Preparation method of N-alkynyl benzimidazole derivatives
A technology of alkynyl benzimidazole and benzimidazole is applied in the field of preparation of N-alkynyl benzimidazole derivatives, and can solve the problems of complicated steps, high environmental pollution of metal salts, and difficulty in obtaining raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of N-alkynylbenzimidazole derivatives, comprising the following steps:
[0025] Under the atmosphere of protective gas, lithium hexamethyldisilazide, diethyl chlorophosphate and lithium hexamethyldisilazide were sequentially added to 1-R shown in general formula 2 1 Formylmethyl 2-R 2 Reaction in the anhydrous solvent of basebenzimidazole, obtains the N-alkynylbenzimidazole derivative shown in general formula 1;
[0026]
[0027] Among them, when adding LiHMDS to react, the temperature is controlled at -78~0°C; adding ClP(O)(OEt) 2 Afterwards, the system is heated to 10-30°C for reaction, R 1 For phenyl, 4-methylphenyl, 2-methylphenyl, 4-methoxyphenyl, 2,5-dimethoxyphenyl, 3,4-methylenedioxybenzene, 4- One of fluorophenyl, 4-chlorophenyl, 4-bromophenyl, 4-iodophenyl, 2-naphthyl, 6-methoxy-2-naphthyl, thienyl, tert-butyl, R 2 For furyl, H, propyl, phenyl, 4-methylphenyl, 4-methoxyphenyl, 3,4-methylenedioxybenzene, 4-chlorophenyl, 4-nitropheny...
Embodiment 1
[0044] R 1 =Ph, R 2 =2-furyl
[0045] Dissolve 2-furylbenzimidazole (184.06mg, 1.0mmol) and α-bromoacetophenone (237.6mg, 1.2mmol) in anhydrous acetone (20mL), and add anhydrous potassium carbonate (165.6mg, 1.2mmol), reflux, TLC tracking reaction (about 12h), after all the raw materials have reacted, the mixture was poured into a large amount of water to wash, extracted