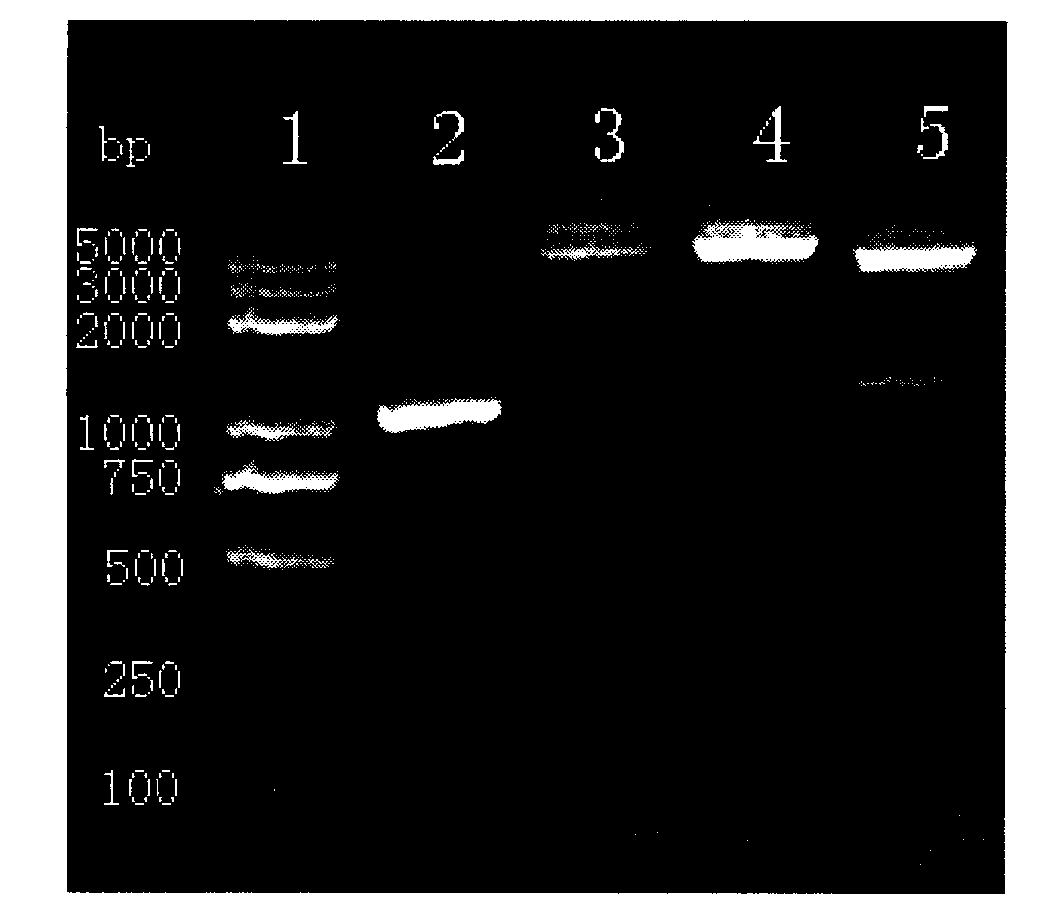
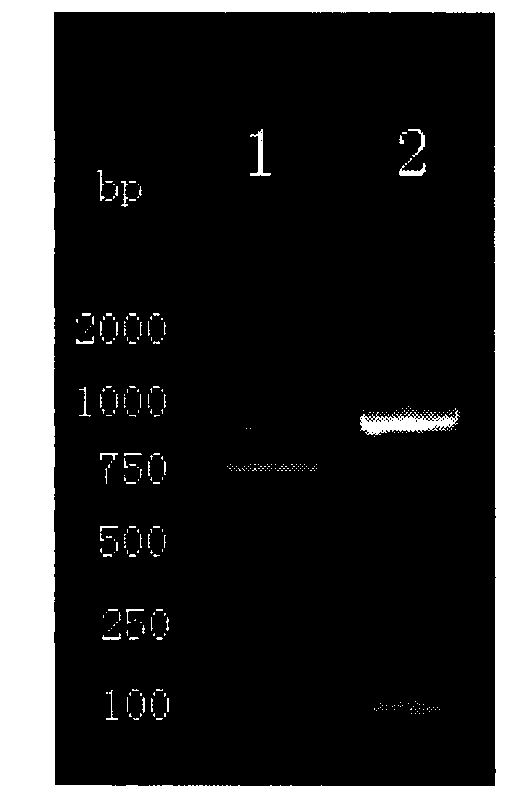
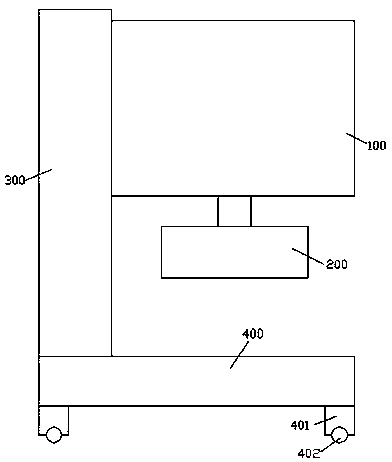
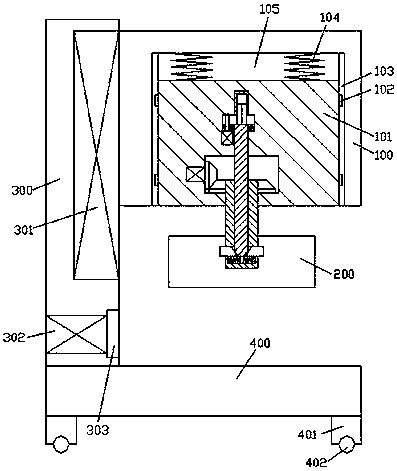
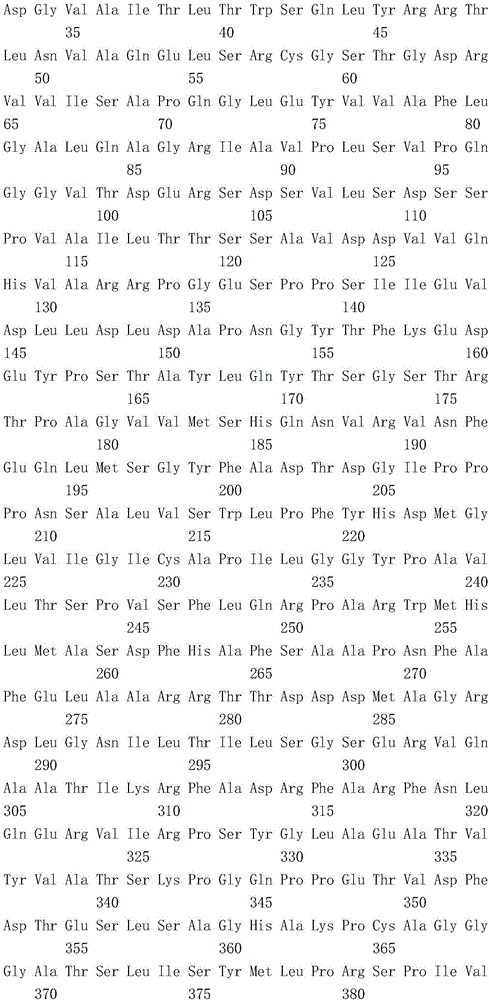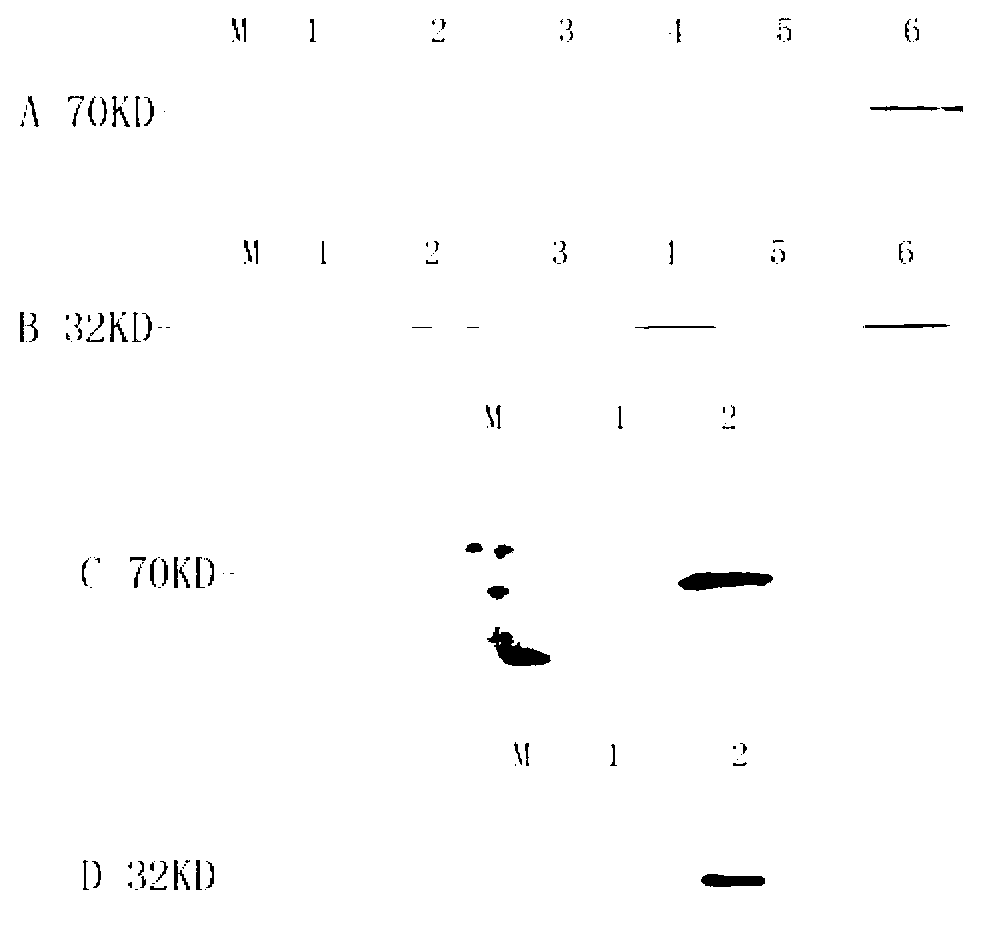Patents
Literature
42 results about "Tuberculosis prevention" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The best way to prevent tuberculosis is to quarantine the infected person to prevent any contact with others that may spread the infection. When it's necessary for an infected person to be around others, it's best to keep in mind that tuberculosis is primarily spread through the air, by way of coughing and sneezing.
