Intramolecular charge transfer type red luminescent material and preparation and application thereof
A technology of charge transfer and red light emission, which is applied to the preparation of the above-mentioned materials, and the application of the above-mentioned materials in organic electroluminescent devices, to achieve the effects of good electron transport performance, simple separation, and simple device preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
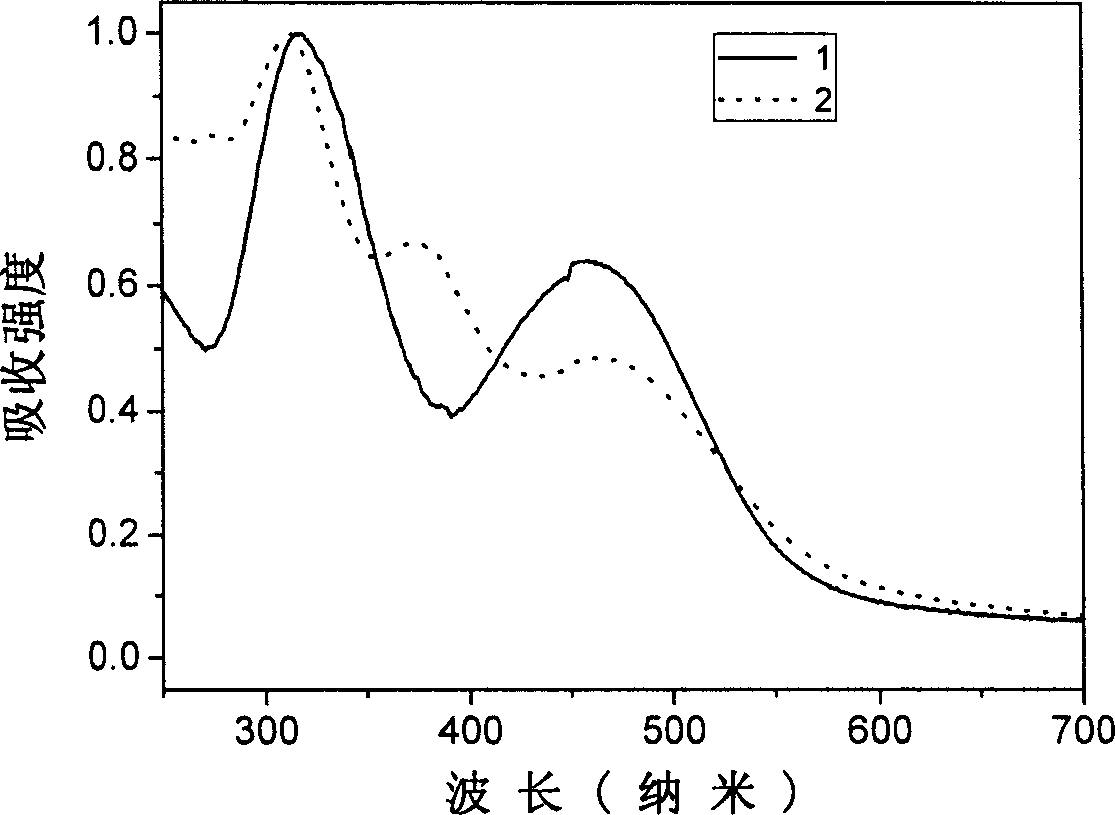
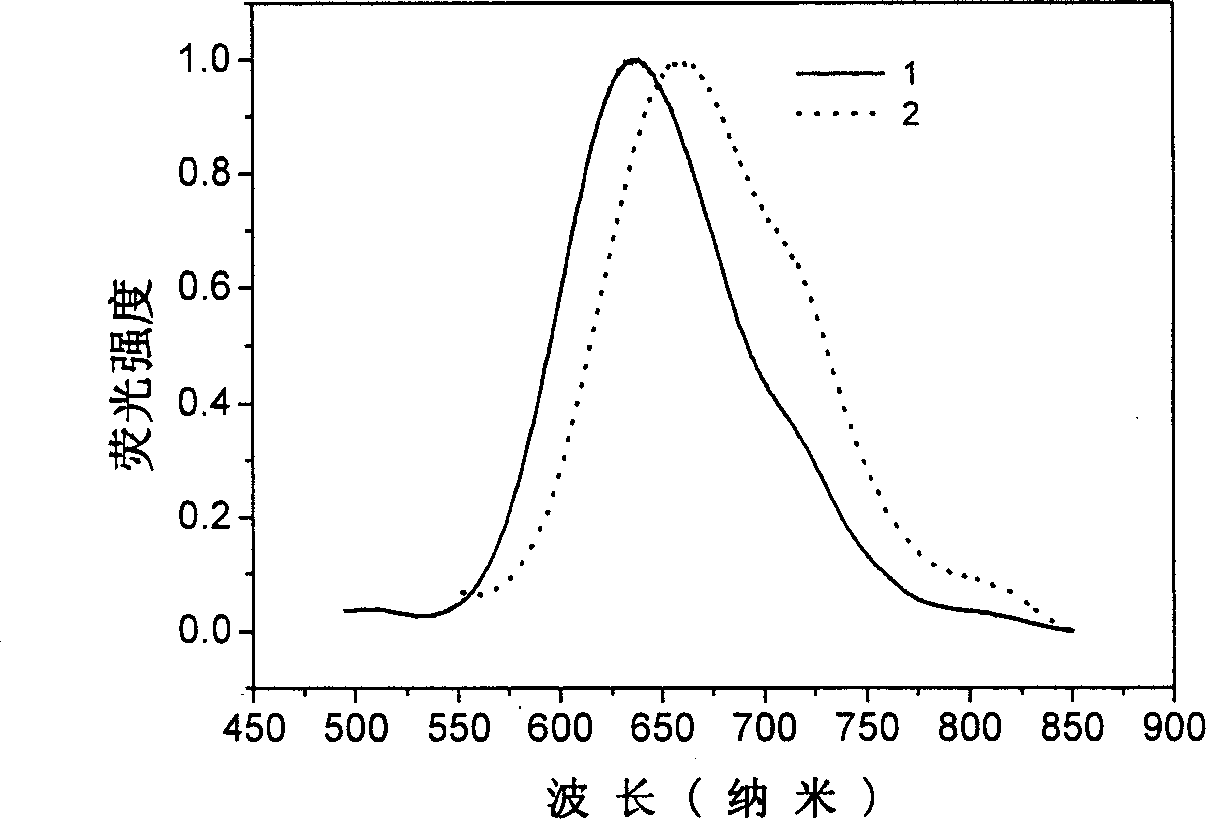
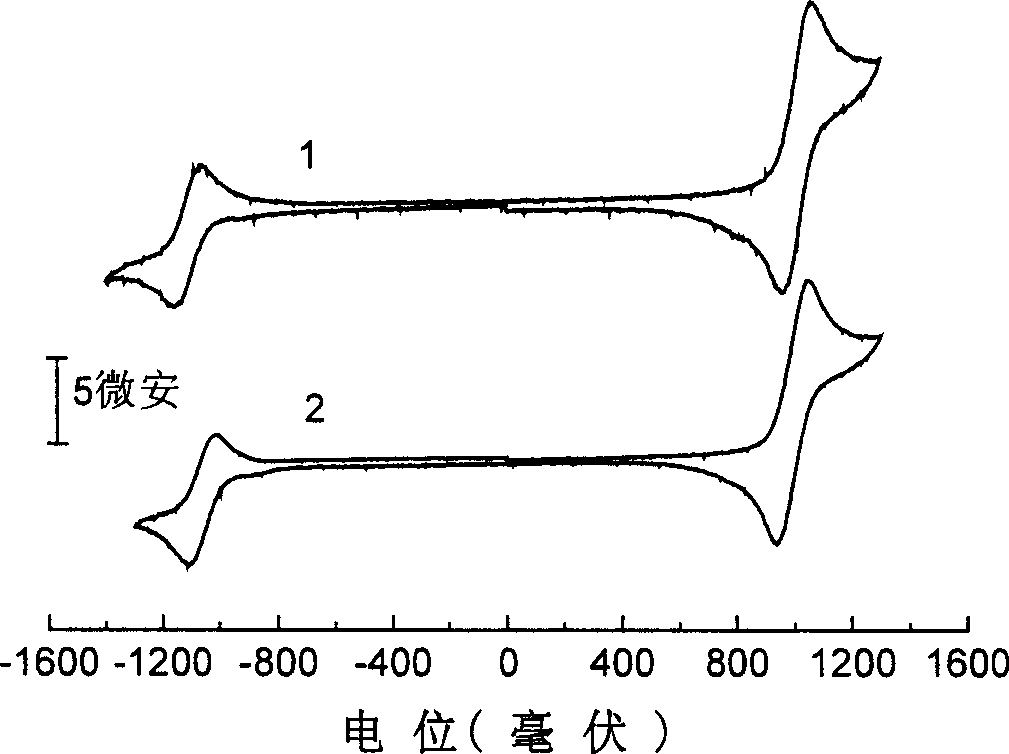
[0039] Example 1: 2,3-dicyano-5,6-bis-(4'-diphenylamino)-phenyl-pyrazine (compound 1)
[0040]
[0041] Step a: In a 100ml three-necked flask, place 4,4'-dibromodiphenylethanedione (2mmol) and 4-tri-n-butyltinphenyl-diphenylamine (4mmol) in about 20ml