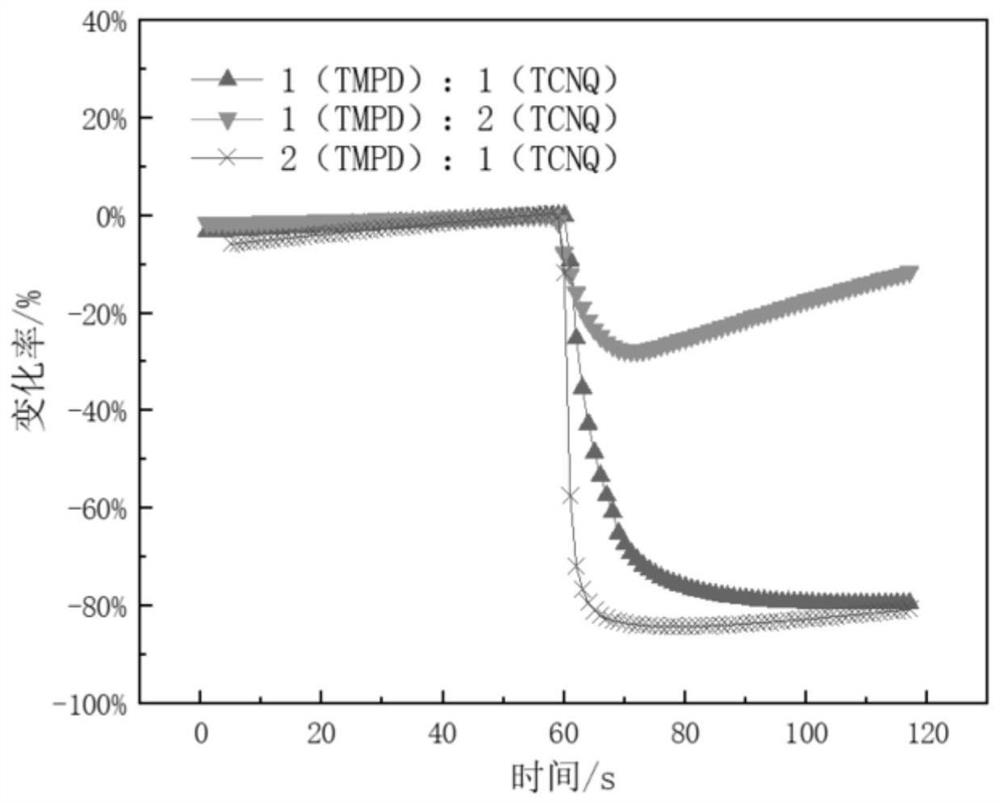
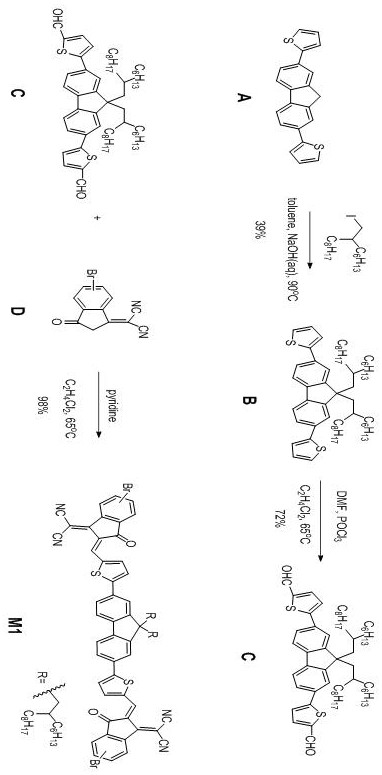
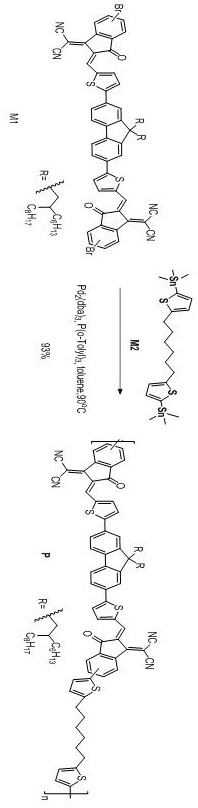
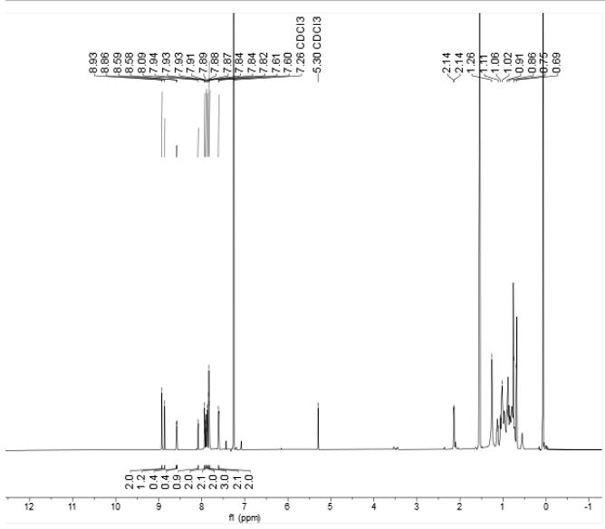
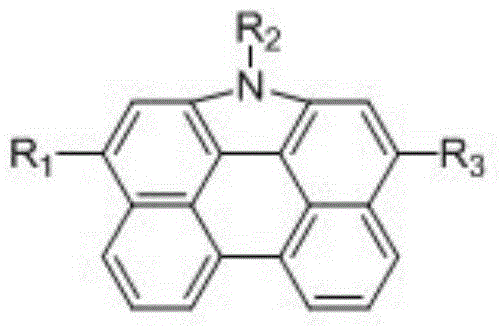
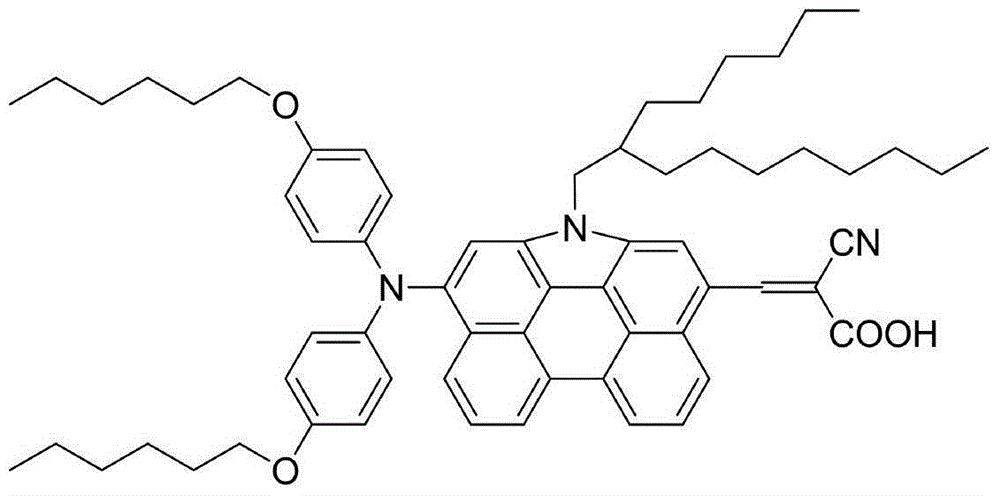
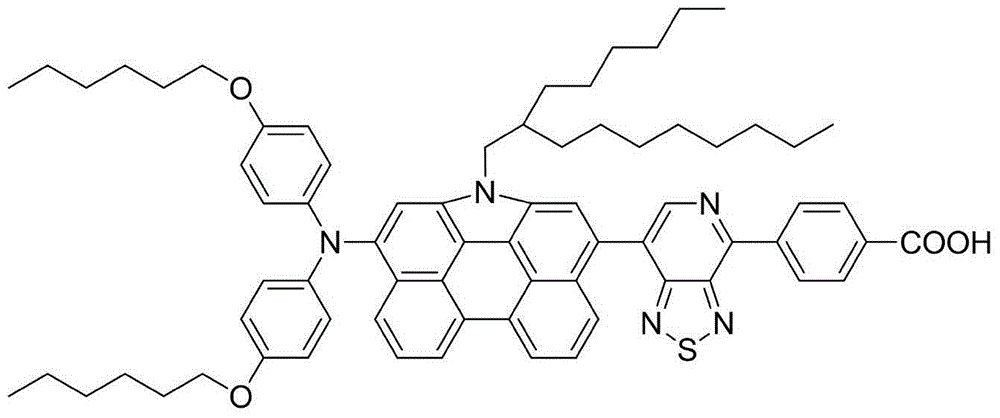
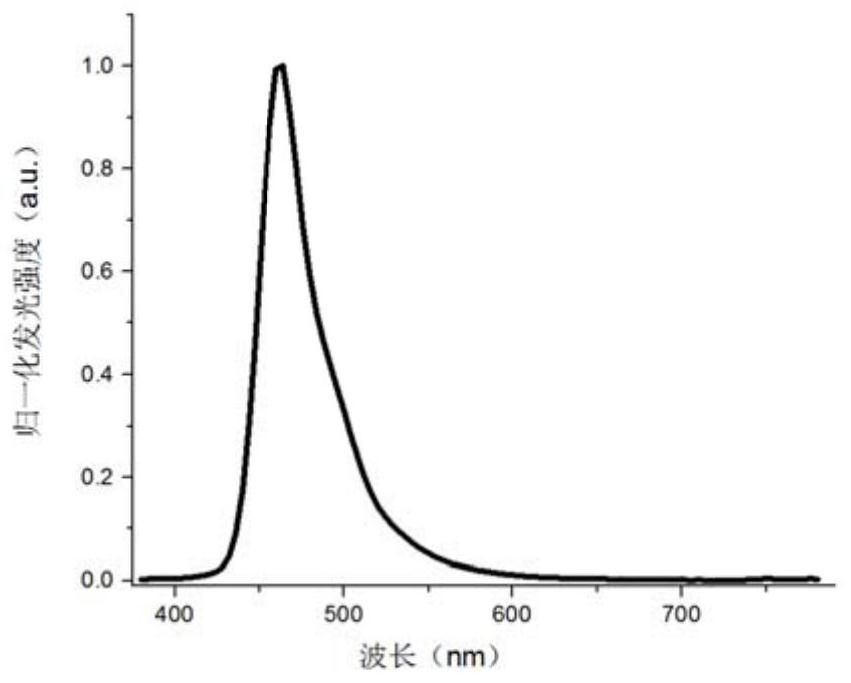
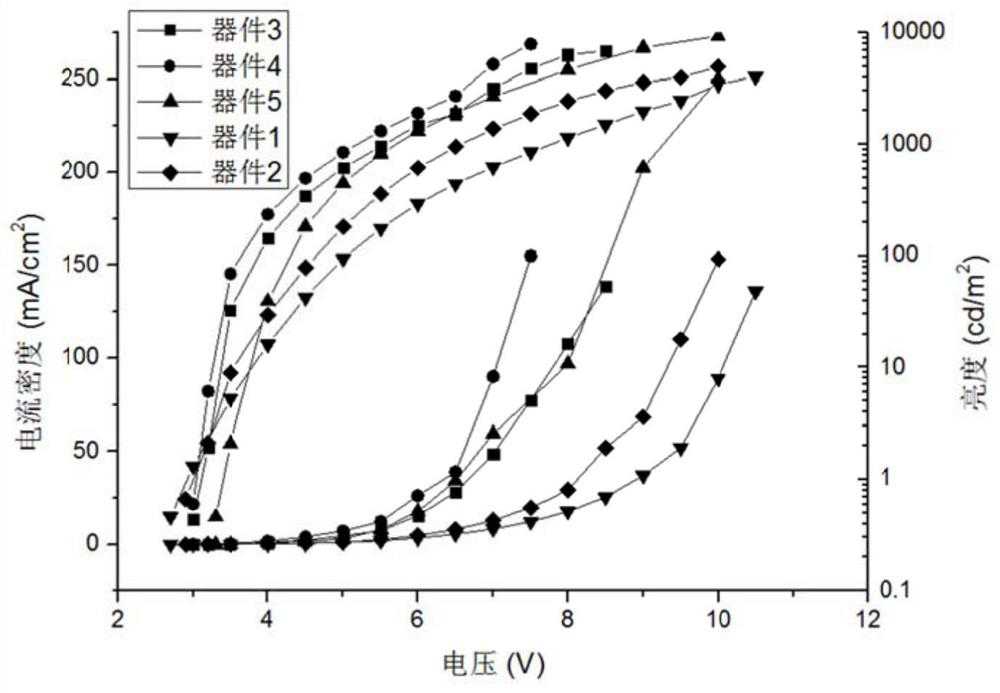
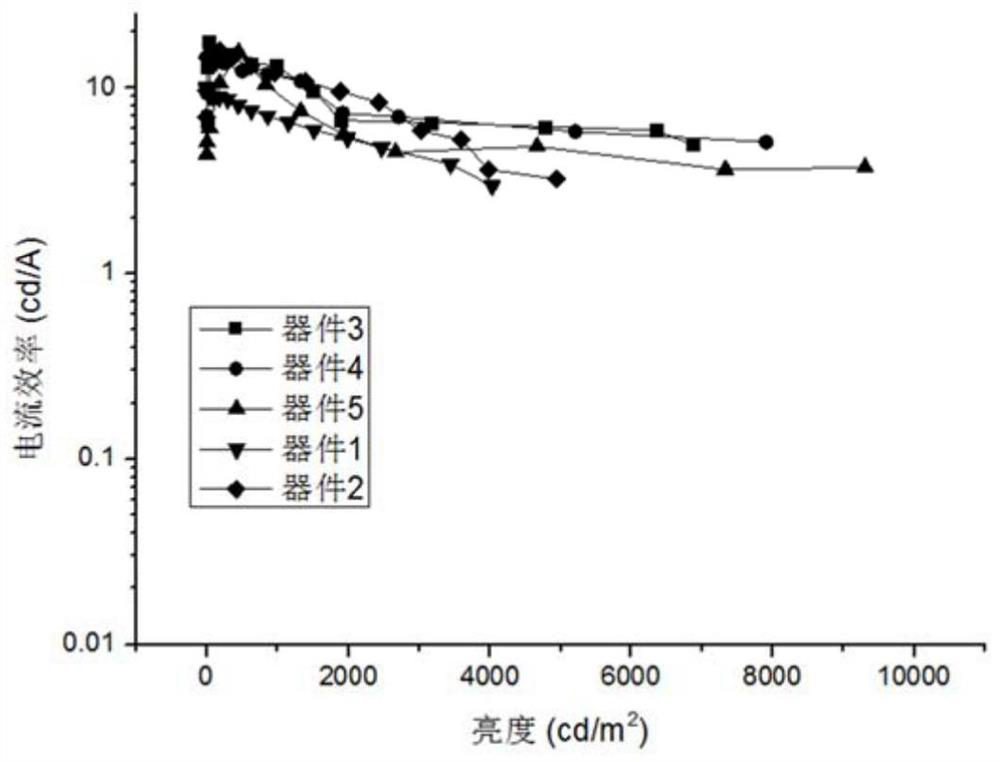
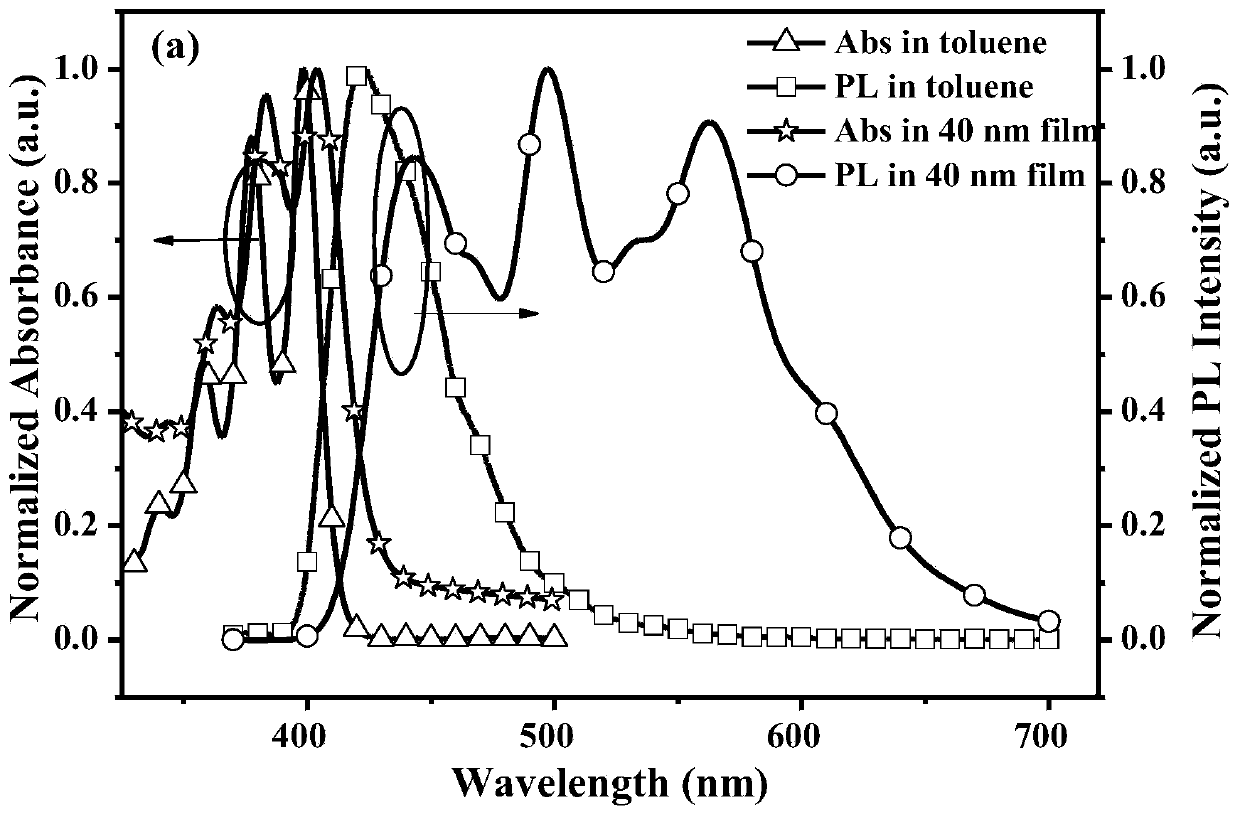
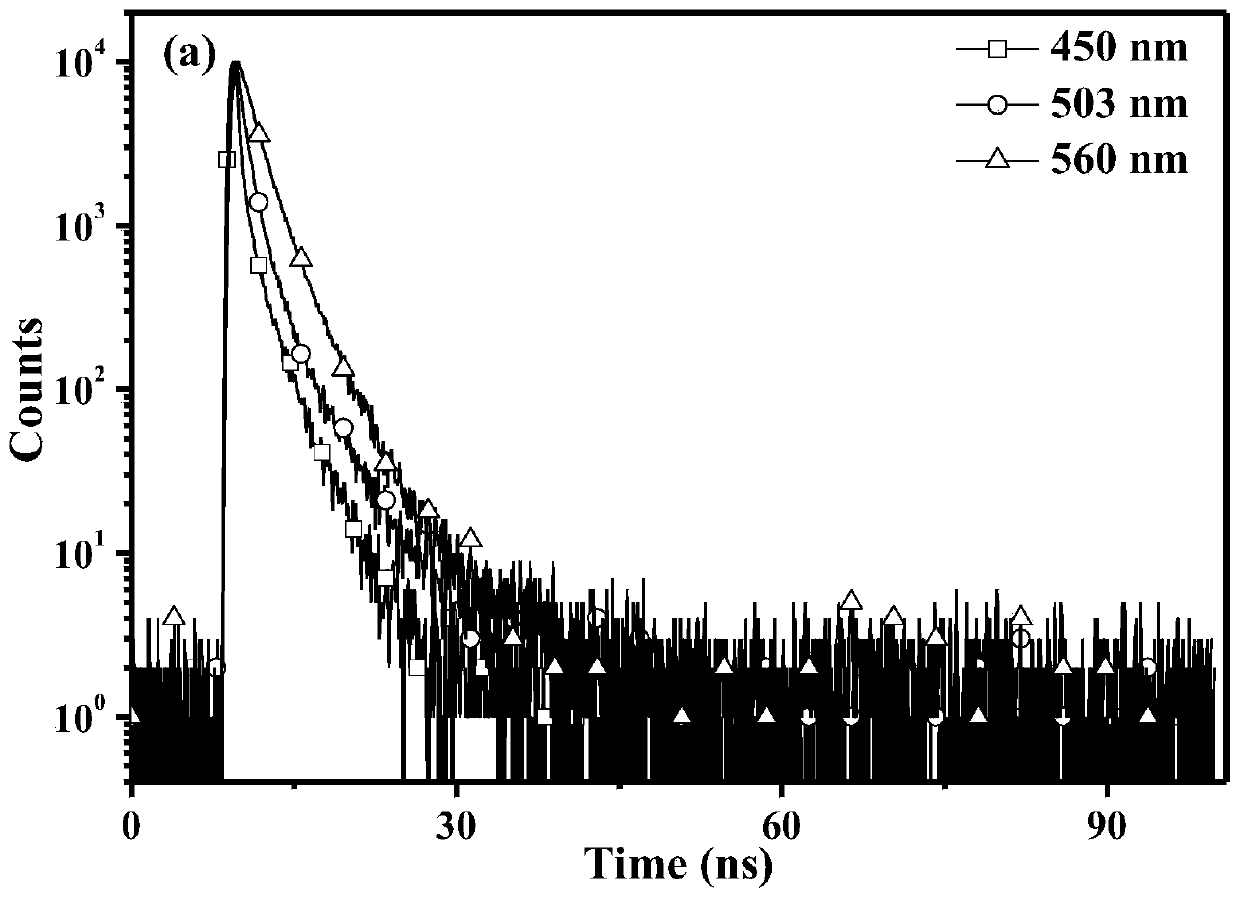
Patents
Literature
33 results about "Intermolecular charge transfer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenanthro-carbazole donor-acceptor organic dye and application thereof in dye-sensitized solar cell
ActiveCN103819929AImprove solubilityRealize step-by-step regulationLight-sensitive devicesAzo dyesSolubilityQuantum yield
The invention provides a phenanthro-carbazole donor-acceptor organic dye and application thereof in a dye-sensitized solar cell, and belongs to the field of organic dyes. The organic dye utilizes the characteristics of phenanthro-carbazole structural units such as a large plane conjugated system and higher fluorescence quantum yield to be connected with the structural units with different electronic withdrawing abilities so as to realize step-by-step regulation and control to the dye molecular energy level; meanwhile, a molecular non-conjugated skeleton is connected with a long alkyl chain, so that the solubility of phenanthro-carbazole units is improved, the difficulty in molecular synthesis and device processing is reduced, and charge transfer among molecules caused by molecular aggregation is reduced; meanwhile, raw materials of phenanthro-carbazole compounds are abundant in source, low in price and easy to cut structurally.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
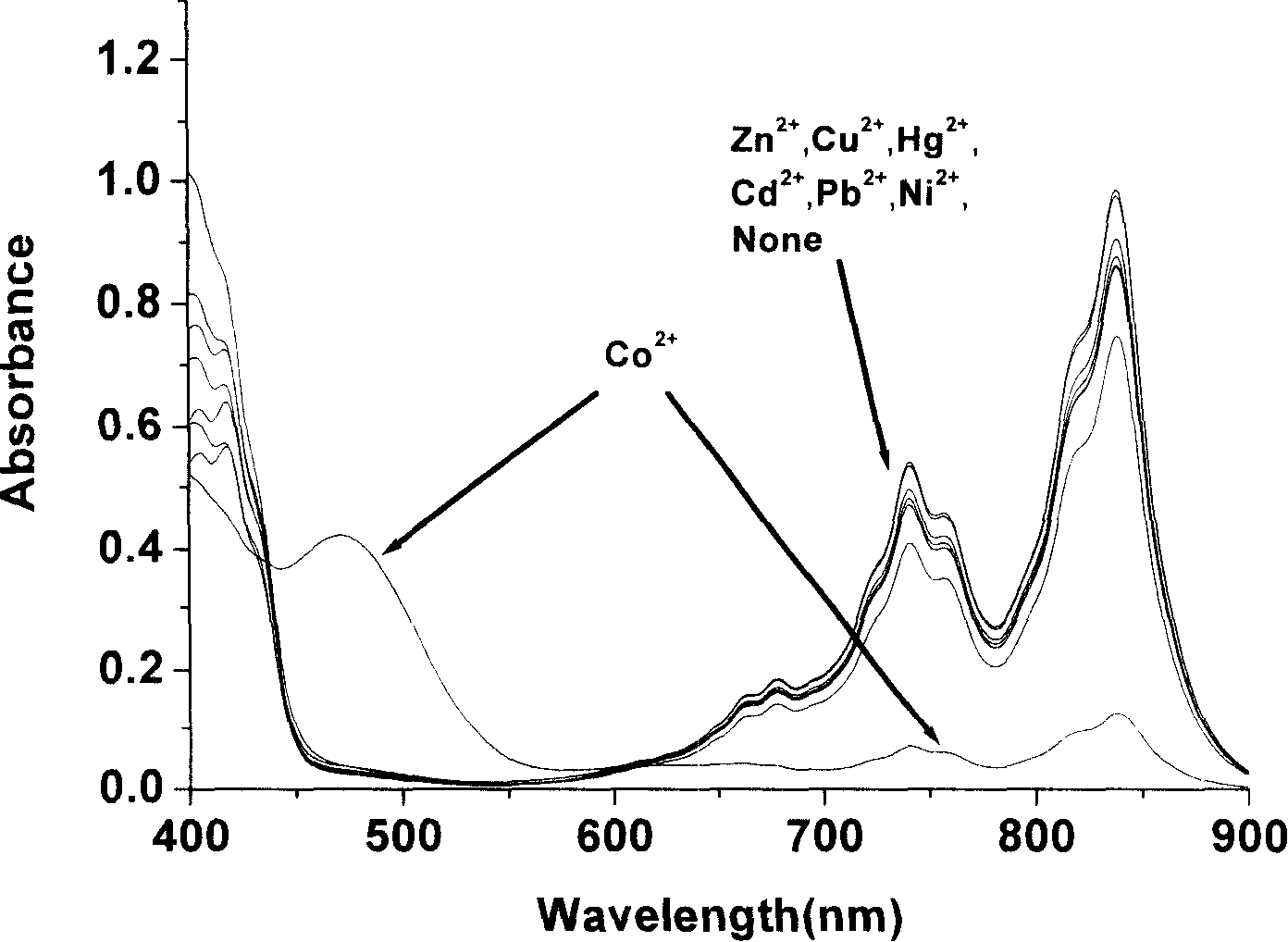
Color matching detecting and analyzing method of cobalt ion
InactiveCN101196473AObvious change in absorption spectrumMeet the test requirementsMaterial analysis by observing effect on chemical indicatorBiological testingCharge-transfer complexElectron donor
