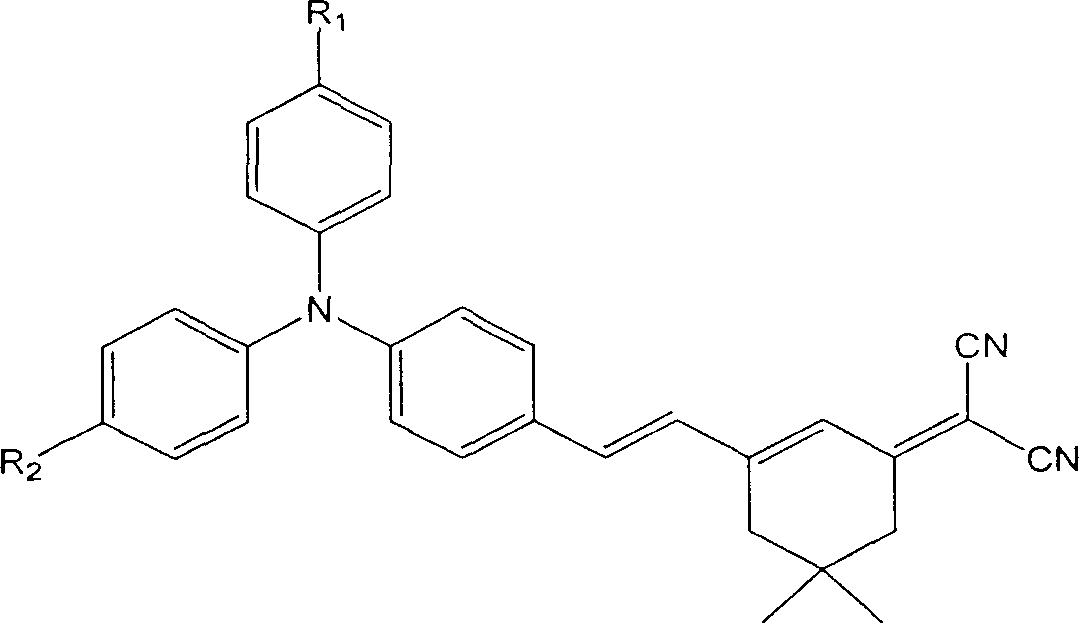
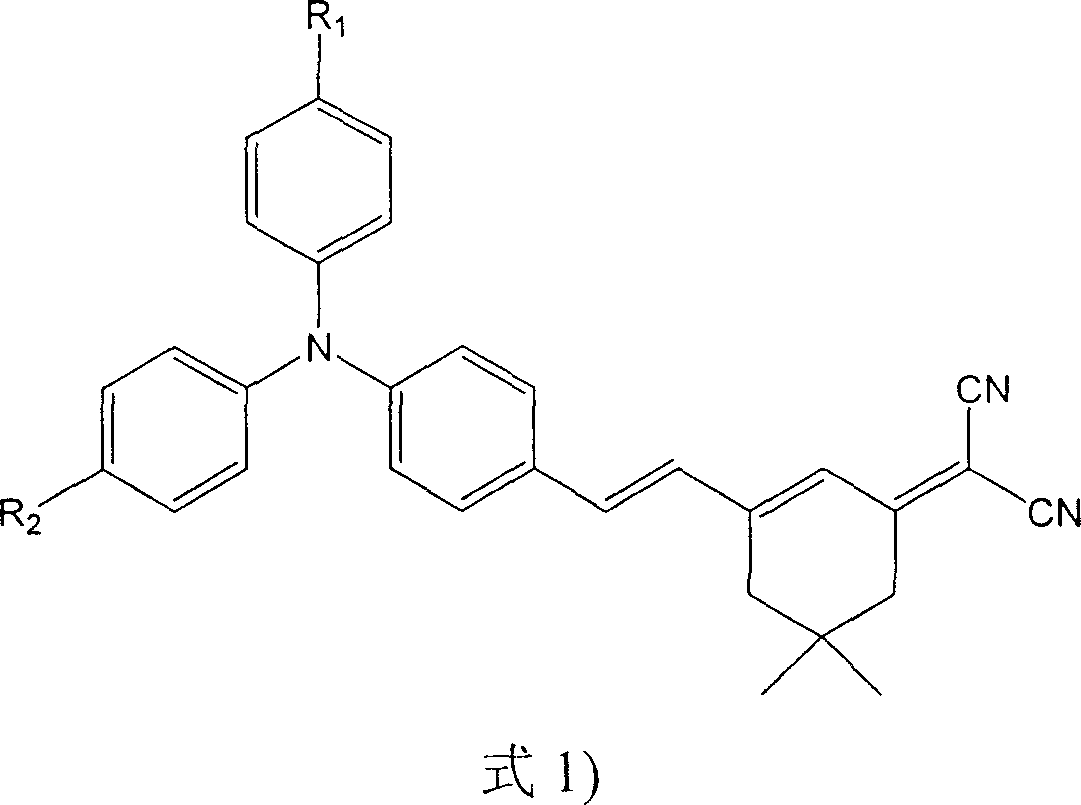
Intramolecular charge transfer chromophore containing triphenylamine group and its synthesis method
A triphenylamine-based, charge transfer technology, applied in chemical instruments and methods, preparation of organic compounds, luminescent materials, etc., can solve the problems of reducing fluorescence quenching concentration, poor thermal stability, large chromophore interaction, etc. The effect of increasing fluorescence quenching concentration, reducing interaction, good thermal stability and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
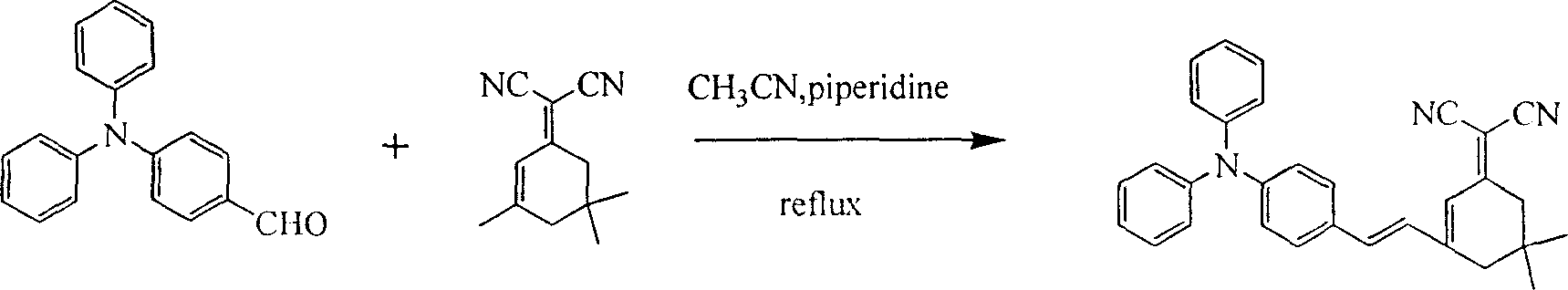
[0018] 4-(Diphenylamine)benzaldehyde (0.273 g, 1.0 mmol) and 2-(3,5,5-trimethylcyclohexen-2-enylidene)malononitrile (0.186 g, 1.0 mmol) were dissolved in acetonitrile solution (20 mL). Piperidine was added dropwise as a catalyst, the mixture was refluxed for 10 hours, cooled to room temperature, the product was precipitated from the acetonitrile solution, the product was washed with deionized water, and dried to obtain pure dark red needle-like crystals, which were triphenylamine-containing The intramolecular charge transfer chromophore 4-[2-(3-dicyanomethylvinyl-5,5-dimethyl-1-cyclohexene)-ethylene]triphenylamine (BHT).
[0019] Yield: 85%
