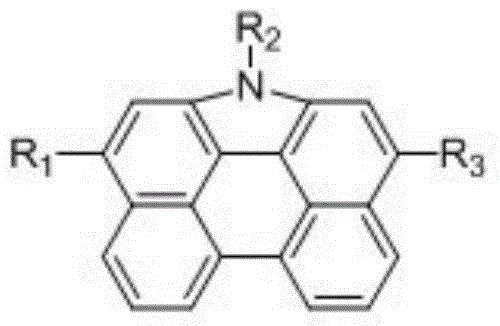
Phenanthro-carbazole donor-acceptor organic dye and application thereof in dye-sensitized solar cell
A technology of phenanthrocarbazole and organic dyes, applied to phenanthrocarbazole donor-acceptor organic dyes and its application in dye-sensitized solar cells, can solve problems such as narrow spectral response range, and achieve low price , Reduce the charge transfer between molecules, the effect of rich sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
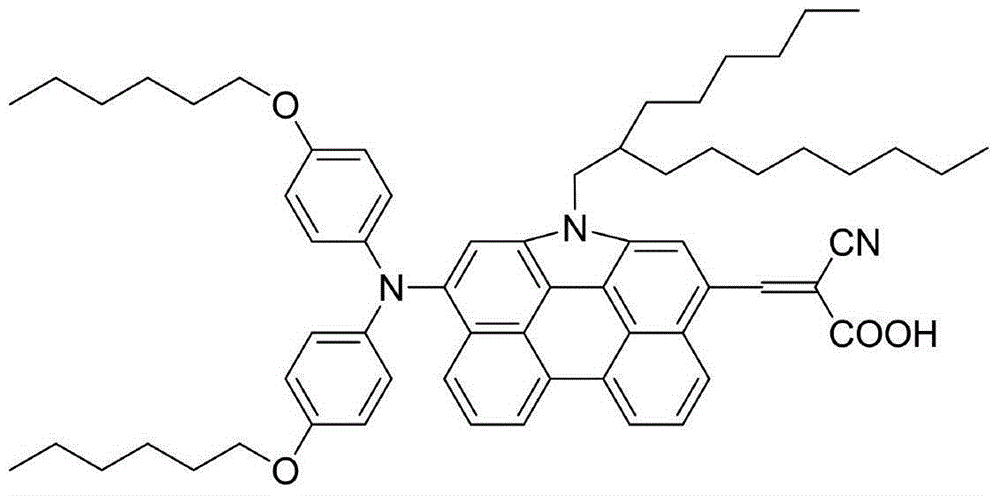
[0029] Embodiment 1: the preparation of organic dye (Ⅰ)
[0030] The synthetic route is as follows:
[0031]
[0032] The synthesis reference of starting material 1 is Org. Lett. 2006, 8, 2221-2224.
[0033] The synthesis reference of starting material 2 is J. Mater. Chem. 2012, 22, 8394-8398.
[0034] The synthesis reference of starting material 4 is J.Org.Chem.2008, 73, 7369-7372.
[0035] Synthesis of Intermediate 3:
[0036]
[0037] In a dry Schlenk reaction flask, 2 g of compound 1 and 2.86 g of compound 2 were dissolved in 20 ml of toluene, and 60 mg of the catalyst Pd(dba) was added in the glove box 2 , 0.24 ml P(t-Bu) 3 (mass fraction 10% in hexane) and 1.49 grams of NaOt-Bu, under the protection of argon, heated to reflux to react overnight, after the reaction was complete, the temperature of the system was lowered to room temperature, extracted three times with chloroform, combined the organic phase, and the organic phase was anhydrous sulfuric acid The s...
Embodiment 2
[0077] Embodiment 2: the preparation of organic dye (II)
[0078]
[0079] Synthesis reference of starting material 9 Bioorg. Med. Chem. Lett, 2012, 22, 415-420.
[0080] Synthesis of Intermediate 10:
[0081]
[0082]In a dry Schlenk reaction flask, 2.13 g of compound 9 and 2.9 ml of hexamethylditin were dissolved in 20 ml of toluene, and 809 mg of Pd (PPh 3 ) 4 , heated to reflux for 2 hours, after the reaction was complete, the temperature of the system was lowered to room temperature, extracted three times with chloroform, combined the organic phases, dried the organic phases with anhydrous sodium sulfate for 2 hours, filtered out the desiccant, concentrated the filtrate, and used ethyl acetate / petroleum Ether (volume ratio 1 / 20) was used as a developer for column chromatography to obtain 2.10 g of white solid intermediate 10 with a yield of 85%.
[0083] NMR characterization data of Intermediate 10:
[0084] 1 HNMR (400MHz, CDCl 3 )δ: 7.93(d, J=7.7Hz, 2H), 7.5...
Embodiment 3
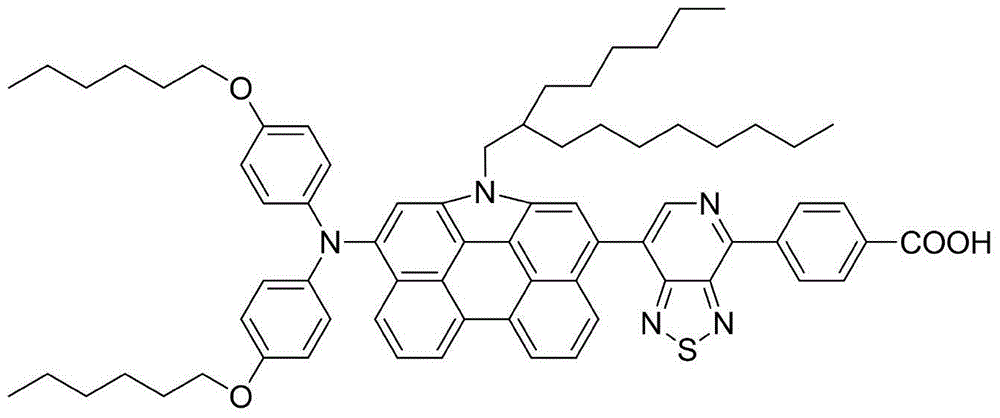
[0114] Embodiment 3: the preparation of organic dye (III)
[0115]
[0116] Synthesis of Intermediate 14:
[0117]
[0118] In a dry Schlenk reaction flask, 200 mg of intermediate 10 and 861 mg of 4,7-dibromobenzothiadiazole were dissolved in 20 ml of toluene, and 49 mg of Pd(PPh 3 ) 2 Cl 2 , heated to reflux to react overnight, after the reaction was complete, the temperature of the system was lowered to room temperature, extracted three times with chloroform, combined the organic phases, dried the organic phases with anhydrous sodium sulfate for 2 hours, filtered out the desiccant, concentrated the filtrate, and used chloroform / petroleum ether (volume Ratio 1 / 1) was used as a developing solvent for column chromatography to obtain 176 mg of white solid intermediate 14 with a yield of 76%. NMR characterization data of Intermediate 14:
[0119] 1 HNMR (400MHz, CDCl 3 )δ:8.08(d,J=8.4Hz,2H),7.88(d,J=8.4Hz,2H),7.83(d,J=7.6Hz,1H),7.52(d,J=7.6Hz,1H) ,1.60(s,9H). 13 CNM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com