The invention relates to a color comparison testing and analyzing method of cobalt ion. The method uses 8-hydroxyquinoline as electron donor, 7, 7, 8, 8-tetracyanoquinodimethane as electron acceptor. Colored charge transfer complex is formed in polar solvent through the charge transferring effect among molecule and is made as testing reagent. Water-soluble cobalt salt are made into water solution of definite viscosity, a definite volume of water solution and the testing reagent with same volume are mixed. The color of the solution changes obviously, and other metal ion doesn't interfere cobalt ion. With room temperature testing, the color comparison reaction of the testing reagent to cobalt ion can be identified by eyes or tested by using ultraviolet-visible spectrophotometer. The invention has very professional color comparison identifying capability to cobalt ion the preparation of solution is convenient, the operation of the color comparison is very simple, and the color changing speed of color comparison reaction is quick, which can finish the color comparison test to the tested object in very short time.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Organic light-emitting diode
ActiveCN109638170ALife expectancyImprove external quantum efficiencySolid-state devicesSemiconductor/solid-state device manufacturingHigh energyDisplay device
The invention provides an organic light-emitting diode. A luminescent layer of the organic light-emitting diode (OLED) is composed by mixing a donor material, an acceptor material and one of a fluorescence luminescent material or phosphorescence luminescent material or thermal-delayed fluorescence material or quantum dot luminescent material or perovskite material. The donor material and the acceptor material with the triplet state energy level being higher than 2.7eV and the glass transition temperature being greater than 120 DEG C are combined, the excited state of charge transfer between high energy molecules is obtained, and the energy is higher than 2.75eV. Through the high-efficiency anti-interstitial traversal and the fluorescence resonance energy transfer between the excited stateand a guest material, the complete utilization of singlet state and triplet state energy for the guest material is realized, and the obtained organic light-emitting diode has the advantages of high efficiency, low operating voltage and long service life, can be used in a display device or light source device and has a good commercial application prospect.