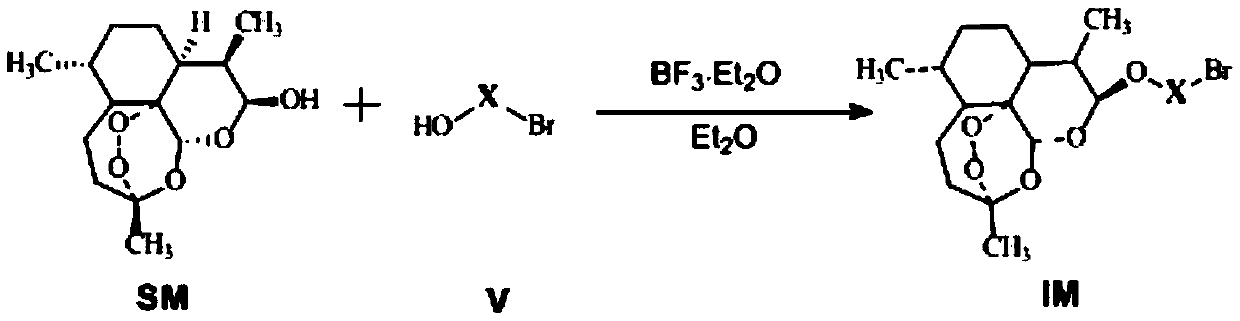
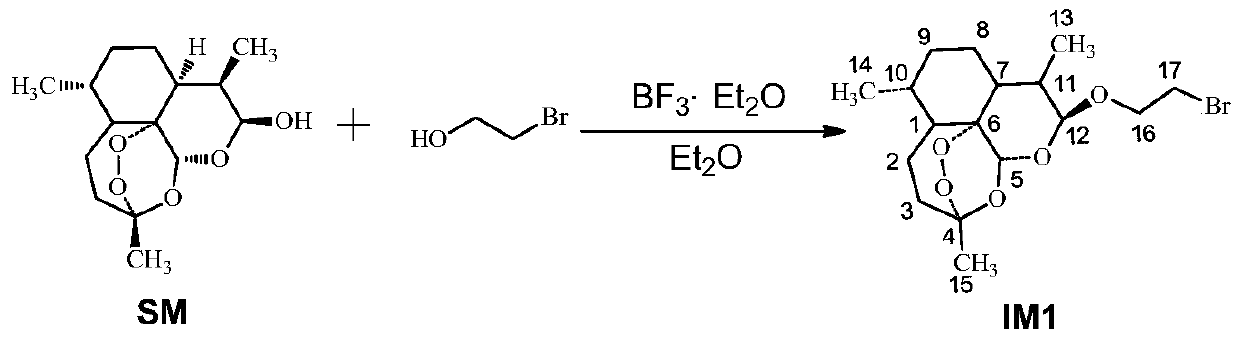
Conjugates of dihydroartemisinin and quinolones compounds as well as preparation method and application thereof
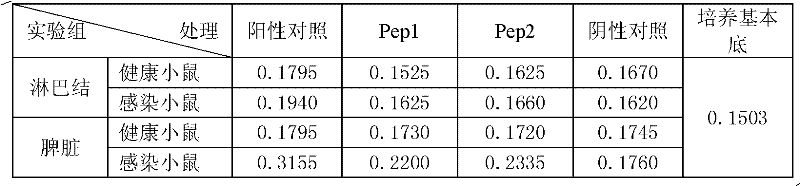
ActiveCN104418864AGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsAntituberculosis drugDihydroartemisinin
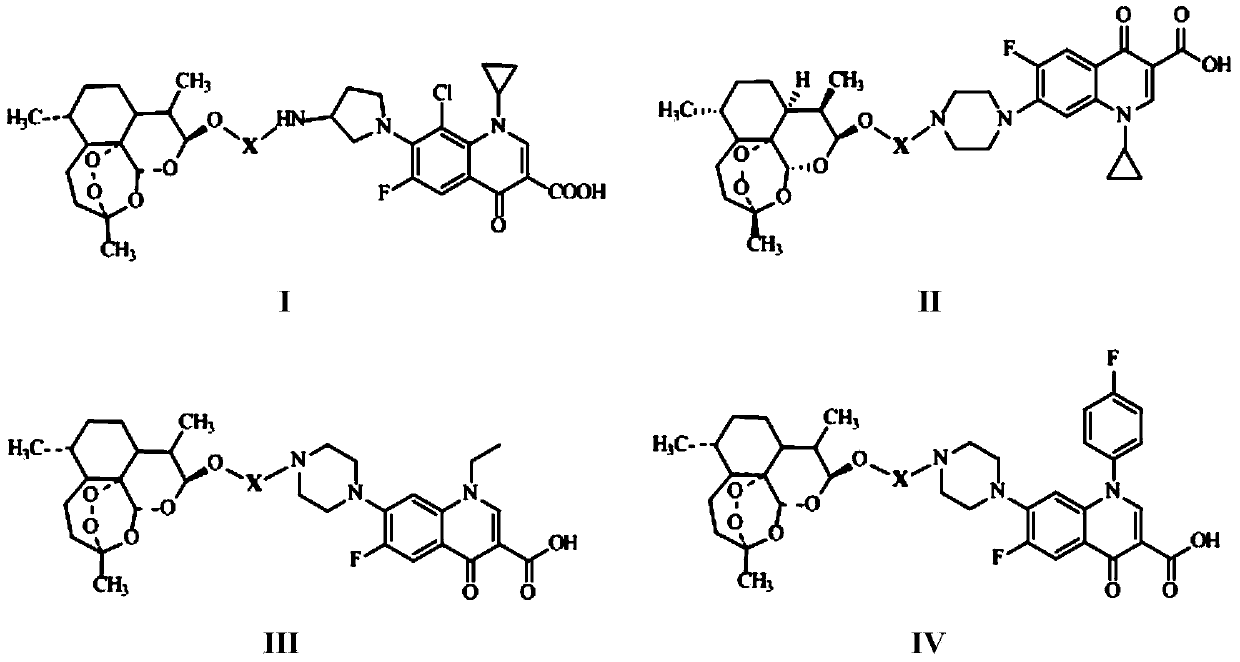
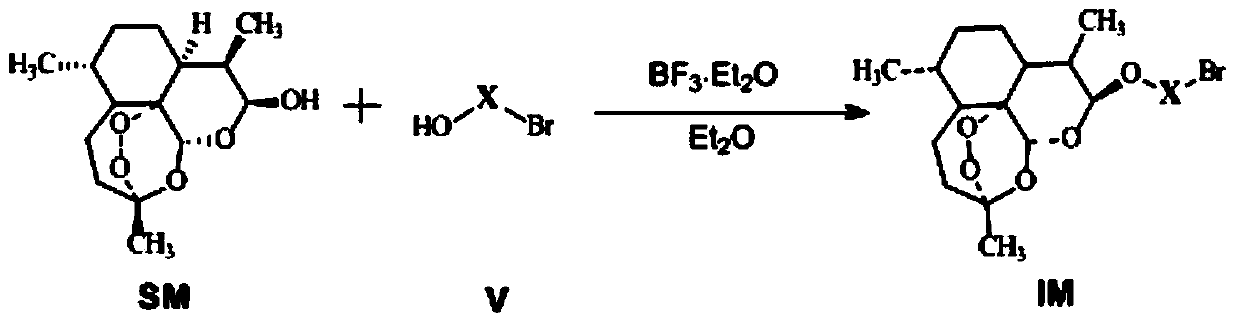
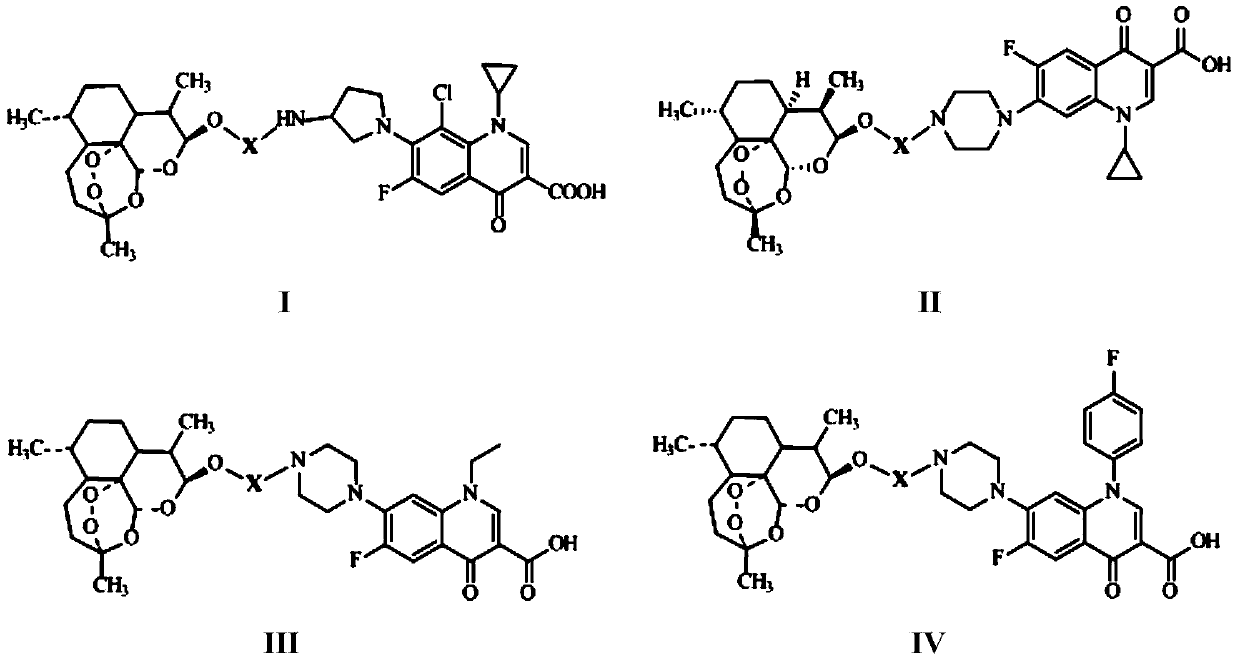
