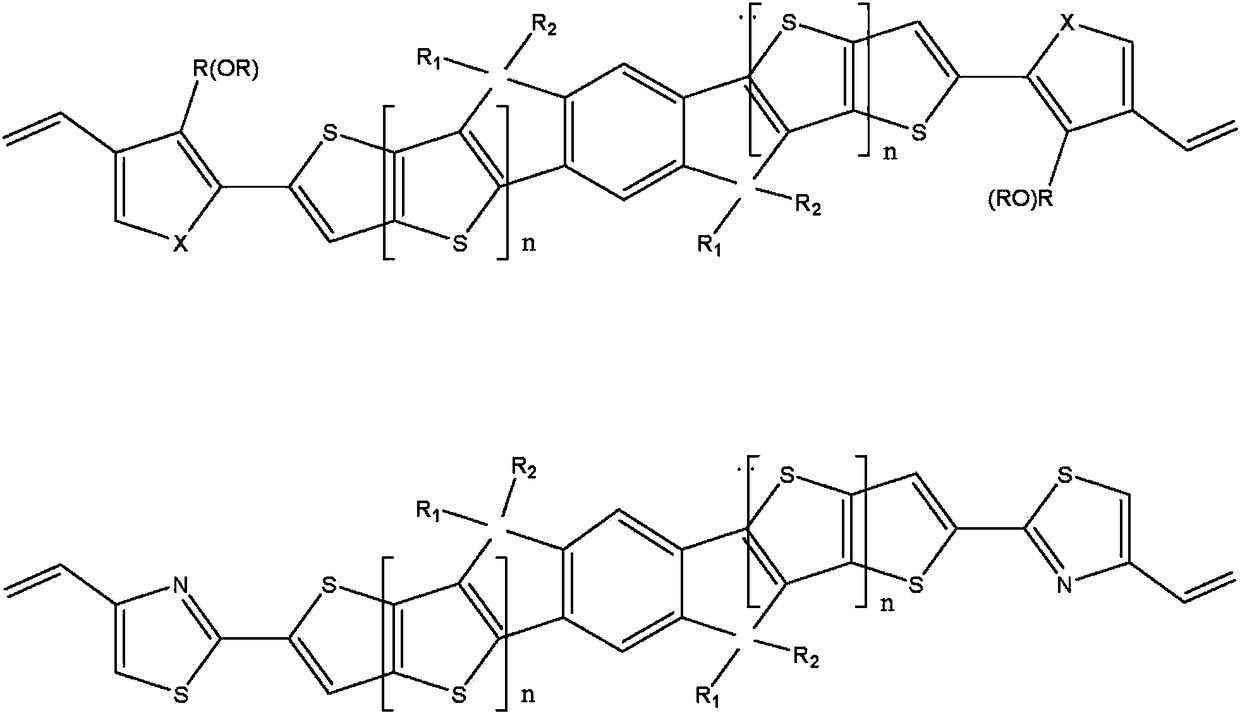
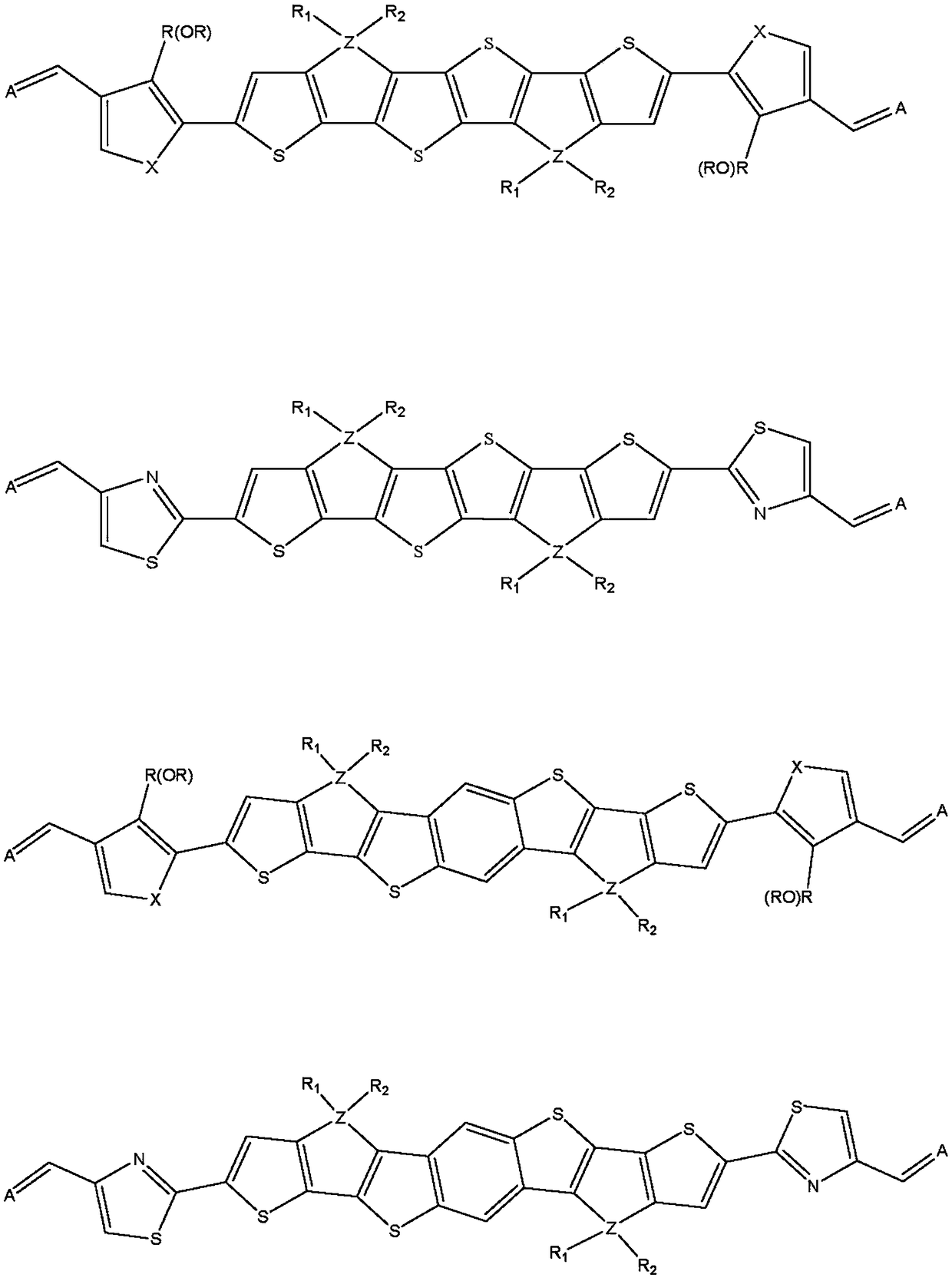
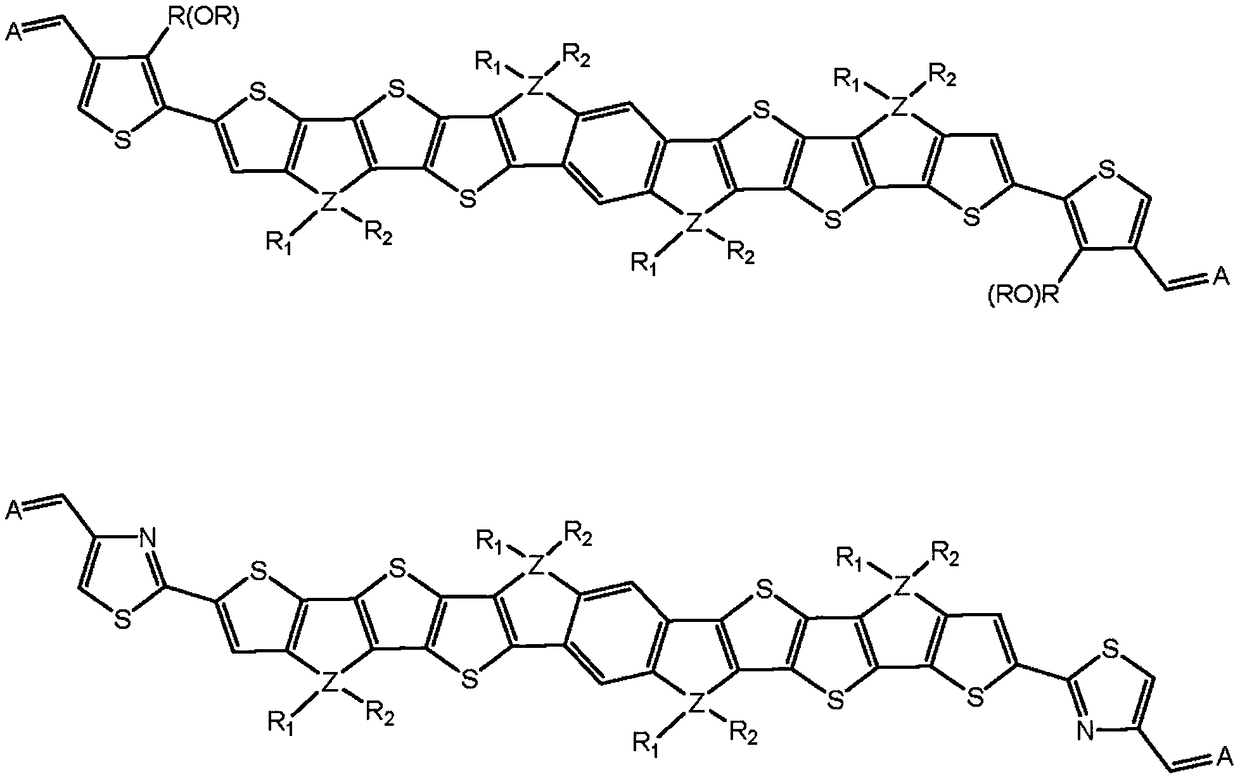
Novel n type organic semiconductor material
A new type of alkyl technology, applied in the field of n-type organic semiconductor materials, can solve the problem that the performance of porphyrin-like acceptor materials is not very good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The synthetic route of the aldehyde compound containing fused ring unit and five-membered heterocycle is as follows:
[0091]
[0092] Add compound A (0.12mmol), compound B (0.3mmol) and 10mL of freshly steamed toluene into the reaction flask, evacuate nitrogen back and forth three times, add Pd(PPh 3 ) 4 (7.6mg), the temperature was 110°C, stirred for 48 hours; cooled to room temperature, added deionized water, CHCl 2 Extraction is carried out, and the obtained organic