Owner:SHANGHAI BLUESHINE OPTOELECTRONICS TECH CO LTD
Intramolecular charge transfer type red luminescent material and preparation and application thereof
InactiveCN1763149AEasy to separateHigh yieldElectrical apparatusElectroluminescent light sourcesElement analysisElectron donor
The present invention relates to serial intermolecular charge transfer type red light emitting materials with unparallel dipole system and its preparation process. The serial red light emitting materials are prepared with triphenylamine and diphenyl naphthylamine with hole transferring radical or their derivative as electron donor and nitrile group with powerful electrophilicity as electronic receptor and through simple Stille coupling reaction and condensation. Their molecular structure, electrochemical properties and physical optical property have been researched and characterized, and devices with the material as light emitting layer and electron transferring layer have been prepared. Tests show that these materials are active red light emitting materials with excellent application foreground.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Polyimide material and preparation method thereof
The invention relates to a polyimide material and a preparation method thereof. The polyimide material comprises diamine monomers, namely 2-(3,5-diaminophenyl)-4 and 5,6,7-(tetrafluorohydrazine isoindoline)-1,3-diketone. The preparation method comprises the steps of mixing the monomers and other aromatic diamines containing strong electron withdrawing groups, and then, reacting the mixture and fatty acid dianhydride to obtain polyimide. Fluorine atoms and the strong electron withdrawing groups are introduced to the structures of the diamine monomers, and meanwhile the diamines containing the strong electron withdrawing groups and the fatty acid dianhydride are used as polymerization monomers, so that the formation of intramolecular / intermolecular charge transfer complexes (CTC) is further inhibited, and the transparency of the polyimide is remarkably improved. The polyimide material prepared by using the preparation method has the advantages of high transparency, high glass transition temperature and favorable solubility and heat resistance and the like and has a wide application prospect in the relevant fields of flexible transparent substrates, flat panel displays and the like.
