Intramolecular charge transfer type red luminescent material and preparation and application thereof
A technology of charge transfer and red light emission, which is applied to the application of the above materials in organic electroluminescent devices and the preparation of the above materials, to achieve the effects of high yield, simple separation, and good electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
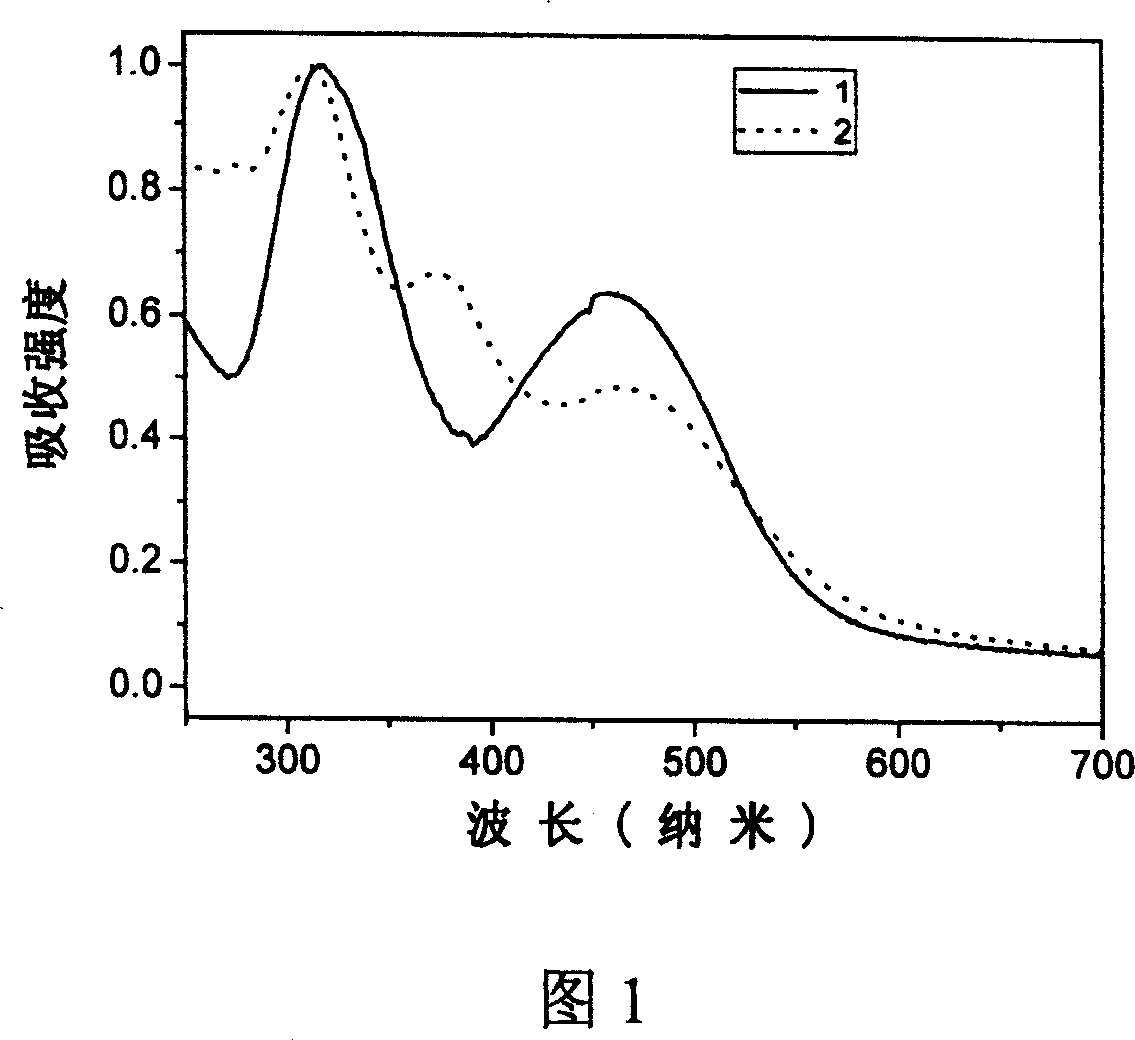
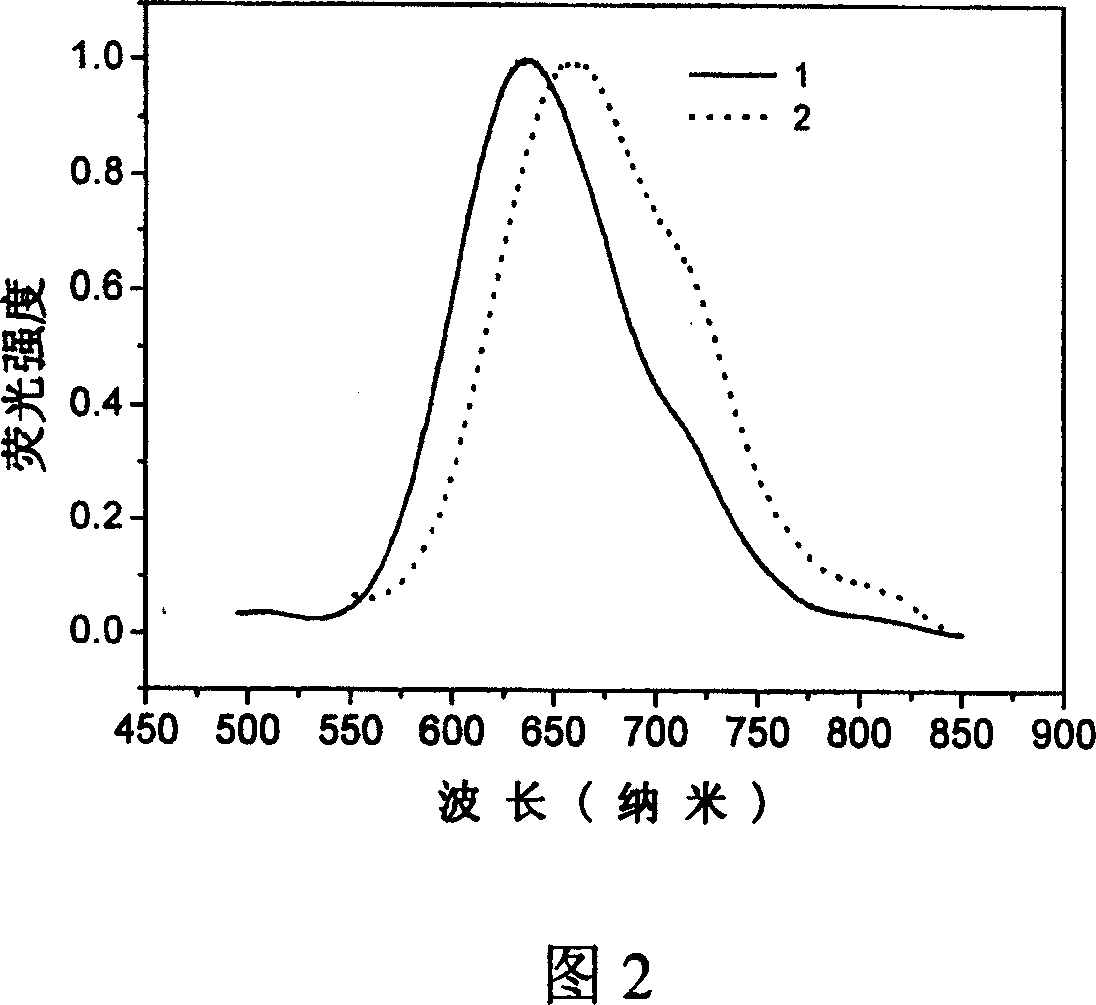
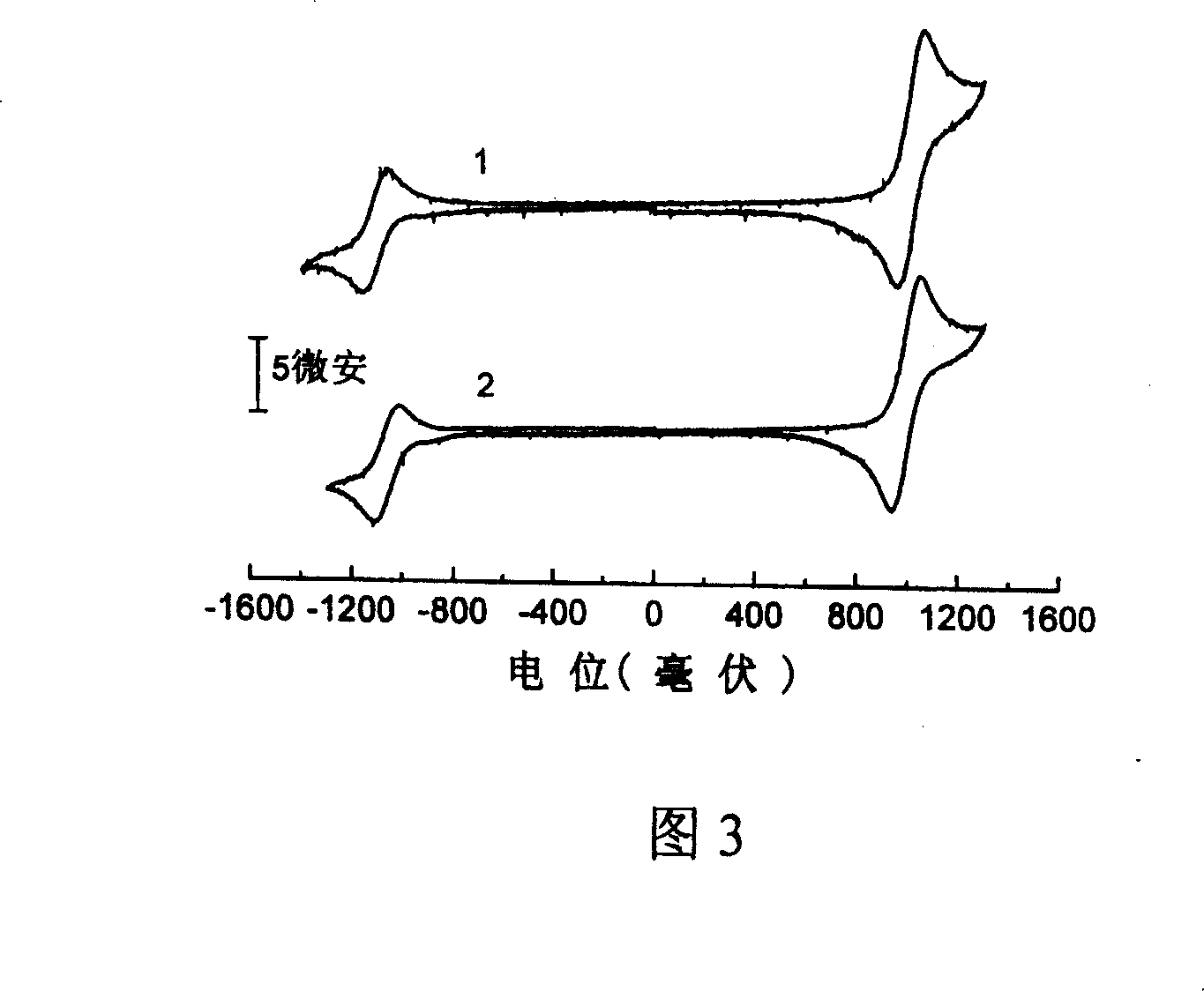
[0039] Example 1: 2,3-dicyano-5,6-bis-(4'-diphenylamino)-phenyl-pyrazine (compound 1)
[0040]
[0041] Step a: In a 100ml three-necked flask, place 4,4'-dibromodiphenylethanedione (2mmol) and 4-tri-n-butyltinphenyl-diphenylamine (4mmol) in about 20ml redistilled N, In N-dimethylformamide, then