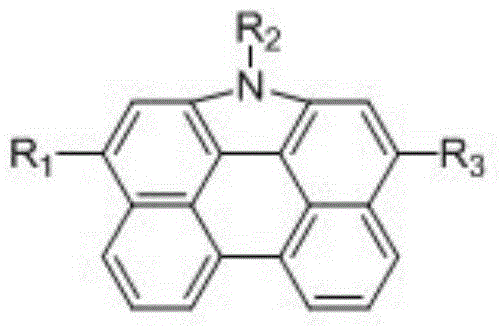
Phenanthro-carbazole donor-acceptor organic dye and application thereof in dye-sensitized solar cell
A technology of phenanthrene carbazoles and organic dyes, applied in the field of phenanthrene carbazoles donor and acceptor organic dyes and its application in dye-sensitized solar cells, which can solve the problems of narrow spectral response range and achieve low price , reduce intermolecular charge transfer, reduce the effect of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
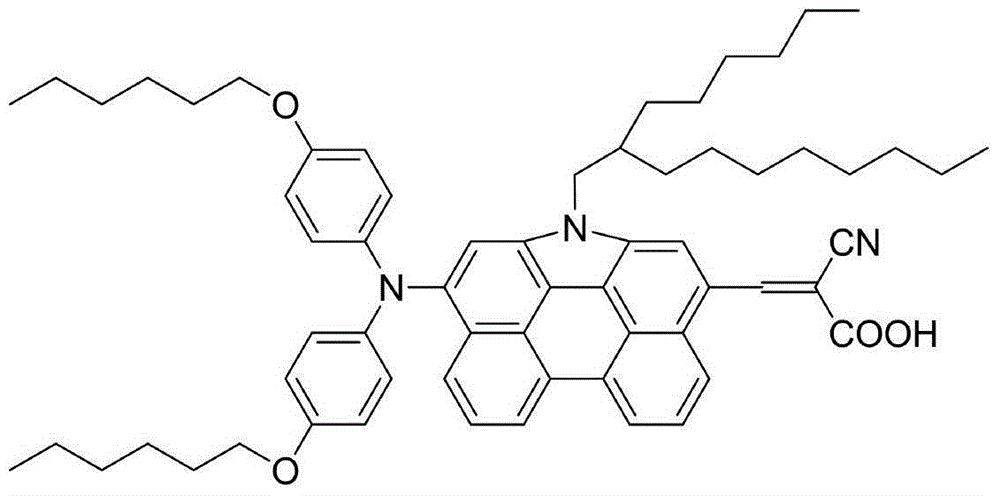
[0029] Example 1: Preparation of organic dye (I)
[0030] The synthetic route is as follows:
[0031]
[0032] Synthesis of starting material 1 is referenced in Org. Lett. 2006, 8, 2221-2224.
[0033] Synthesis of starting material 2 is referenced in J. Mater. Chem. 2012, 22, 8394-8398.
[0034] Synthesis of starting material 4 is referenced in J. Org. Chem. 2008, 73, 7369-7372.
[0035] Synthesis of Intermediate 3:
[0036]
[0037] In a dry Schlenk reaction flask, dissolve 2 g of compound 1 and 2.86 g of compound 2 in 20 ml of toluene, add 60 mg of catalyst Pd(dba) in the glove box 2 , 0.24 ml P(t-Bu) 3 (10% mass fraction in hexane) and 1.49 g NaOt-Bu, heated and refluxed overnight under argon protection, after the reaction was completed, the temperature of the system was lowered to room temperature, extracted with chloroform three times, the organic phases were combined, and the organic phase was treated with anhydrous sulfuric acid. After drying over sodium for ...
Embodiment 2
[0077] Embodiment 2: the preparation of organic dye (II)
[0078]
[0079] Synthesis reference of starting material 9 Bioorg. Med. Chem. Lett, 2012, 22, 415-420.
[0080] Synthesis of Intermediate 10:
[0081]
[0082]In a dry Schlenk reaction flask, 2.13 g of compound 9 and 2.9 ml of hexamethylditin were dissolved in 20 ml of toluene, and 809 mg of Pd (PPh) were added under argon atmosphere. 3 ) 4 , heated and refluxed for 2 hours, after the reaction was completed, the temperature of the system was lowered to room temperature, extracted with chloroform three times, the organic phases were combined, the organic phases were dried with anhydrous sodium sulfate for 2 hours, the desiccant was filtered out, the filtrate was concentrated, and the ethyl acetate / petroleum Ether (volume ratio 1 / 20) was used as a developing solvent for column chromatography to obtain 2.10 g of intermediate 10 as a white solid with a yield of 85%.
[0083] NMR characterization data of intermedia...
Embodiment 3
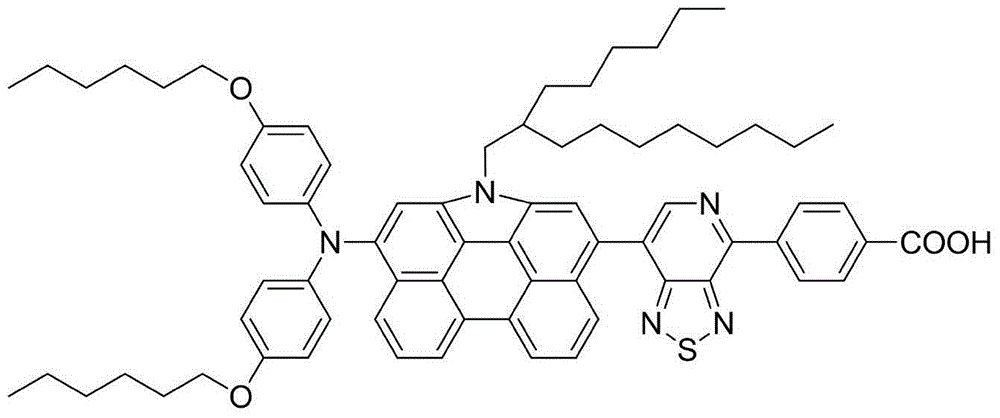
[0114] Example 3: Preparation of organic dye (III)
[0115]
[0116] Synthesis of Intermediate 14:
[0117]
[0118] In a dry Schlenk reaction flask, 200 mg of intermediate 10 and 861 mg of 4,7-dibromobenzothiadiazole were dissolved in 20 ml of toluene, and 49 mg of Pd (PPh 3 ) 2 Cl 2 , heated and refluxed overnight, after the reaction was completed, the temperature of the system was lowered to room temperature, extracted with chloroform three times, the organic phases were combined, the organic phases were dried with anhydrous sodium sulfate for 2 hours, filtered to remove the desiccant, and the filtrate was concentrated and washed with chloroform / petroleum ether (volume ratio 1 / 1) as a developing solvent column chromatography to obtain 176 mg of intermediate 14 as a white solid with a yield of 76%. NMR characterization data of intermediate 14:
[0119] 1 HNMR (400MHz, CDCl 3 )δ:8.08(d,J=8.4Hz,2H),7.88(d,J=8.4Hz,2H),7.83(d,J=7.6Hz,1H),7.52(d,J=7.6Hz,1H) , 1.60 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com