Owner:VALIANT CO LTD
Diamine monomer containing tetraphenylethylene-diarylamine structure, preparation method and application of the diamine monomer in polyamide synthesis
ActiveCN110606810AImprove transmittanceReduce transferOrganic compound preparationTenebresent compositionsAggregation-induced emissionPolyamide
The invention discloses a diamine monomer containing a tetraphenylethylene-diarylamine structure, a preparation method and application of the diamine monomer in polyamide synthesis, belonging to the technical field of organic compounds. A polyamide material generally has the problems of dark color, poor fluorescent effect and the like, and the fluorescence effect is weak when a triphenylamine group is directly introduced into the polyamide material, so that a tetraphenylethylene structure and a triphenylamine group are combined together to obtain the diamine monomer containing the tetraphenylethylene-diarylamine structure and introduced into polyamide in the form of the diamine monomer, the twisted structure of the diamine monomer can weaken intermolecular charge transfer and improve the transmittance of polyamide, and the unique aggregation-induced emission phenomenon of the diamine monomer can greatly improve the fluorescence effect.
Owner:JILIN UNIV
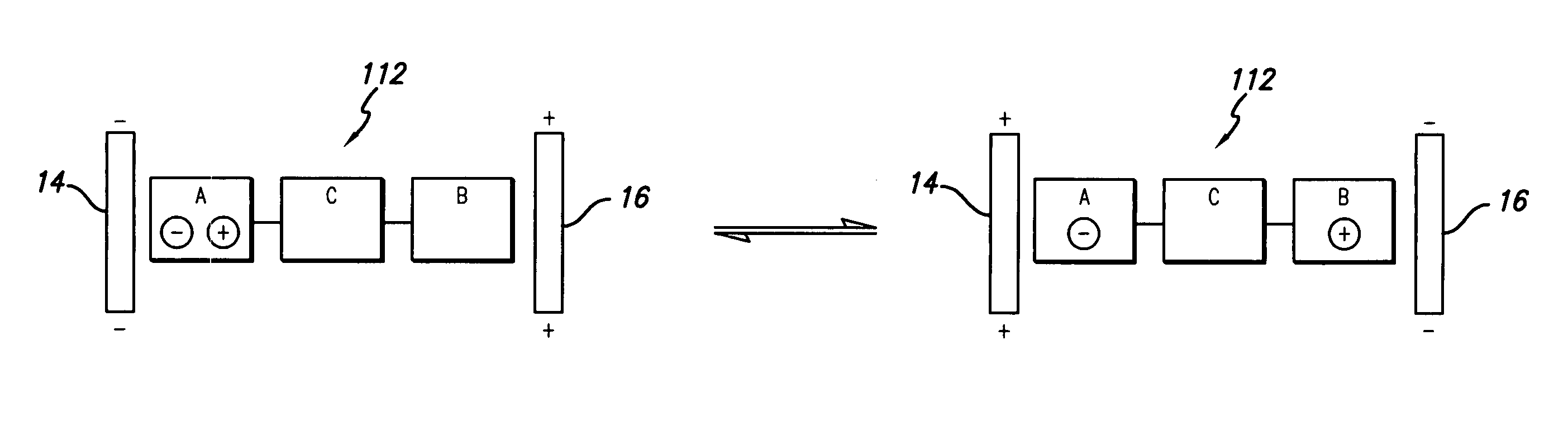
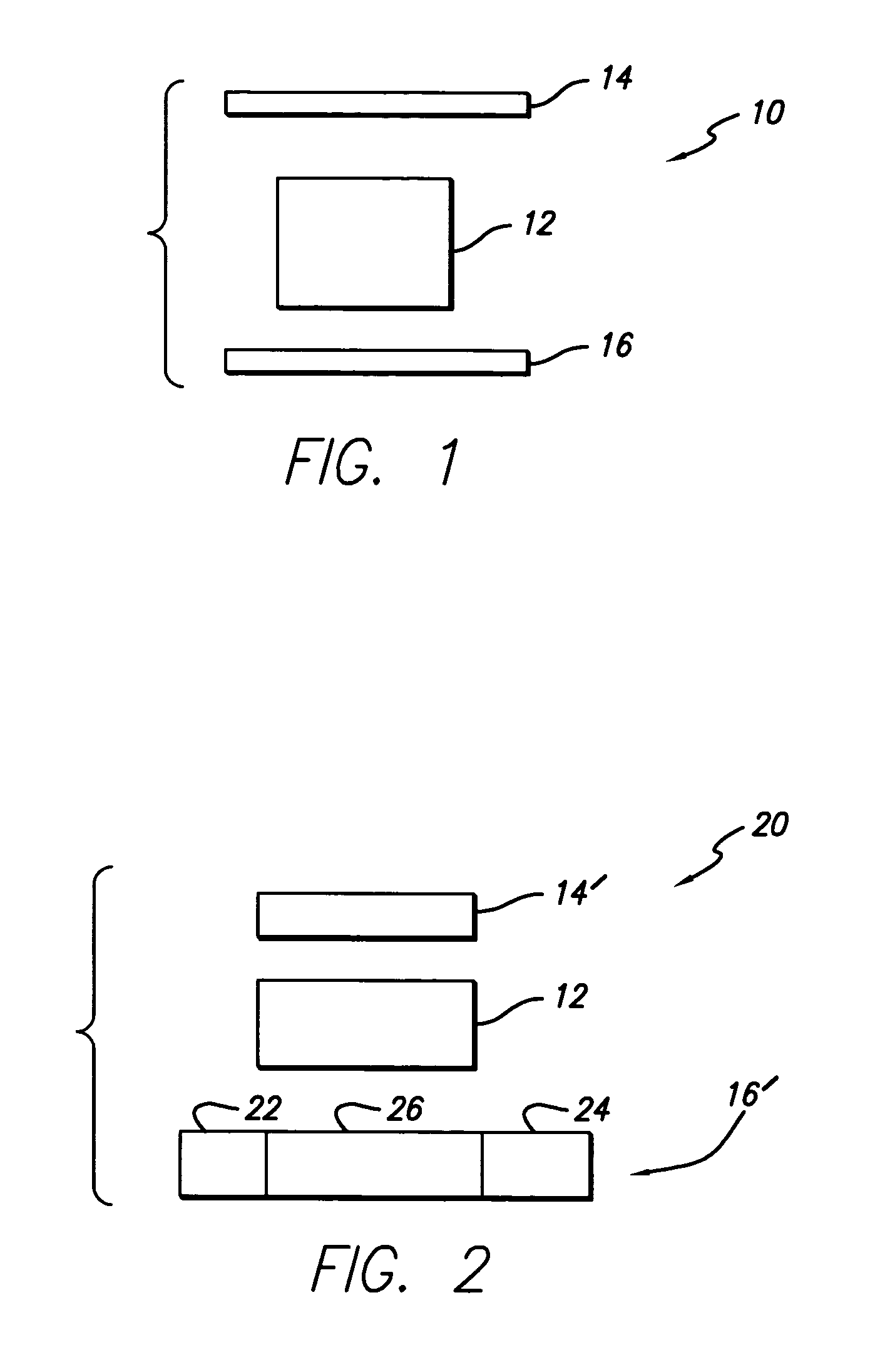
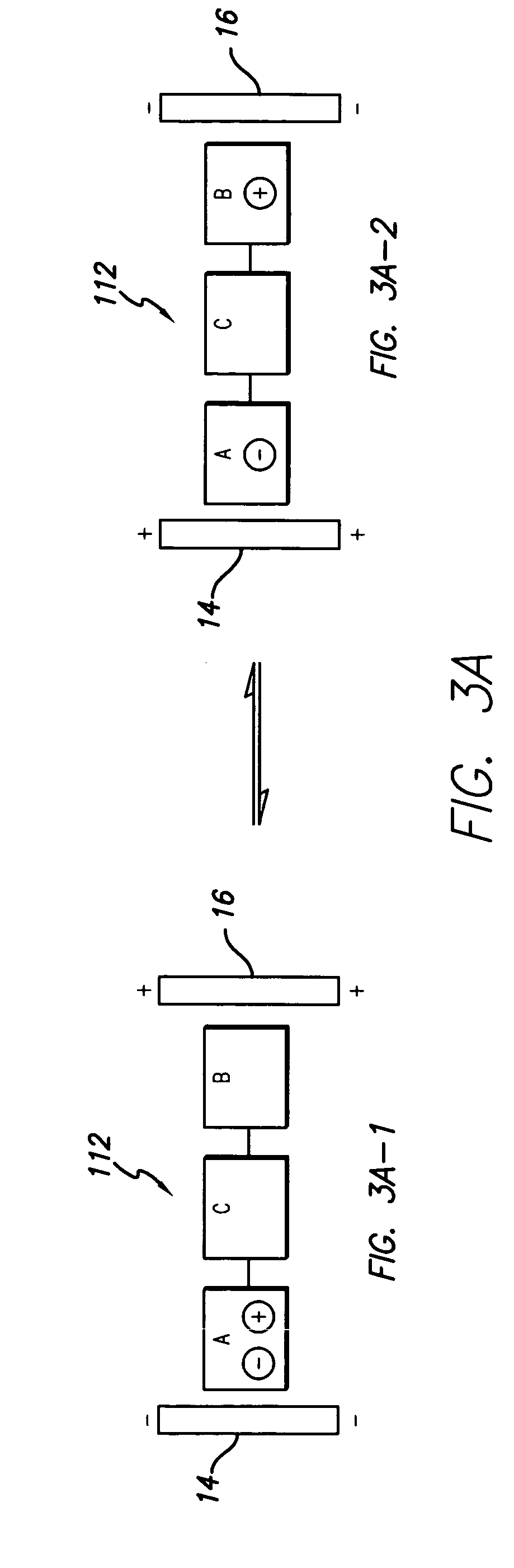
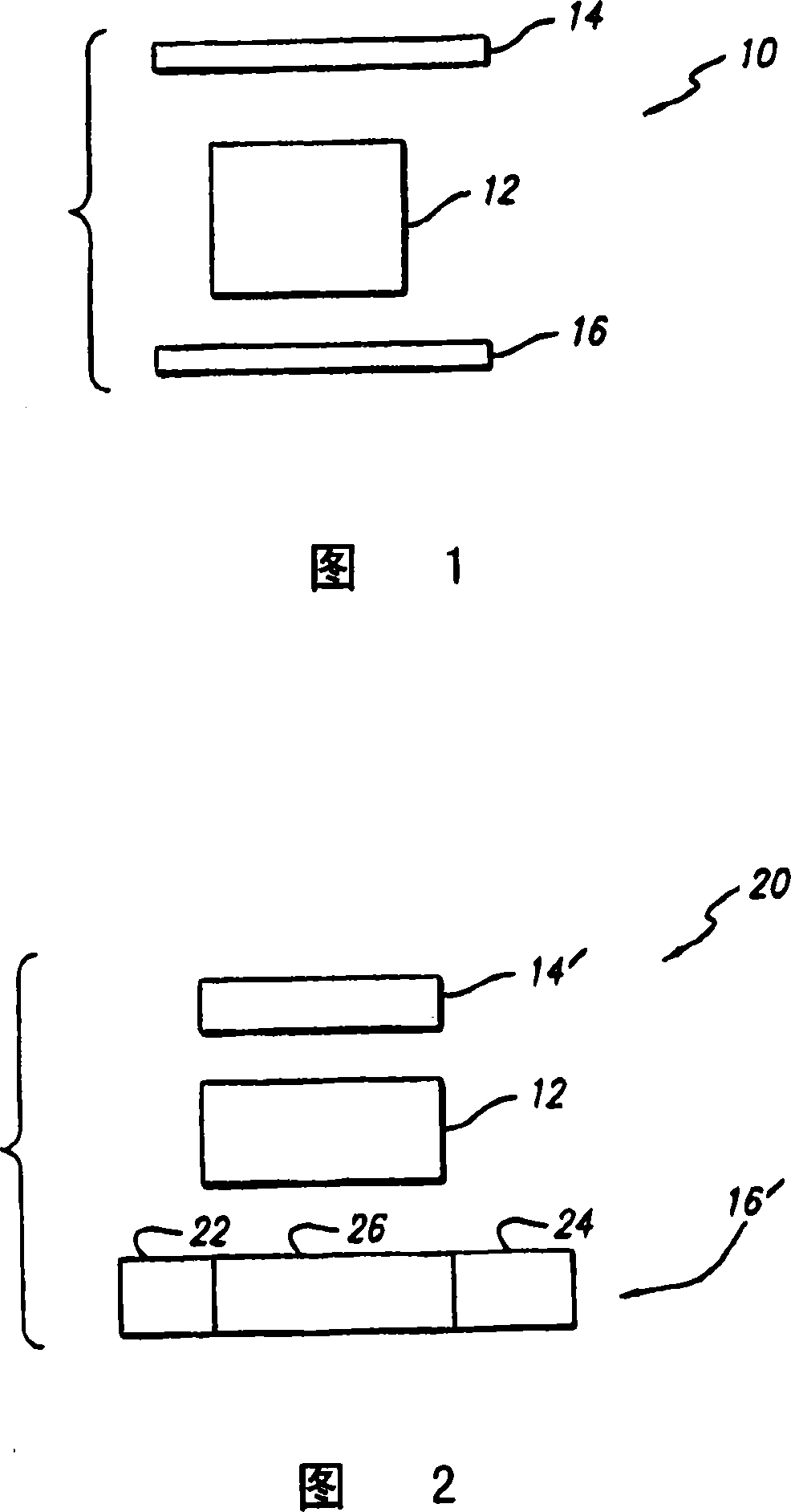
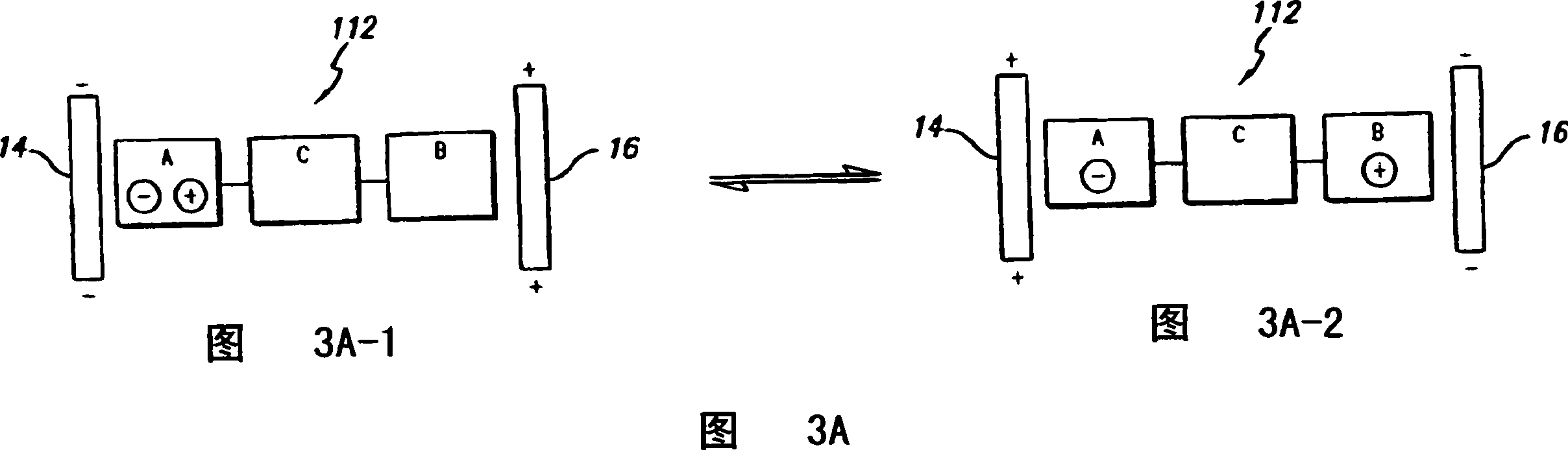
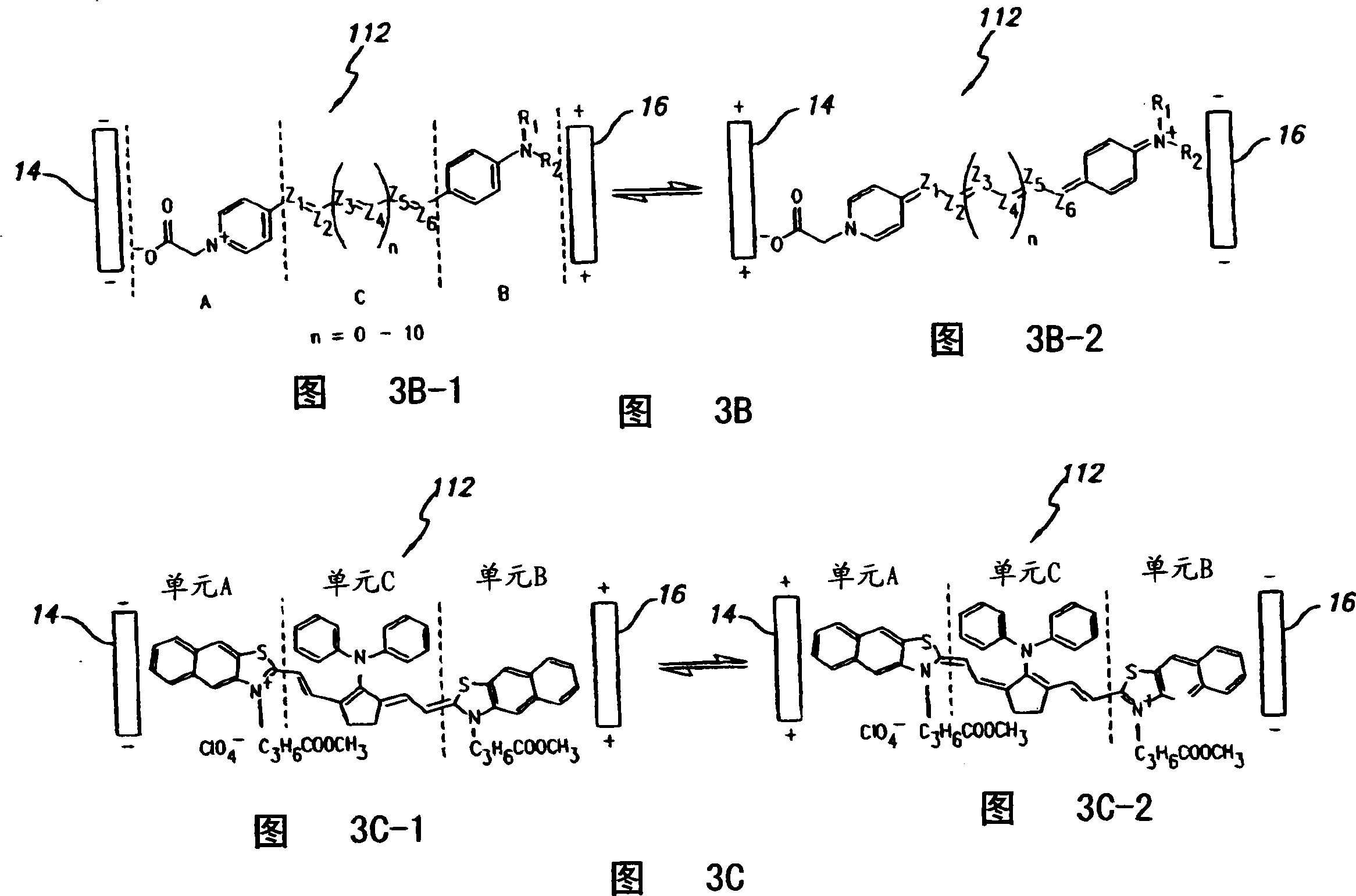
Composition of matter which results in electronic switching through intra- or inter- molecular charge transfer, or charge transfer between molecules and electrodes induced by an electrical field
A composition of matter is provided that results in a change of electrical properties through intra-molecular charge transfer or inter-molecular charge transfer or charge transfer between a molecule and an electrode, wherein the charge transfer is induced by an electric field.
