Receptor material based on di(bithiophene)six-membered heterocycle as well as preparation method and application of receptor material
A six-membered heterocycle and dithiophene technology, which is applied in the field of organic photovoltaic materials, can solve the problems of poor electron donating ability and light absorption ability, and achieve the effects of good thermal stability, broadened absorption spectrum, and strong light absorption ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Preparation of compound 2
[0057] Weigh compound 1 (740mg, 2.97mmol) in 100mL Schlenk, vacuum dry for 0.5h, change argon 3 times during this period, add 10mL of anhydrous Et 2 O dissolved. The freshly prepared LDA (3.56mmol) was placed in a -78°C cryostat, TMSCl (0.6mL, 6.53mmol) was dropped into the LDA, stirred for 10min, the solution of compound 1 was added dropwise, and the reaction was allowed to rise to room temperature overnight. Add CH dropwise at low temperature (-90℃~-60℃) 3 The reaction was quenched with OH, the solvent was spin-dried, extracted with DCM, dried, filtered and spin-dried to obtain a crude product. 300-400 mesh silica gel column chromatography, using HEX as eluent, obtained 791 mg of compound 3 with a yield of 83%.
[0058]
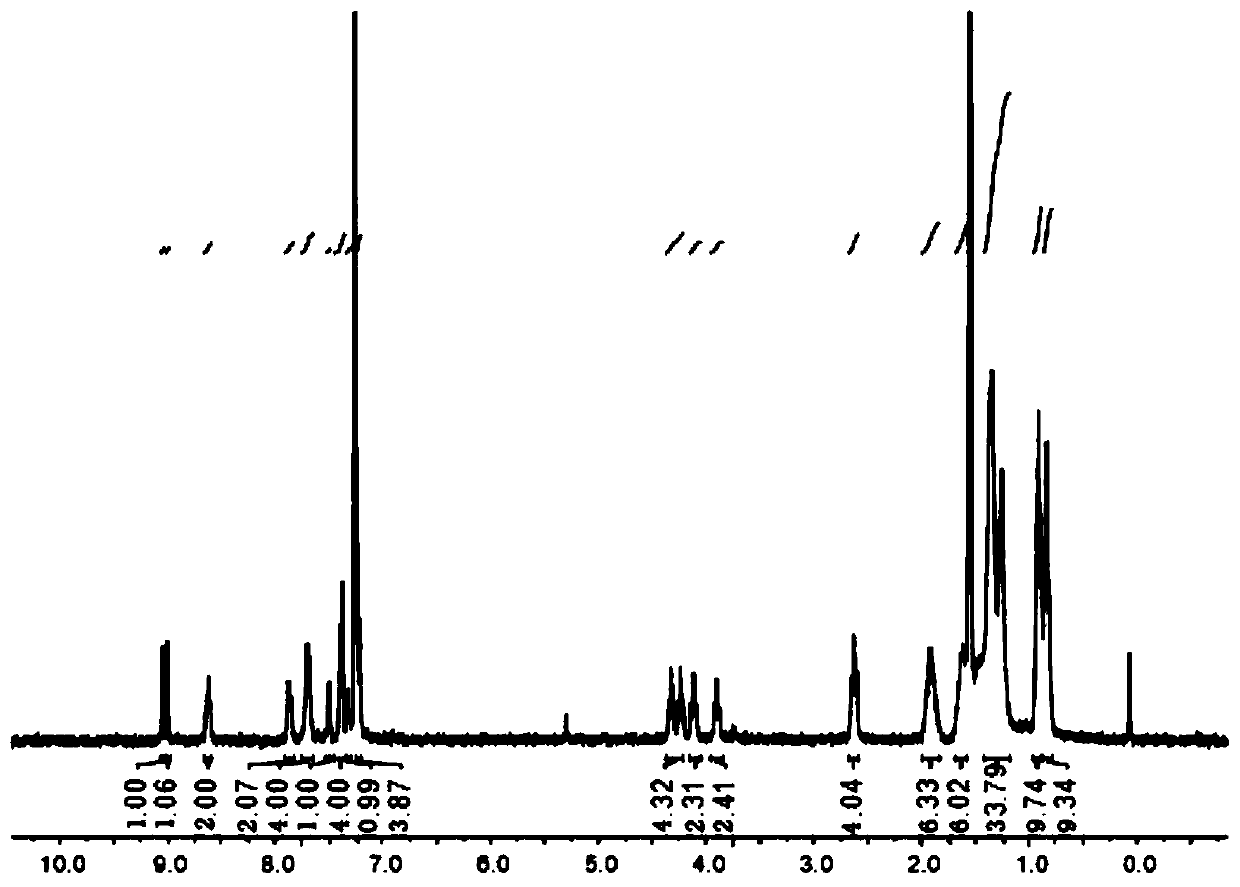
[0059] Characterization data of compound formula 2: 1 H NMR (300MHz, CDCl 3 )δ7.20(s,1H),4.13(s,3H),0.37(s,9H). 13 C NMR (75MHz, CDCl 3 )δ148.09, 142.68, 137.98, 133.22, 126.02, 95.25, 77.67, 77.25, 76.82, 59...
