Fluorenyl cyano indanone non-conjugated polymer receptor and preparation method thereof
A technology of fluorenyl cyanoindanone and non-conjugated polymers, which is applied in the field of polymer solar cell acceptor materials, can solve the problems of lagging development of polymer acceptors, and achieve good physical and chemical properties, good application prospects, and low price low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of fluorenyl cyanoindanone non-conjugated polymer
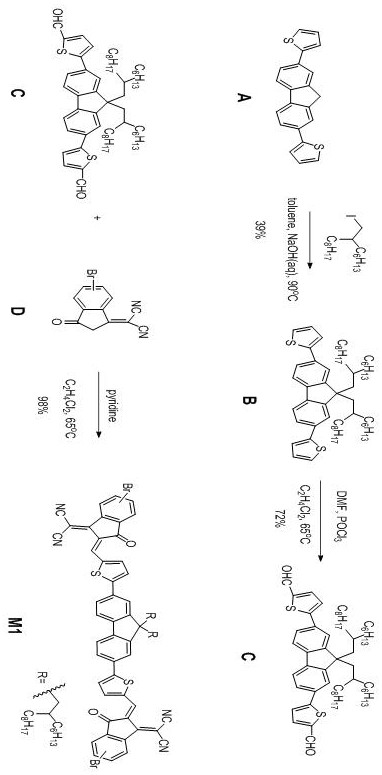
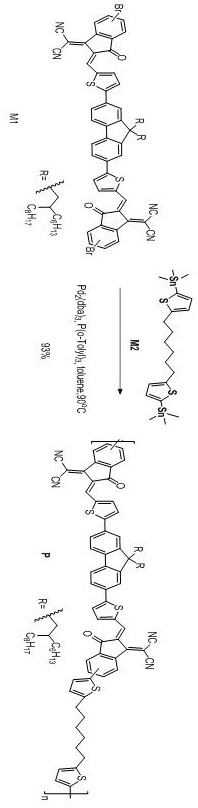
[0044] This example provides a fluorenyl cyanoindanone non-conjugated polymer. Firstly, monomer M1 is synthesized. For the synthesis route, see figure 1 ; Re-synthesis of polymer P, its synthetic route see figure 2 .
[0045] 1.1 Preparation of monomer M1
[0046] The preparation of monomer M1 specifically comprises the following steps:
[0047] 1.1.1 Synthesis of intermediate compound C
[0048] The synthesis of intermediate compound C specifically comprises the following steps
[0049] (a) Synthesis of Intermediate Compound B
[0050] The structural formula of intermediate compound B is as follows
[0051]
[0052] In an argon atmosphere, weigh compound A (2.70mmol, 892.35mg) and tetrabutylammonium bromide (0.27mmol, 87.68mg) into a two-necked bottle, then add toluene (31mL) and 50% mass fraction of NaOH solution (31 mL). After deoxygenating the raw material mixture by bubbling...
Embodiment 2
[0070] Example 2, thermal stability, gel permeation chromatography, ultraviolet-visible-near-infrared absorption spectrum, electrochemical properties and photoluminescent properties of polymer P
[0071] 2.1 Thermal stability of polymers
[0072] Figure 5 It is given that polymer P is raised from room temperature to 540°C at a rate of 20°C / min under nitrogen atmosphere. The Td (weight loss 5%) of the polymer is 381° C., indicating that the thermal stability of the polymer is good, which lays a foundation for the application of the material in polymer photovoltaic devices.
[0073] 2.2 Gel Permeation Chromatography of Polymer P
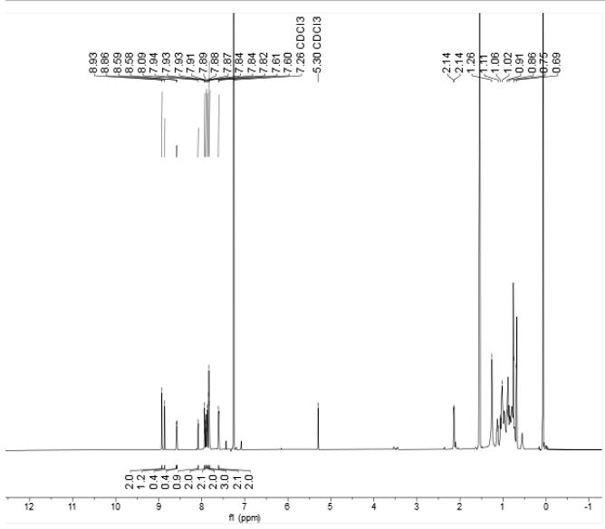
[0074] Image 6 The number average molecular weight (Mn) measured by gel permeation chromatography (GPC) of polymer P is 3.91KDa, the weight average molecular weight (Mw) is 7.00KDa, and the distribution coefficient PDI is 2.19.
[0075] 2.3 UV-Vis-NIR absorption spectrum of polymer P
[0076] Figure 7 The ultraviolet-visible-near-infrared abso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Open circuit voltage | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com