Owner:HEWLETT PACKARD DEV CO LP
Compound for organic optoelectronic device and organic optoelectronic component containing compound
ActiveCN109593081AHas a glass transition temperatureImprove external quantum efficiencyGroup 4/14 element organic compoundsSolid-state devicesDisplay deviceHole transport layer
The invention provides a compound for an organic optoelectronic device and an organic optoelectronic element containing the compound and application and specifically discloses a compound shown as a chemical formula 1. A hole injection layer, a hole transmission layer, an electron blocking layer or a light emitting layer of an organic light emitting device (OLED) contains the compound. A donor group with high thermal stability and a receptor group are connected through a non-conjugated linkage system and the obtained compound can form an intermolecular charge transfer excitation state to realize light emission. Meanwhile, the compound has high glass transition temperature and good thermal and light stability. A preparation method of the compound is simple and large-scale production is easyto realize; the compound is used as a functional layer and is applied to an OLED element; the obtained element has the advantages of high efficiency, low operation voltage and long service life, can be applied to a display device or a light source device and has a very good commercial application prospect.
Owner:SHANGHAI BLUESHINE OPTOELECTRONICS TECH CO LTD
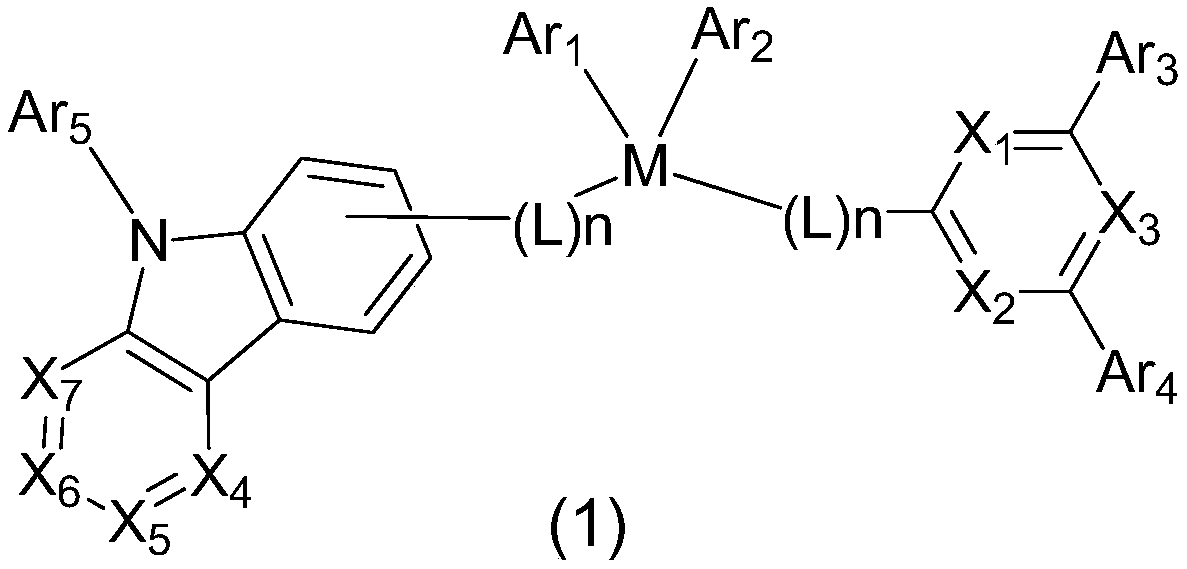
Intermolecular charge transfer type fluorescent dyes and use thereof
InactiveCN1699502AStrong electrophileEasy to synthesizeElectrical apparatusElectroluminescent light sourcesElectricityCarbazole
The invention provides an intermolecular charge transfer type fluorescent dyes and its preparation, which comprises using fine cavity transmission groups of triphenylamine, carbazole and fentiazin as electron donors, using nitrile radicals with strong pro-electron capacity as electron donors, forming C=N conjugated pi bond connection through simple condensation reaction, thus preparing a series of type P luminescent materials. Experiment has proved that, these materials are red electroluminescent materials having pure emission.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Intramolecular charge transfer chromophore containing triphenylamine group and its synthesis method
InactiveCN1865240AImprove thermal stabilityImprove solubilityCarboxylic acid nitrile preparationOrganic compound preparationPhotoluminescenceSynthesis methods
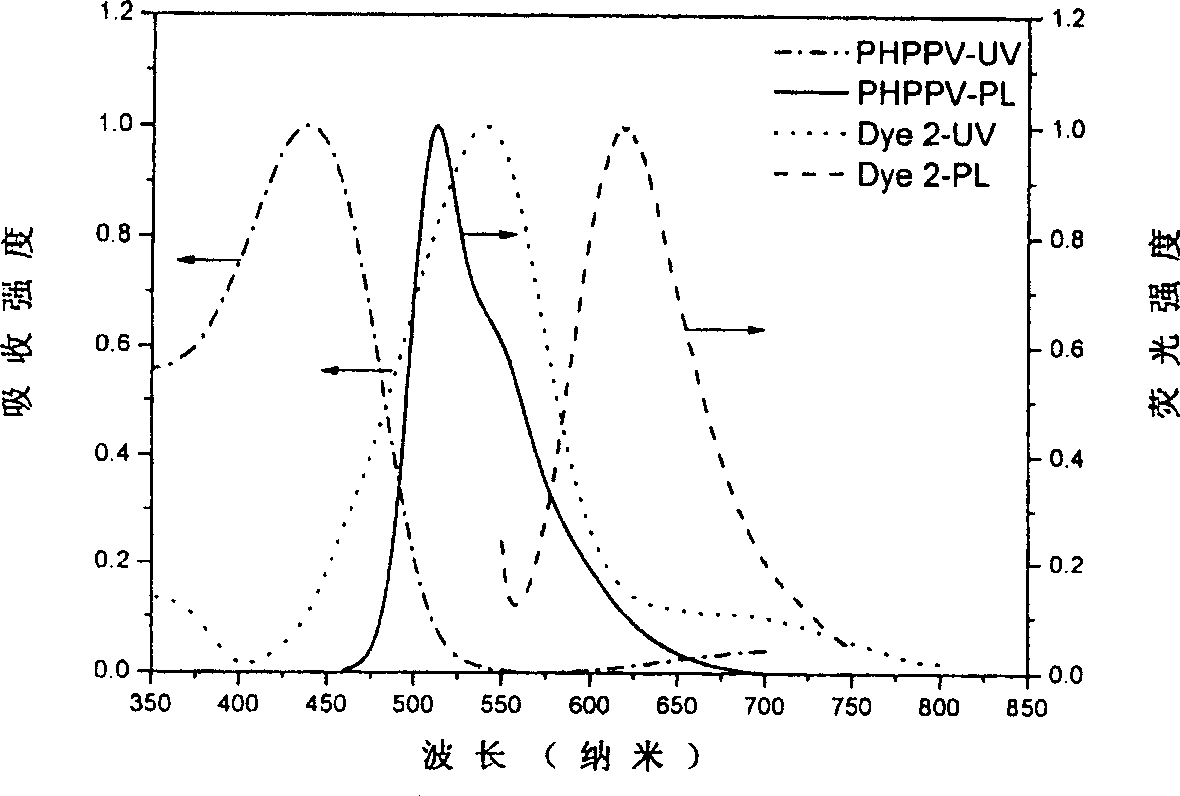
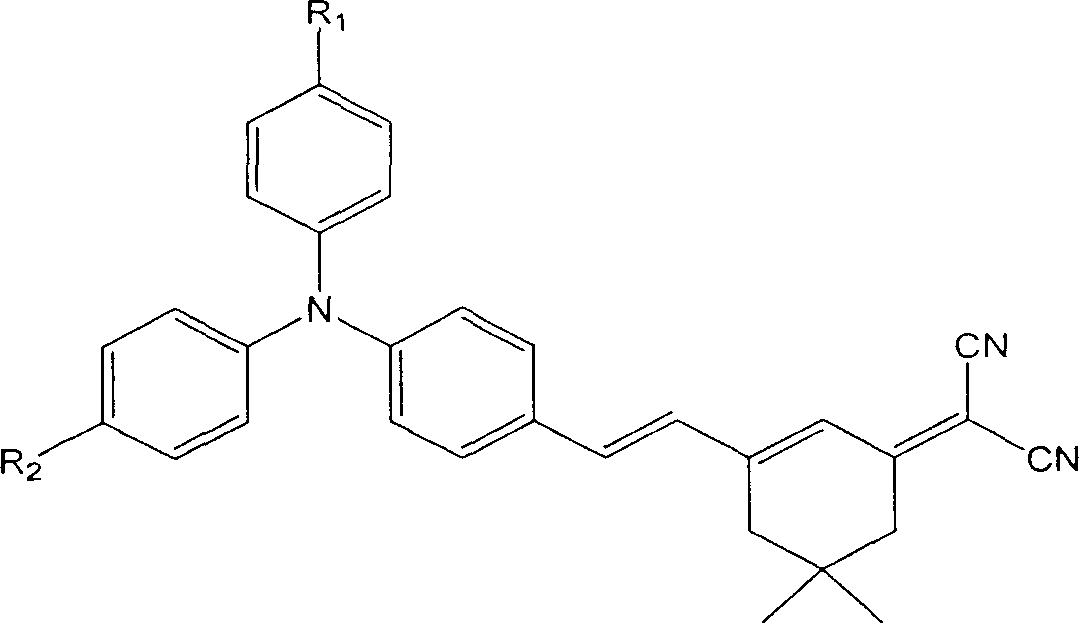
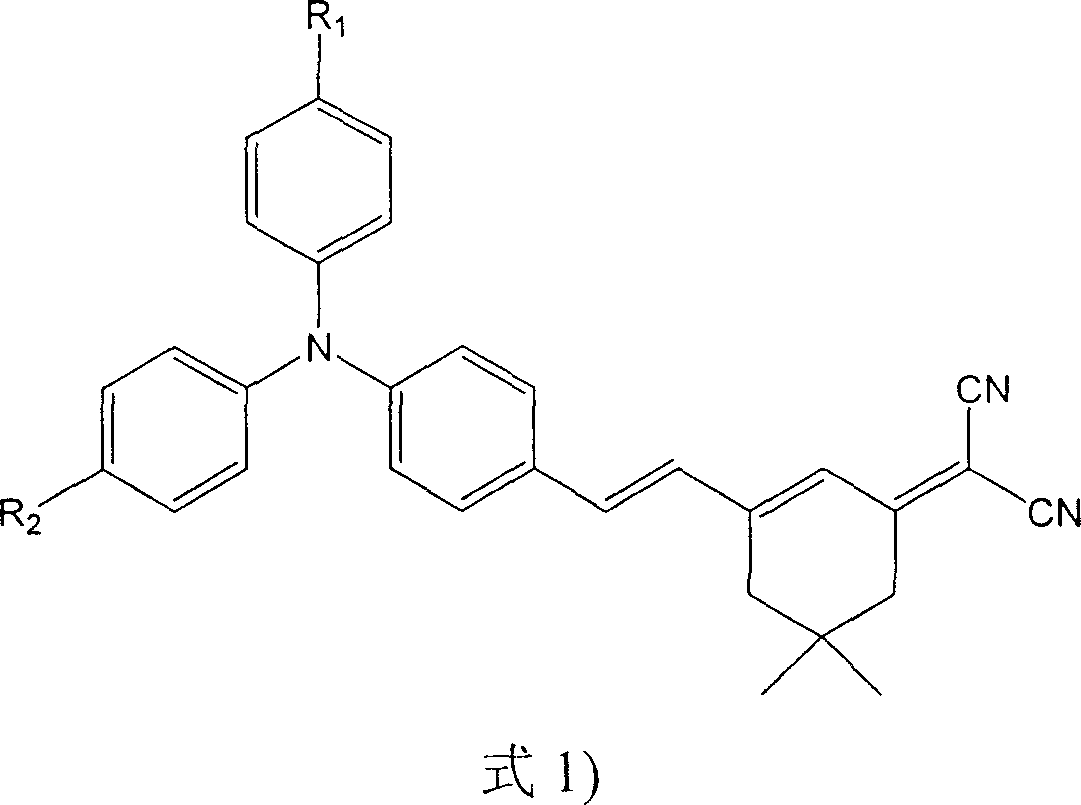
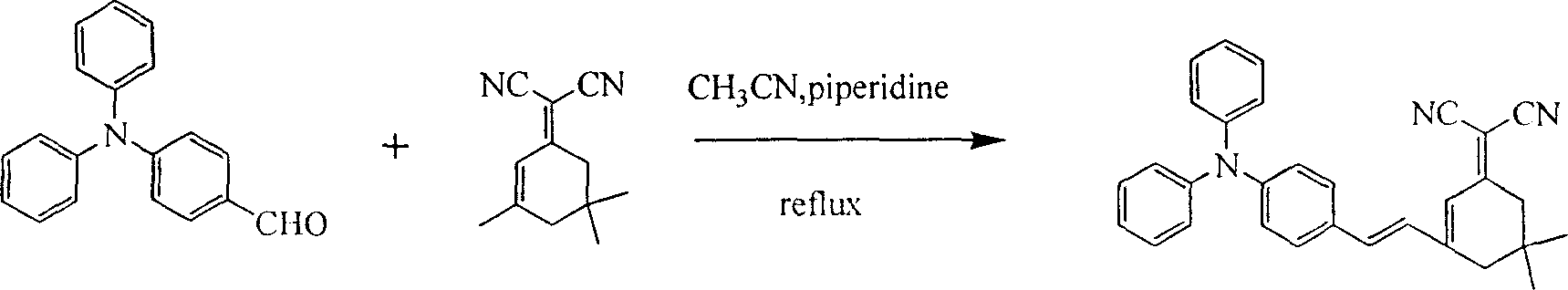
This invention discloses an intermolecular charge transfer chromogen with triphenylamine group, with a formula indicated by (1), wherein: R1 is hydrogen atom or methyl group or methoxyl group, R2 is hydrogen atom or 2-(5,5-dimethyl-3-vinylcyclohexenyl-2-enylidene)propanedinitrile group. This invention applies mild condition uses substituent group triphenylamine aldehyde or triphenylamine dialdehyde as raw material to react by heating with 2-(5,5-dimethyl-3-vinylcyclohexenyl-2-enylidene)propanedinitrile group to produce the intermolecular charge transfer chromogen with high decomposition temperature, non-coplanar structure, and triphenylamine group. By changing R1 and R2, it can obtain different absorption peak value and fluorescent emission peak, which could be used in photoluminescence, electroluminescence, data storage, light communication and bio-imaging.
Owner:ZHEJIANG UNIV
OLED device, display panel and display device
ActiveCN109742251ASolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceElectron transfer
The invention relates to the technical field of OLEDs, and discloses an OLED device, a display panel and a display device. The OLED device comprises an anode, a light emitting layer, a cathode, a charge transfer and hole transmission structure and / or a charge transfer and electron transmission structure, wherein the charge transfer and hole transmission structure is located between the anode and the light emitting layer and comprises a first photo-induction electron transfer material and a hole transmission material; the first photo-induction electron transfer material is configured to generate intermolecular charge transfer with the hole transmission material under optical excitation; the charge transfer and electron transmission structure is located between the cathode and the light emitting layer and comprises a second photo-induction electron transfer material and an electron transmission material; and the second photo-induction electron transfer material is configured to generateintermolecular charge transfer with the electron transmission material under optical excitation. The OLED device is capable of improving the light emitting performance of devices.