Embodiment 2
[0085] (1) Preparation of compound 8-1: the method is the same as in Example 1;
[0086] (2) Preparation of compound 9-2:
[0087] Weigh compound formula 8-1 (77mg, 0.0617mmol) and bisfluorocyanindanone (57mg, 0.25mmol) into 50mL Schlenk, vacuum dry for 0.5h, change argon 3 times during this period, add 6mL of anhydrous CHCl 3 And 0.2mL pyridine, react overnight at room temperature. Add CH to the reaction solution 3 OH, until a large amount of blue solids are precipitated, and the solids are washed with CH 3 OH washed several times, 300-400 mesh silica gel column chromatography, HEX:CHCl 3 =1:2 was the eluent, and 76 mg of compound 9-2 was obtained with a yield of 74%.
[0088]
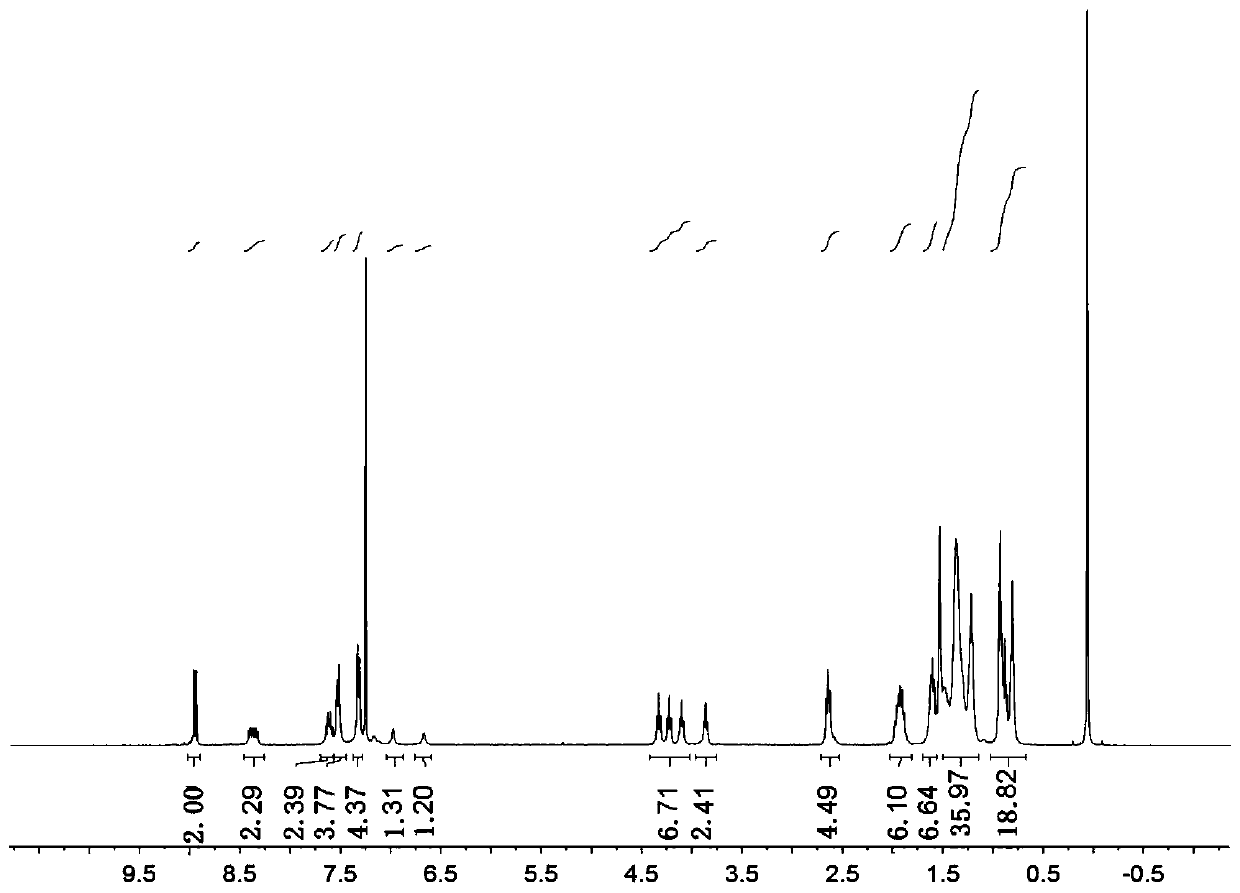
[0089] Characterization data of compound formula 9-2: 1 H NMR (400MHz, CDCl 3 )δ8.96(d, J=10.1Hz, 2H), 8.45-8.32(m, 2H), 7.62(dt, J=10.9, 7.6Hz, 2H), 7.53(d, J=7.9Hz, 4H), 7.33(d, J=8.0Hz, 4H), 6.98(s, 1H), 6.68(s, 1H), 4.34(t, J=7.2Hz, 2H), 4.23(t, J=7.0Hz, 2H), 4.11(t, J=7.0Hz, 2H), 3.87...