redistilled N, In N-dimethylformamide, then add the catalyst Pd (PPh 3 ) 2 Cl 2 (0.2mmol) (the catalyst can also be Pd(PPh 3 ) 4 or PhCH 2 Pd(PPh 3 ) 2Cl), under normal pressure, nitrogen atmosphere, temperature controlled at 80-100° C., heated to reflux for about 24 hours; after the reaction stopped, cooled to room temperature. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent: dichloromethane / petroleum ether 1:1 v / v) to obtain an orange-red solid (yield 90%).
[0042] Mass spectrum (MALDI-TOF-MS): m / z calculated value 696.28, measured value 696.3 (M + );
[0043] H NMR spectrum ( 1 H-NMR) (400MHz, CDCl 3 , ppm): δ=8.04(d, J...
Embodiment 2
[0050] Example 2: 6,7-dicyano-2,3-bis-(4'-diphenylamino)-phenyl-quinoxaline (compound 2)
[0051]
[0052] The heating reflux time of step a in embodiment 1 is 16 hours, all the other conditions are the same as embodiment 1, the product 4,4'-(4'-diphenylamino)-diphenylethanedione (0.2mmol) obtained by reaction, 4,5-diamino-phthalocyanine (0.2 mmol) and acetic acid (10 mL) were added into a 50 ml three-neck flask and refluxed for 12 hours under nitrogen atmosphere. After cooling and filtering, the obtained red precipitate was precipitated with dichloromethane and petroleum ether to obtain pure product 2 (yield 93.7%).
[0053] Mass Spectrum (MALDI-TOF-MS) m / z: calculated value 818.32; measured value 818.0 (M + );
[0054] H NMR spectrum ( 1 H-NMR) (400MHz, CDCl 3 , ppm): δ=8.63(s, 2H), 7.70(d, J=8.3Hz, 4H), 7.63(d, J=8.3Hz, 4H), 7.53(d, J=8.5Hz, 4H), 7.28 -7.32 (m, 8H), 7.14-7.16 (m, 12H), 7.07 (t, 4H).
[0055] NMR spectrum ( 13 C-NMR) (100MHz, CDCl 3 , ppm): δ=157....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com