Owner:BOE TECH GRP CO LTD +1
A composition of matter which results in electronic switching through intra- or inter- molecular charge transfer between molecules and electrodes induced by electricity
A composition of matter is provided that results in a change of electrical properties through intra-molecular charge transfer or inter-molecular charge transfer or charge transfer between a molecule (12) and an electrode (14, 16; 14', 16'), wherein the charge transfer is induced by an electric field.
Owner:HEWLETT PACKARD DEV CO LP
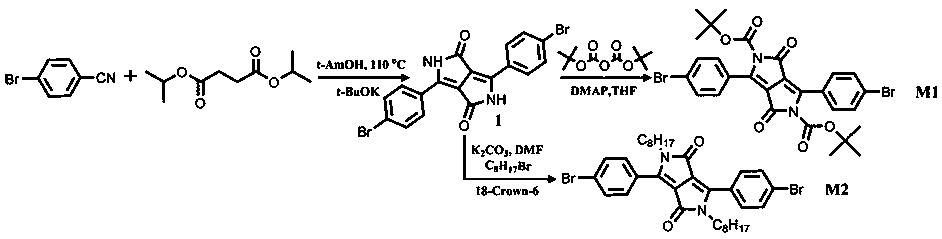
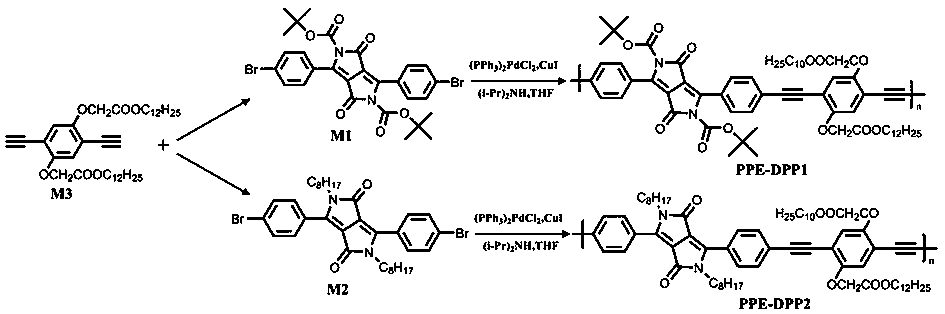
Fluorescent conjugated polymer containing DPP building unit as well as preparation method and application thereof
ActiveCN110003449AAmplify the response effectImprove responsivenessLuminescent compositionsFluorescenceBackbone chain
The invention discloses a fluorescent conjugated polymer containing a DPP building unit as well as a preparation method and application of the fluorescent conjugated polymer containing the DPP building unit. By using a Sonogashira coupling method, the fluorescent conjugated polymer of which the main chain is of a DPP structure and the side chain has different structures is synthesized. In the invention, DPP is taken as an electron-deficient system, an intermolecular charge transfer can be formed between DPP and strong electron-donating anions, and electron donating abilities of different anions are different, thereby further affecting the fluorescence of a conjugated polymer to realize response detection of the anions. The fluorescent conjugated polymer containing the DPP building unit isapplied to sensing detection of common anions in a tetrahydrofuran solution system, thereby having the effect of responsive detection. After being detected, the fluorescent conjugated polymer has goodresponse to OH<->, F<->, Cl<->, Br<->, I<-> and AcO<-> and can visually observe color changes of the polymer solution and changes of fluorescence colors, thereby being convenient to realize the response detection of the anions.
Owner:SUZHOU UNIV
Electroluminescent material, method for manufacturing same, and light emitting device
ActiveUS11322694B2Extend your lifeEnhance electron-withdrawing propertyOrganic chemistrySolid-state devicesElectron donorFluorenone
The present application provides an electroluminescent material, a method for manufacturing an electroluminescent material, and a light emitting device, by employing the strong electron-withdrawing group such as cyano, pyridine, pyrimidine, or s-triazine to enhance the electron-withdrawing property of the fluorenone receptor unit, a captodative electron effect between the electron donor unit and the electron acceptor unit in the molecule is enhanced, so that the intermolecular charge transfer property is enhanced while the red light shifts, thereby further reducing the energy level difference between the single-line energy level and the triplet energy level of the target molecule, to realize a long life span, red light emitted electroluminescent material, a method for manufacturing the electroluminescent material and a light emitting device.
Owner:WUHAN CHINA STAR OPTOELECTRONICS SEMICON DISPLAY TECH CO LTD
Electroluminescent material, method for manufacturing same, and light emitting device
The present application provides an electroluminescent material, a method for manufacturing an electroluminescent material, and a light emitting device, by employing the strong electron-withdrawing group such as cyano, pyridine, pyrimidine, or s-triazine to enhance the electron-withdrawing property of the fluorenone receptor unit, a captodative electron effect between the electron donor unit and the electron acceptor unit in the molecule is enhanced, so that the intermolecular charge transfer property is enhanced while the red light shifts, thereby further reducing the energy level difference between the single-line energy level and the triplet energy level of the target molecule, to realize a long life span, red light emitted electroluminescent material, a method for manufacturing the electroluminescent material and a light emitting device.