Embodiment 3
[0091] (1) Preparation of compound 7-1: the method is the same as in Example 1;
[0092] (2) Preparation of compound 8-2:
[0093] Weigh compound 7-1 (115mg, 0.13mmol), 5-bromo-3-hexyloxythiophene-2-carbaldehyde (84mg, 0.2879mmol), K 2 CO 3 (90mg, 0.654mmol) and Pd(PPh 3 ) 4 (8mg, 0.0065mmol) in 100mL Schlenk, vacuum-dry for 0.5h, change argon 3 times during this period, add 10mL of anhydrous THF and 5mL of oxygen-free water, transfer to 90°C oil bath and reflux overnight. The solvent was spin-dried, extracted with DCM, dried, filtered and spin-dried to obtain a crude product. 300-400 mesh silica gel column chromatography with DCM as the eluent gave 104.8 mg of compound 8-2 with a yield of 77%.
[0094]
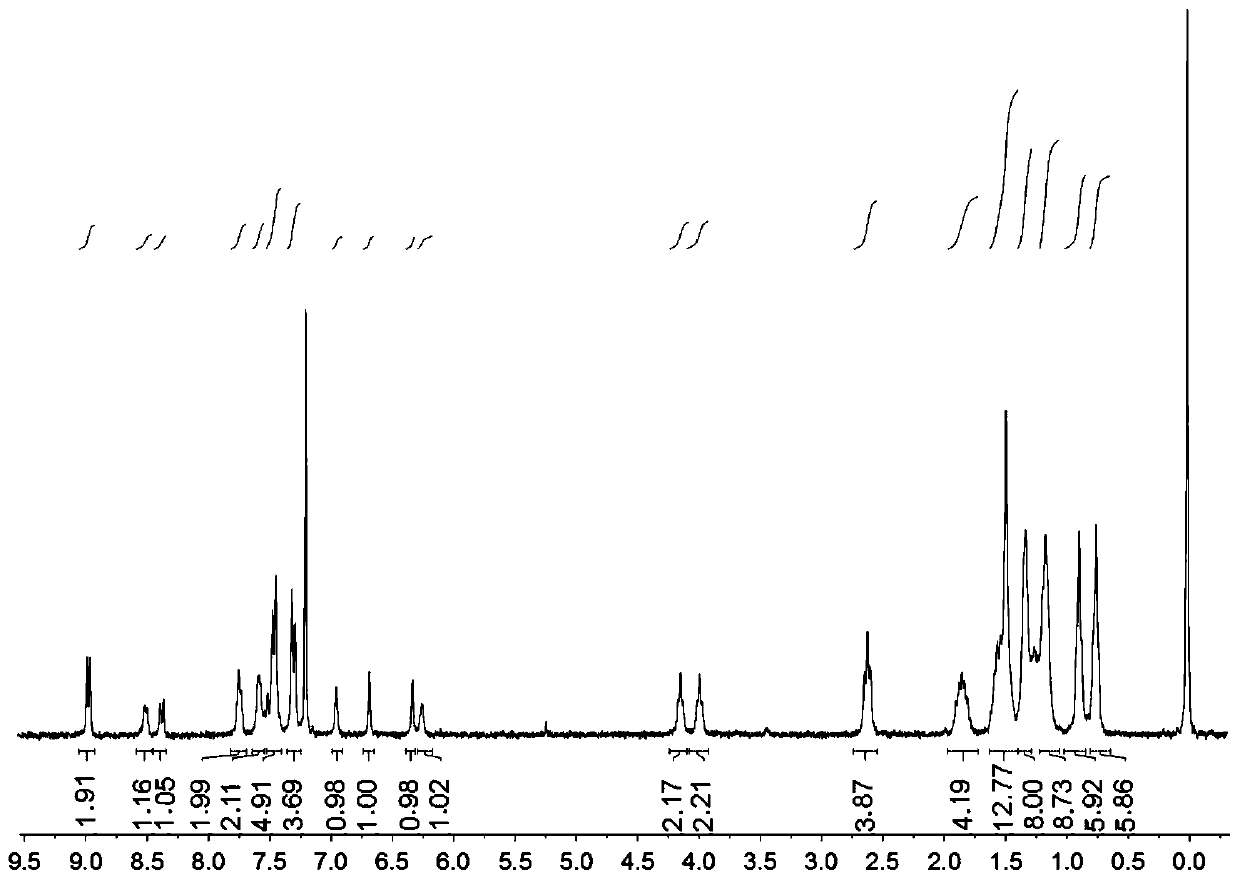
[0095] Characterization data of compound formula 8-2: HRMS (DART) calcd for [C 71 h 91 o 7 S 6 ] 1046.3234, found 1047.33073.
[0096] (3) Preparation of compound formula 9-3:
[0097] Weigh compound formula 8-2 (65mg, 0.064mmol) and cyanoindanone (50mg, 0.26mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com