phase is dried with anhydrous magnesium sulfate; After filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent (volume ratio: 2:1) as eluting agent, silica gel (200-300 mesh) Column chromatography was used for separation and purification to obtain a yellow solid, which was molecule 1 containing a six-membered fused ring and an aldehyde group.
Embodiment 2
[0094] The synthetic route of the A-D-A type conjugated molecule containing fused ring unit is as follows:
[0095]
[0096] Add compound 1 (0.03mmol) and compound C (0.18mmol) and 10mL of distilled chloroform to the reaction flask, pass nitrogen for 30 minutes to remove the air in the reaction flask, then add piperidine, and stir at 65°C for 12 hours; cool to room temperature, pour the reaction solution into methanol, filter the obtained precipitate, use petroleum ether / dichloromethane mixed solvent as eluent, and conduct separation and purification on silica gel (200-300 mesh) column chromatography, which is the compound containing fused ring units. A-D-A type conjugated molecule 2.
Embodiment 3
[0098]
[0099] Add compound A (0.12mmol) and compound D (0.3mmol) and 10mL of freshly steamed toluene into the reaction flask, and add Pd(PPh 3 ) 4 (7.6mg), the temperature was 110°C, stirred for 48 hours; cooled to room temperature, added deionized water, CHCl 2 Extraction is carried out, and the obtained organic phase is dried with anhydrous magnesium sulfate; after filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent as eluent, and perform separation and purification by silica gel (200-300 mesh) column chromatography to obtain a solid The product, namely molecule 3, contains a five-membered fused ring and an aldehyde group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com