Owner:WUHAN CHINA STAR OPTOELECTRONICS SEMICON DISPLAY TECH CO LTD
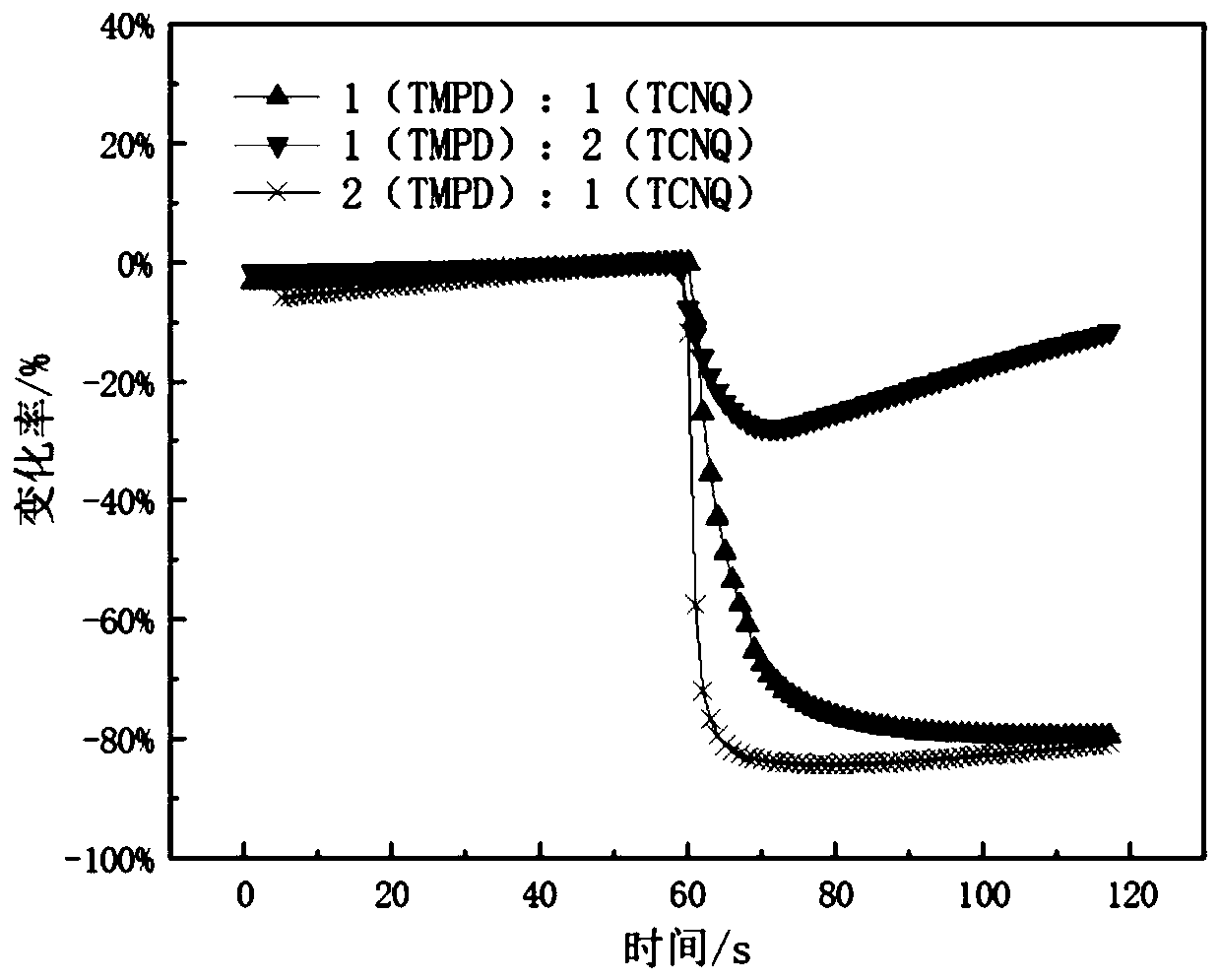
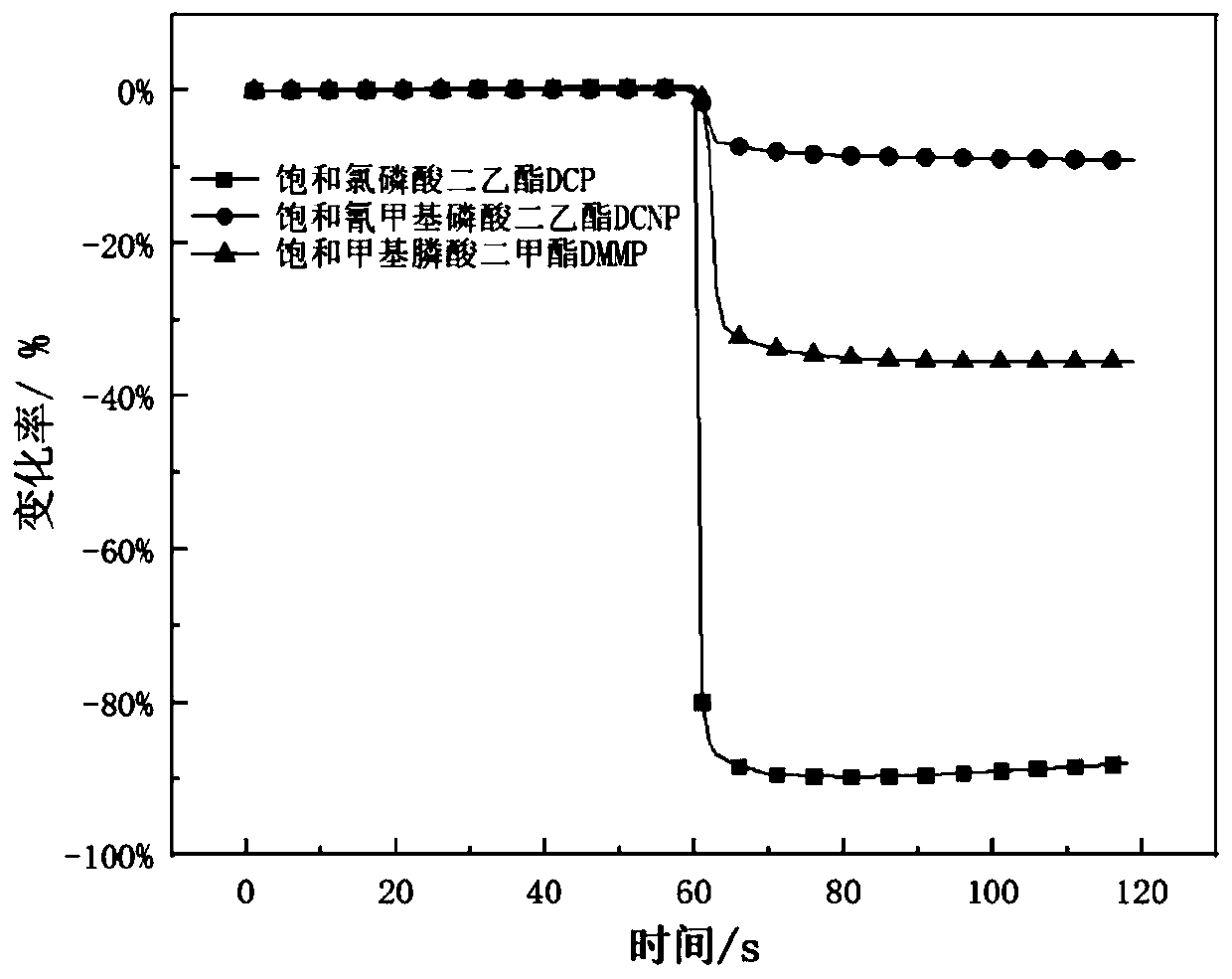
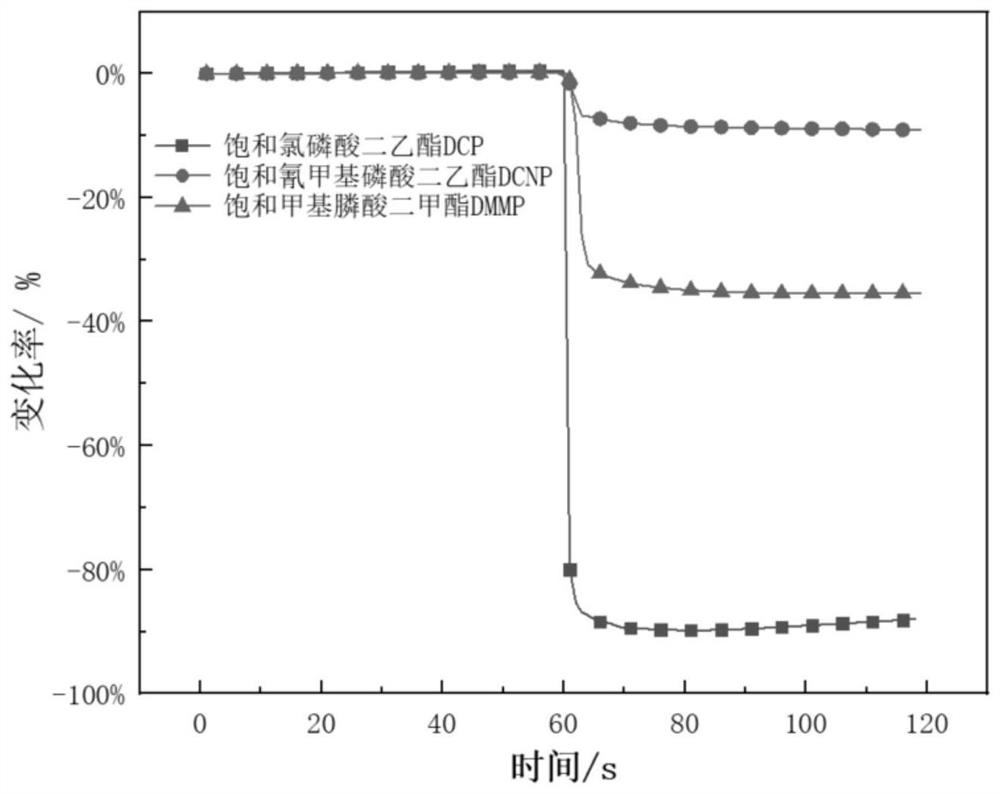
Method for detecting diethyl chlorophosphate gas and/or sarin toxic gas based on charge transfer complex
ActiveCN109752417AImprove conductivityGood film formingMaterial resistanceCharge-transfer complexElectrochemistry
The invention relates to a method for detecting diethyl chlorophosphate gas and / or sarin toxic gas based on a charge transfer complex. The method comprises the steps that a first compound containing aN,N dimethyl aromatic amine structure and a second compound containing a tetracyanoquinodimethane structure interacts through intermolecular charge transfer in a solvent to obtain a solution of the charge transfer complex; a sensing material is loaded on the surface of an electrode to form a sensor piece; and the sensor piece is put into the atmosphere containing the diethyl chlorophosphate gas and / or the sarin toxic gas to be detected. According to the method, through the charge transfer complex formed by mixing of the first compound and the second compound in the solvent, the high electronmobility and high hole mobility are achieved, the independent chemical physical properties of the first compound and the second compound are maintained while complexing is conducted, the charge transfer complex can serves as an electrochemical sensing probe to detect the sarin toxic gas, and the problems that in the prior art, detection sensitivity of the sarin toxic gas is low, specificity is low, the means is complex, and the cost is high are solved.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
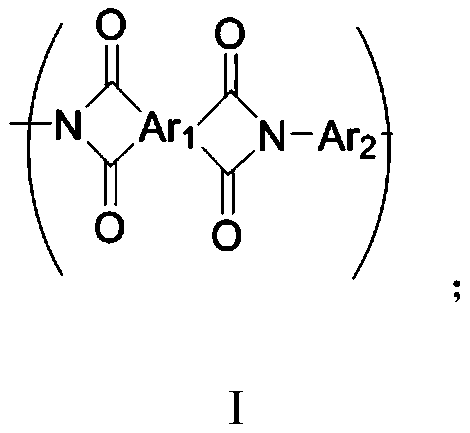
Polyimide, polyimide film and preparation method thereof
The invention discloses polyimide, a polyimide film and a preparation method thereof, and belongs to the technical field of film materials. The structural general formula of the polyimide is shown inthe specification, wherein Ar1 represents a tetravalent organic group with a C10-C64 aromatic ring group or alicyclic group; Ar2 represents a divalent organic group having a C6-C60 aromatic ring group. According to the polyimide disclosed in the embodiment of the invention, a coplanar rigid structure and a twisted non-coplanar rigid structure are introduced into a main chain structure; compared with the prior art, the advantages are that the intermolecular acting force of polyimide can be improved, the intermolecular conjugation effect is obvious, intramolecular and intermolecular charge transfer is obvious, meanwhile, molecular chains are uniformly arranged, and the rigidity effect of the polyimide molecular chains and the coloring degree of the film are improved, so that the polyimide film with excellent heat resistance and mechanical strength can be prepared.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Novel n type organic semiconductor material
The invention discloses a novel n type organic semiconductor material. The novel n type organic semiconductor material comprises five-parallel (six-parallel, seven-parallel or eleven-parallel) fused ring unit-based A-D-A type conjugated micromolecules and porphyrin micromolecules, wherein the five-parallel (six-parallel, seven-parallel or eleven-parallel) fused ring unit-based A-D-A type conjugated micromolecules are used for changing the position where a terminal electron-withdrawing group is linked with a penta aromatic heterocyclic ring according to structural characteristics of an A-D-A type receptor material, steric hindrance can be reduced, a material is enabled to have better steric configuration, and isotropic charge transmission can be favorably formed, so that the performance ofa device can be increased; the porphyrin micromolecules enable an electron-withdrawing terminal group A to be linked to a beta position of a penta heterocyclic ring or a meta position of a hepta heterocyclic ring according to the structure characteristics of a porphyrin compound, and better intermolecular charge transfer exists between a porphyrin core and the electron-withdrawing terminal group,so that the compound has better charge transmission performance; meanwhile, the steric hindrance can be reduced, and the material is enabled to have better steric configuration, so that the performance of the device can be increased.
