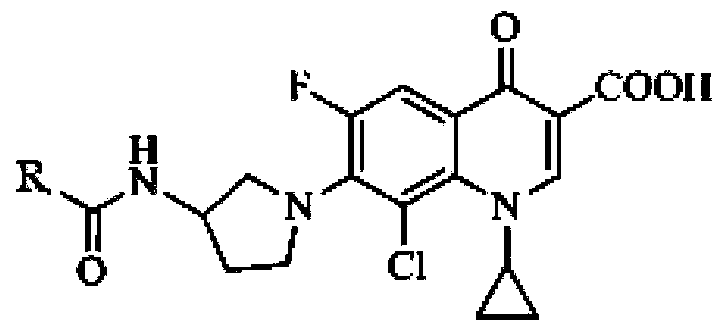
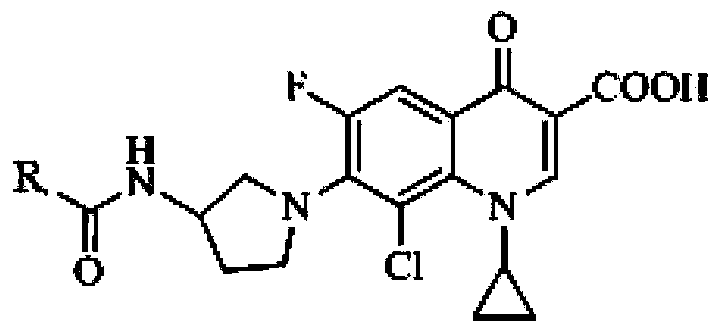
Application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments
A derivative, star amino technology, applied in medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve unclear problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
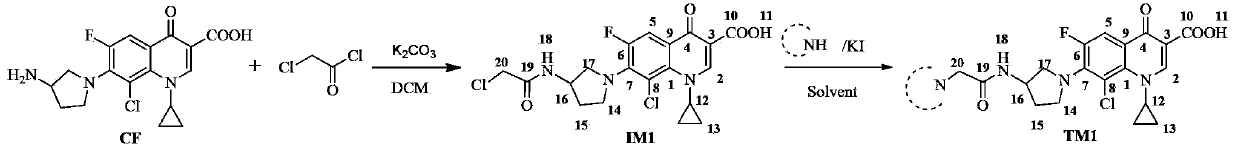
[0018] Embodiment 1, the synthesis of compound TM1
[0019]
[0020] Add clinfloxacin (CF), K 2 CO 3 and an appropriate amount of dichloromethane (DCM), drop the DCM solution of chloroacetyl chloride under ice bath, CF, K 2 CO 3 The molar ratio of feeding to chloroacetyl chloride is 10:25:15-25. After dropping, stir the reaction under ice bath, and monitor the progress of the reaction by TLC (thin layer chromatography). After the reaction was completed, a yellow-green turbid liquid was obtained, which was filtered with suction, and the filter cake was washed with DCM. The washing liquid and the filtrate were combined, and rotary evaporated to dryness to obtain intermediate IM1.
[0021] Add the amine component, K 2 CO 3 And catalytic amount of KI and appropriate amount of solvent, after stirring at room temperature for 30 minutes, add intermediate IM1, IM1, K 2 CO 3 The molar ratio of the feed to the amine component is 1:3:2, the temperature is controlled at 30-45°C,...
Embodiment 2
[0035] The synthesis of embodiment 2, compound TM2
[0036]
[0037] Add CF and an appropriate amount of chloroform to the reaction flask, and after stirring evenly, slowly add a chloroform solution of bis(trichloromethyl)carbonate (BTC) dropwise. Triethylamine (TEA), the feeding molar ratio of CF, BTC and TEA is 17:6:10, dropwise is finished, reacts at room temperature, TLC monitors reaction process. After the reaction was completed, filter with suction, wash the filter cake with DCM, combine the washing liquid and the filtrate, add water, adjust the pH to 4-5 with 2N HCl, then extract twice with DCM, collect the organic phase, wash twice with saturated saline, no Dry over sodium sulfate, filter, concentrate the filtrate by rotary evaporation, and purify by flash column chromatography to obtain intermediate IM2.
[0038] Add chloroform, amine component, and K to the reaction vial 2 CO 3 , after stirring at room temperature for 0.5 hours, add intermediate IM2, amine comp...
Embodiment 3
[0048] The synthesis of embodiment 3, compound TM3
[0049]
[0050] Add 12 mmol of amino acid Boc-AA-OH whose amino group is protected by tert-butoxycarbonyl (Boc) and 20 mL of DCM to the reaction flask, stir for 1 min, add 1-hydroxybenzotriazole (HOBt) 12 mmol and bicyclic Hexylcarbodiimide (DCC) 15mmol, stirred for 1min, then added dropwise TEA 1mL, stirred in ice bath for about 30min, until all the raw materials were activated esters, filtered with suction, washed the filter cake with DCM, combined the lotion and filtrate, added Clinfloxacin 10mmol was stirred at room temperature for reaction, and the reaction progress was monitored by TLC. After the reaction is completed, filter with suction, wash the filter cake with DCM, combine the washing liquid and the filtrate, and use 10% (w / w) citric acid solution, 0.5mol / L NaHCO 3 solution, saturated NaCl solution, the organic phase was collected, anhydrous NaCl 2 SO 4 Drying, rotary evaporation, and purification by column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com