[0020] 1 H NMR (500MHz, CDCl 3 , δppm): δ1.07(s 6H, -(CH 3 ) 2 ), δ2.46(s 2H, -(CH 2 )-), δ2.59(s 2H, -(CH 2 )-), δ6.79(s 1H, -(CH)=), δ6.84-6.87(d 1H, CH=CH), δ6.99-7.02(t 3H, ArH), δ7.09-7.14( m 1H, ArH), δ7.26, 7.31(d 1H, CH=CH), δ7.29-7.30(d 3H, ArH), δ7.34-7.36(d 2H, ArH).
[0021] Elemental analysis: theoretical value C 31...
Embodiment 2
[0026] 4,4'-(anilinediyl)benzaldehyde (0.301g, 1.0mmol) and 2-(3,5,5-trimethylcyclohexen-2-enylidene)malononitrile (0.558g , 3.0mmol) was dissolved in N,N-dimethylformamide solution (20mL), piperidine, acetic acid and acetic anhydride were added dropwise as a catalyst, the mixture was refluxed for 10 hours, after cooling to room temperature, the reaction mixture was poured into water and used dichloromethane extraction. Extraction with anhydrous MgSO 4 After drying, dichloromethane was removed under reduced pressure. The initial product is purified by silica gel column chromatography with petroleum ether: dichloromethane (1:3, V / V) as the eluent, and finally obtains a deep red product with metallic luster, which is an intramolecular compound containing a triphenylamine group. The charge transfer chromophore 4,4'-bis[2-(3-dicyanomethylvinyl-5,5-dimethyl-1-cyclohexene)-ethylene]triphenylamine (BDHT).
[0027] Yield: 58%
[0028] 1 H NMR (500MHz, CDCl 3 , δppm): δ1.08(s 12H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com