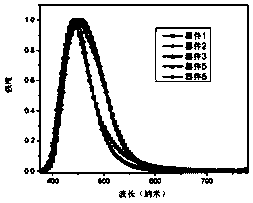
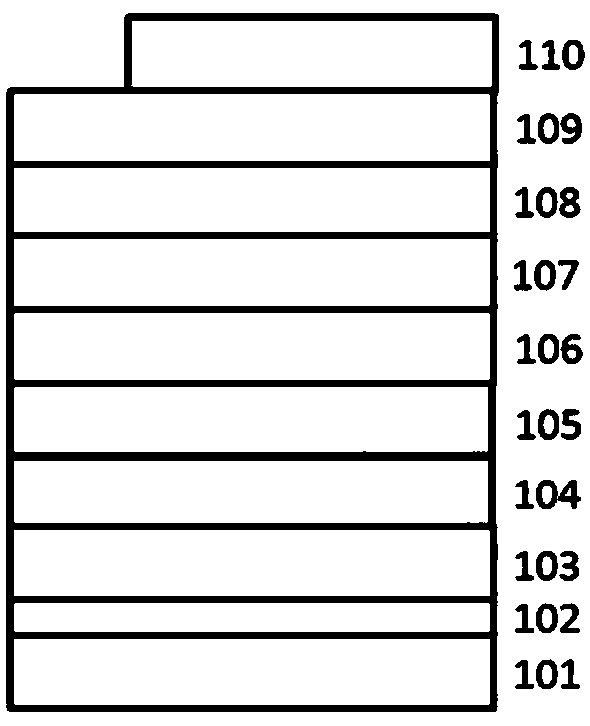
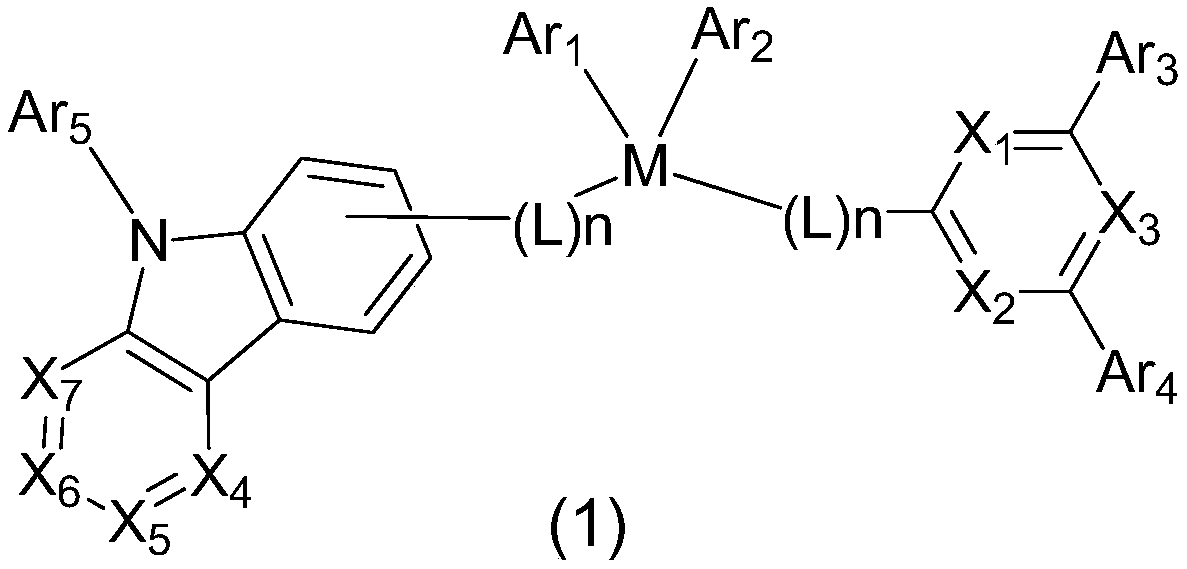
Compound for organic optoelectronic device and organic optoelectronic component containing compound
A kind of organic optoelectronic device, optoelectronic device technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the synthesis of C-8-1
[0071]
[0072] a) Under nitrogen atmosphere, bis(4-bromophenyl)diphenylmethane (4.4g) and 9-phenylcarbazole-3-boronic acid (3g) were completely dissolved in 120mL tetrahydrofuran in a 250ml round bottom flask , 60 mL of 2M aqueous sodium carbonate solution was added, and then tetrakis-(triphenylphosphine)palladium (0.3 g) was added, and the mixture was heated and stirred for 12 hours. After cooling to room temperature, the aqueous layer was removed. Add 100 mL of dichloromethane, and wash twice with 30 mL of saturated brine. The dichloromethane layer was dried over anhydrous magnesium sulfate, and concentrated in vacuo. Then dichloromethane:ethyl acetate (20:1~2:1) was used as eluent to purify and separate on a silica gel column to obtain (4-(9-phenylcarbazol-3-yl)phenyl)- (4-Bromophenyl)diphenylmethane, white solid 5.8 g (90% yield); MS (ESI): 640.2 (M+H).
[0073] b) Dissolve (4-(9-phenylcarbazol-3-yl)phenyl)-(4-bromophen...
Embodiment 2
[0075] Embodiment 2: the synthesis of S-8-1
[0076]
[0077] Similar to the synthesis conditions of Example 1, using bis(4-bromophenyl)diphenylsilane as a raw material, the final product S-8-1 can be obtained through the same reaction conditions and separation and purification steps, MS (ESI) : 809.3(M+H)
Embodiment 3
[0078] Embodiment 3: the synthesis of S-8-31
[0079]
[0080] Similar to Example 2, 9-phenylcarbazole-3-boronic acid was replaced with 9-biphenylcarbazole-3-boronic acid, and S-8-31 could be obtained through the same reaction conditions and separation steps. MS (ESI): 885.3 (M+H) glass transition temperature 143 degrees.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com