Owner:NANJING UNIV OF TECH
Compound for organic photoelectric device and organic photoelectric device comprising same
ActiveCN109593081BHas a glass transition temperatureImprove external quantum efficiencyGroup 4/14 element organic compoundsSolid-state devicesPhoto stabilityDisplay device
Owner:SHANGHAI BLUESHINE OPTOELECTRONICS TECH CO LTD
Fluorescent conjugated polymer containing pyrrolopyrrolidone building block, preparation method and application
ActiveCN110003449BImplement Responsive DetectionAmplify the response effectLuminescent compositionsFluorescencePyrrolidinones
The invention discloses a fluorescent conjugated polymer containing a pyrrolopyrrolidone building unit, a preparation method and an application. Fluorescent conjugated polymers with pyrrolopyrrolidone structure as the main chain and different structures in the side chains were synthesized by Sonogashira coupling method. In the present invention, since pyrrolopyrrolidone is an electron-deficient system, intermolecular charge transfer can be formed with strong electron-donating anions, and the electron-donating abilities of different anions are different, which further affects the fluorescence of the conjugated polymer, thereby Responsive detection of anions is achieved. It is applied to the sensing and detection of common anions in tetrahydrofuran solution system, and has the effect of responsive detection. After testing, its pair of OH ‑ , F ‑ , Cl ‑ 、Br ‑ , I ‑ 、AcO ‑ It has a good response effect, and the color change of the polymer solution and the change of the fluorescent color can be observed with the naked eye, and the response detection to the anion can be realized conveniently.
Owner:SUZHOU UNIV
A method for detecting clodronate gas and/or sarin gas based on charge transfer complex
ActiveCN109752417BImprove conductivityGood film formingMaterial resistanceCharge-transfer complexElectrochemistry
The invention relates to a method for detecting diethyl clodronate gas and / or sarin poisonous gas based on a charge transfer complex, comprising the steps of: comprising a first compound containing N,N dimethyl aromatic amine structure and a compound containing tetracyanide The second compound of the dimethyl-p-benzoquinone structure obtains a solution of the charge-transfer complex through intermolecular charge-transfer interactions in a solvent; the sensing material is loaded on the surface of the electrode to form a sensing device; the sensing device is placed in a chlorophosphine-containing detection in the atmosphere of diethyl acetate gas and / or sarin gas. In the present invention, the charge transfer complex formed by mixing the first compound and the second compound in a solvent has high mobility of electrons and holes, and maintains the independent chemical and physical properties of the first compound and the second compound while compounding , can be used as an electrochemical sensing probe to detect sarin poisonous gas, which solves the problems of low detection sensitivity, low specificity, complicated means and high cost of sarin poisonous gas in the prior art.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
9-naphthanthracene derivative monomolecular white light material as well as preparation and application thereof
ActiveCN108069816AGuaranteed yieldHigh yieldSolid-state devicesSemiconductor/solid-state device manufacturingChemical structureAnthracene
The invention discloses a 9-naphthanthracene derivative. The 9-naphthanthracene derivative uses a dianthracene group as a center and uses the effect of the naphthaline group on molecular arrangement,so that the molecules show the typical blue light emission of the anthracene group in the monomolecular state, forms the intermolecular charge transfer state during the molecular accumulation, shows the green light emission, and forms yellow light emission with excimers; through the three joint effects, the compound is the monomolecular white light material. Through the improvement on the key chemical structure of the 9-naphthanthracene derivative and the preparation process, a novel intermolecular charge transfer state combining excimer 9-naphthanthracene derivative monomolecular white lightmaterial is obtained; the 9-naphthanthracene derivative can be synthesized by a simple synthesis method, and can be used as an electroluminescent material, so that the technical problems of preparation complexity, light color instability and the like of the existing monomolecular white light material are solved.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Fluorenyl cyano indanone non-conjugated polymer receptor and preparation method thereof
PendingCN113861392ASimple structureEasy to synthesizeSolid-state devicesSemiconductor/solid-state device manufacturingPolymer scienceBackbone chain
The invention relates to a fluorenyl cyano indanone non-conjugated polymer receptor and a preparation method thereof. The invention relates to a conjugated polymer based on thiophene and benzothiadiazole and a preparation method thereof. According to the invention, a non-trapezoidal condensed ring micromolecule monomer is designed and synthesized by using fluorene and cyano indanone structural units, and then the non-conjugated polymer receptor with good solubility is synthesized by a Stille copolymerization method. According to the invention, non-trapezoidal fused ring small molecule receptors are bridged through a non-conjugated alkyl chain to form a main chain non-conjugated polymer receptor, the main chain non-conjugated polymer receptor has wide and strong absorption in a visible-near infrared region, and a flexible side chain can provide good solubility for a material. The effective intermolecular charge transfer can occur between the polymer and a donor, and the polymer has good physicochemical properties. Therefore, the material has a potential application prospect in polymer solar cells as a semiconductor active layer donor material.
Owner:FUZHOU UNIV
A kind of oled device, display panel and display device
ActiveCN109742251BSolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceEngineering
The invention relates to the technical field of OLEDs, and discloses an OLED device, a display panel and a display device. The OLED device includes an anode, a light-emitting layer, and a cathode; it also includes: a charge transfer and hole transport structure, located between the anode and the light-emitting layer, including a first photo-induced electron transfer material and a hole transport material, and the first photo-induced electron transfer material configured to undergo intermolecular charge transfer between the hole-transport material and the charge-transfer material under photoexcitation; and / or, a charge-transfer and electron-transport structure located between the cathode and the light-emitting layer, including a second light-induced electron transfer material and electron The transport material, the second photo-induced electron transfer material is configured to undergo intermolecular charge transfer between the electron transport material and the photo-excited material. The above-mentioned OLED device can improve the luminous performance of the device.
Owner:BOE TECH GRP CO LTD +1
Intramolecular charge transfer type red luminescent material and preparation and application thereof
InactiveCN100341977CEasy to separateHigh yieldElectrical apparatusElectroluminescent light sourcesElement analysisElectron donor
The present invention relates to serial intermolecular charge transfer type red light emitting materials with unparallel dipole system and its preparation process. The serial red light emitting materials are prepared with triphenylamine and diphenyl naphthylamine with hole transferring radical or their derivative as electron donor and nitrile group with powerful electrophilicity as electronic receptor and through simple Stille coupling reaction and condensation. Their molecular structure, electrochemical properties and physical optical property have been researched and characterized, and devices with the material as light emitting layer and electron transferring layer have been prepared. Tests show that these materials are active red light emitting materials with excellent application foreground.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Receptor material based on di(bithiophene)six-membered heterocycle as well as preparation method and application of receptor material
ActiveCN111018884AExtended conjugate lengthEnhanced light absorptionOrganic chemistrySolid-state devicesMolecular orbital energyReceptor
The invention relates to an acceptor material based on a di(bithiophene)six-membered heterocycle as well as a preparation method and an application of the acceptor material. According to the small molecule acceptor material, an asymmetric five-membered fused ring structure composed of two fused dithiophenes and a six-membered ring containing an oxygen group heteroatom is used as a parent nucleus;conjugated groups are introduced into the two sides of the parent nucleus to serve as pi bridges, electron withdrawing terminal groups which are connected with the pi bridge and have specific structures are designed on the two sides of the parent nucleus; finally, a series of brand-new A-D-A type small molecule receptor materials with good solubility and good thermal stability are formed; the parent nucleus of the acceptor material has strong electron donating capability, the pi bridge effectively prolongs the conjugate length of the molecule; the terminal electron withdrawing group A can effectively adjust the lowest unoccupied molecular orbital (LUMO) energy level of molecules, widens the absorption spectrum of the material, reduces the optical band gap of the material, and enhances theintermolecular charge transfer ability, thereby enhancing the photocurrent, and is especially suitable for preparing solar cells with high short-circuit current and high energy conversion efficiency.
Owner:HENAN UNIVERSITY
Phenanthro-carbazole donor-acceptor organic dye and application thereof in dye-sensitized solar cell
ActiveCN103819929BImprove solubilityRealize step-by-step regulationLight-sensitive devicesAzo dyesSolubilityQuantum yield
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
An organic electro-optic element
ActiveCN109638170BLife expectancyImprove external quantum efficiencySolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceFluorescent light
Owner:SHANGHAI BLUESHINE OPTOELECTRONICS TECH CO LTD
A 9-naphthalene anthracene derivative monomolecular white light material and its preparation and application
ActiveCN108069816BGuaranteed yieldHigh yieldSolid-state devicesSemiconductor/solid-state device manufacturingChemical structureGreen-light
The invention discloses a 9-naphthalene anthracene derivative. The 9-naphthalene anthracene derivative is centered on a bisanthracene group, and utilizes the effect of the naphthalene group on molecular arrangement, so that the molecule presents an anthracene group in a single molecular state. The classic blue light emission of the group, the green light emission of the intermolecular charge transfer state formed when the molecules are stacked, and the yellow light emission of the excimer associations, the three combined effects make this kind of compound a single-molecule white light material. In the present invention, by improving the key chemical structure and preparation process of the 9-naphthalene anthracene derivative, a new single-molecule white light material of the 9-naphthalene anthracene derivative in which an intermolecular charge transfer state is combined with an excimer association is obtained, Moreover, the 9-naphthalene anthracene derivative can be synthesized by a simple synthesis method, and can be used as an electroluminescence material, thereby solving technical problems such as complex preparation of existing single-molecule white light materials, unstable light color, and the like.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Diamine monomer containing tetraphenylethylene-bisarylamine structure, preparation method and application in polyamide synthesis
ActiveCN110606810BImprove transmittanceReduce transferOrganic compound preparationTenebresent compositionsSimple Organic CompoundsPolymer science
The invention discloses a diamine monomer containing a tetraphenylethylene-diarylamine structure, a preparation method and application of the diamine monomer in polyamide synthesis, belonging to the technical field of organic compounds. A polyamide material generally has the problems of dark color, poor fluorescent effect and the like, and the fluorescence effect is weak when a triphenylamine group is directly introduced into the polyamide material, so that a tetraphenylethylene structure and a triphenylamine group are combined together to obtain the diamine monomer containing the tetraphenylethylene-diarylamine structure and introduced into polyamide in the form of the diamine monomer, the twisted structure of the diamine monomer can weaken intermolecular charge transfer and improve the transmittance of polyamide, and the unique aggregation-induced emission phenomenon of the diamine monomer can greatly improve the fluorescence effect.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com