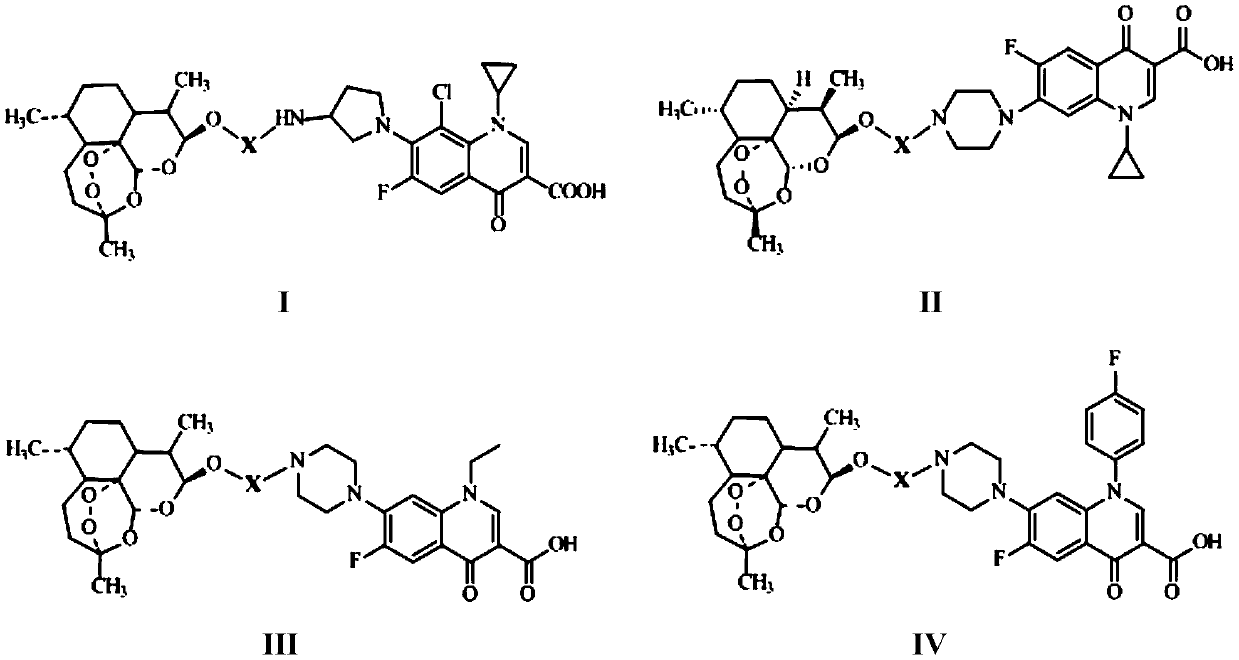
The invention discloses conjugates of dihydroartemisinin and quinolones compounds shown in formulae I to IV and officinal salt thereof, wherein X is -CH2CH2-, -CH2CH2CH2- or -COCH2CH2CO-. The conjugates have a certain antibacterial effect on a mycobacterium tuberculosis standard sensitive strain, a clinical separating sensitive strain and a clinical separating drug resistance strain, and especially the inhibitory effects of conjugates of dihydroartemisinin and clinafloxacin on the mycobacterium tuberculosis standard sensitive strain, the clinical separating sensitive strain and part of the clinical separating drug resistance strain are stronger than those of independent dihydroartemisinin and clinafloxacin. The conjugates can be used for preparing antituberculosis drugs and have a potential application prospect in tuberculosis prevention and cure field. The formulae I to IV are as shown in the specification.