add the catalyst Pd (PPh 3 ) 2 Cl 2 (0.2mmol) (the catalyst can also be Pd(PPh 3 ) 4 or PhCH 2 Pd(PPh 3 ) 2Cl), under normal pressure, nitrogen atmosphere, temperature controlled at 80-100° C., heated to reflux for about 24 hours; after the reaction stopped, cooled to room temperature. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent: dichloromethane / petroleum ether 1:1 v / v) to obtain an orange-red solid (yield 90%).
[0042] Mass spectrum (MALDI-TOF-MS): m / z calculated value 696.28, measured value 696.3 (M + );
[0043] H NMR spectrum ( 1 H-NMR) (400MHz, CDCl 3 , ppm): δ=8.04(d, J...
Embodiment 2
[0050] Example 2: 6,7-dicyano-2,3-bis-(4'-diphenylamino)-phenyl-quinoxaline (compound 2)
[0051]
[0052] The heating reflux time of step a in embodiment 1 is 16 hours, all the other conditions are the same as embodiment 1, the product 4,4'-(4'-diphenylamino)-diphenylethanedione (0.2mmol) obtained by reaction, 4,5-diamino-phthalocyanine (0.2 mmol) and acetic acid (10 mL) were added into a 50 ml three-neck flask and refluxed for 12 hours under nitrogen atmosphere. After cooling and filtering, the obtained red precipitate was precipitated with dichloromethane and petroleum ether to obtain pure product 2 (yield 93.7%).
[0053] Mass Spectrum (MALDI-TOF-MS) m / z: calculated value 818.32; measured value 818.0 (M + );
[0054] H NMR spectrum ( 1 H-NMR) (400MHz, CDCl 3 , ppm): δ=8.63(s, 2H), 7.70(d, J=8.3Hz, 4H), 7.63(d, J=8.3Hz, 4H), 7.53(d, J=8.5Hz, 4H), 7.28 -7.32 (m, 8H), 7.14-7.16 (m, 12H), 7.07 (t, 4H).
[0055] NMR spectrum ( 13 C-NMR) (100MHz, CDCl 3 , ppm): δ=157....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com