with dichloromethane (30mL×3), the organic phase was washed with saturated brine, anhydrous sulfuric acid After drying over sodium, after filtration, the solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography [V (petroleum ether): V (ethyl acetate) = 5:1] to obtain 265.9 mg of substrate 2a, with a yield of 88.2%.
[0046] 2a White solid, 150~151℃
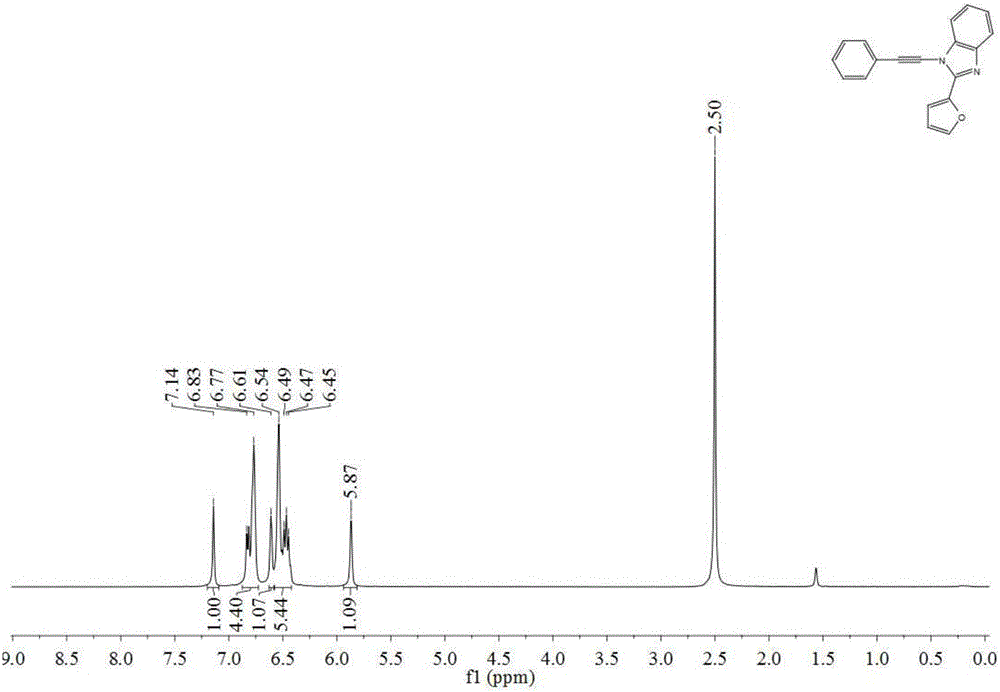
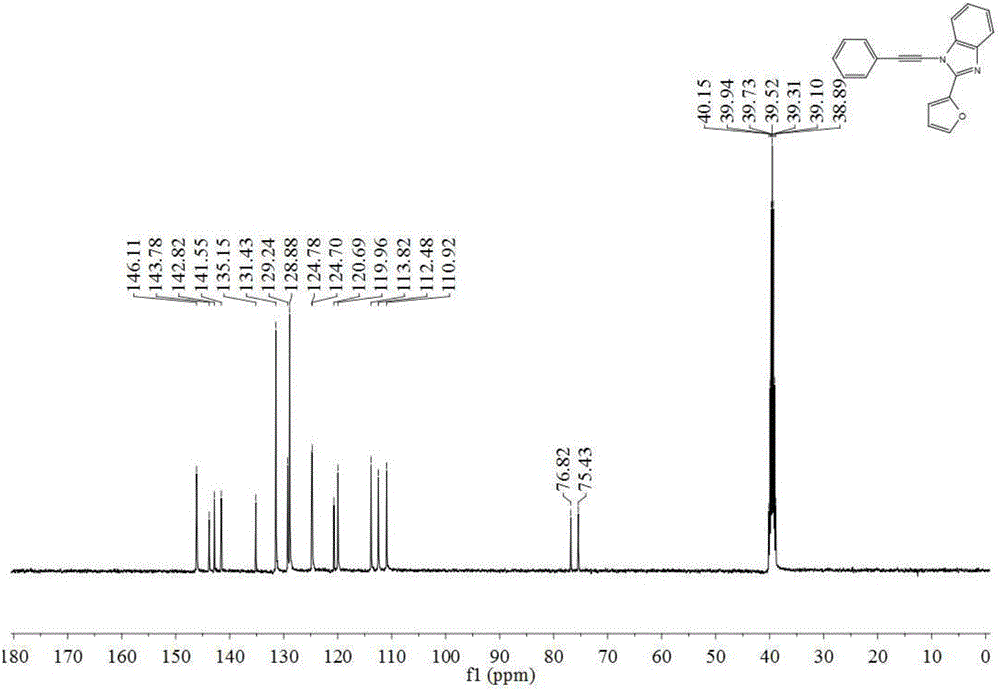
[0047] 1 HNMR (400MHz, DMSO) δ8.17 (d, J = 7.4Hz, 2H), 7.81–7.74 (m, 2H), 7.67 (dt, J = 14.6, 7.2Hz, 4H), 7.27 (d, J = 3.7 Hz,2H),7.18(d,1H),6.67(m,1H),6.27(s,2H).
[0048] MS(EI)m / z(%):302(M + ,100). ...
Embodiment 2
[0050] Preparation 1a
[0051] The chemical structure of 1a is as follows
[0052]
[0053] Under the protection of nitrogen at 0°C, LiHMDS (0.75 mL of 1.0 mol / L solution in THF) was added dropwise to a solution of substrate 2a (151.5 mg, 0.5 mmol) in THF (10 mL), and stirred for 15 minutes. ClP(O)(OEt) 2 (0.13mL, 1.0mmol) was added dropwise into the above reaction system. After the dropwise addition was complete, the cooling device was removed, the temperature was raised to 10° C. and stirring was continued for 2 hours. The reaction was cooled to 0°C again, and then LiHMDS (1.0 mL of tetrahydrofuran solution with a concentration of 1.0 mol / L) was added dropwise to the above reaction system, and stirring was continued at this temperature for 2 hours. The reaction was quenched with saturated ammonium chloride solution, the reaction mixture was poured into water, extracted with ethyl acetate (30mL×3), the organic phase was washed with saturated brine, dried over anhydrous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com