Owner:SOUTHWEST UNIVERSITY
Conjugated product of dihydroartemisinin and quinolones, preparation method and application thereof
ActiveCN104418864BGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsQuinoloneAntituberculosis drug
The invention discloses conjugates of dihydroartemisinin and quinolones compounds shown in formulae I to IV and officinal salt thereof, wherein X is -CH2CH2-, -CH2CH2CH2- or -COCH2CH2CO-. The conjugates have a certain antibacterial effect on a mycobacterium tuberculosis standard sensitive strain, a clinical separating sensitive strain and a clinical separating drug resistance strain, and especially the inhibitory effects of conjugates of dihydroartemisinin and clinafloxacin on the mycobacterium tuberculosis standard sensitive strain, the clinical separating sensitive strain and part of the clinical separating drug resistance strain are stronger than those of independent dihydroartemisinin and clinafloxacin. The conjugates can be used for preparing antituberculosis drugs and have a potential application prospect in tuberculosis prevention and cure field. The formulae I to IV are as shown in the specification.
Owner:SOUTHWEST UNIV
Medicine-taking electronic monitor for patient with tuberculosis
InactiveCN105836313AReduce work stressGuaranteed tightnessSmall article dispensingOral administration deviceMedicineBottle
The invention discloses a machine-taking electronic monitor for a patient with tuberculosis, and mainly relates to the field of medicine-taking management of the patient with tuberculosis. The machine-taking electronic monitor comprises a housing, wherein a refrigerating cavity is arranged in the housing; a medicine storage bottle is arranged in the refrigerating cavity; one end of the medicine storage bottle is provided with a bottleneck; threads are arranged on the bottleneck; the bottom of the refrigerating cavity is provided with a threaded hole adaptive to the bottleneck; a funnel is arranged below the threaded hole; the bottom of the funnel is provided with a guide barrel; a medicine taking barrel is arranged on the side wall of the guide barrel; the bottom of the guide barrel is provided with a medicine outlet channel; and a medicine taking slot is formed in the outer wall of the housing. The medicine-taking electronic monitor has the beneficial effects that: the medicine-taking electronic monitor can replace a supervisor for the patient with tuberculosis to remind the patient to regularly take medicines with proper dosage on time and to take periodic review, so that working pressure of the tuberculosis prevention and treatment institution can be relieved, a proper temperature can be provided for storing anti-tuberculosis medicines, medicine-taking and reviewing moments can be set, the medicines can be stored and can be automatically taken out, and the medicine-taking moments of the patient can be recorded.
Owner:李源
Novel recombinant vaccine used for preventing tuberculosis
InactiveCN101850112AStable expressionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to the construction of a human GM-CSF gene and Mycobacterium tuberculosis ESAT6 gene chimerically expressed GMCSF-ESAT6 protein recombinant Bacillus Calmette Guerin (BCG) vaccine and immunogenicity research thereof, namely the sequence of the human GM-CFS gene and the sequence of the Mycobacterium tuberculosis ESAT6 gene are inserted into the sequence of the same escherichiacoli-Mycobacterium tuberculosis shuttle plasmid pMV361 by gene engineering technology so as to construct a recombinant shuttle plasmid rpMV361GMCSF-ESAT6; and a vector is introduced into a BCG vaccine by an electroporation method so as to construct a recombinant BCG vaccine rBCG:GMCSF-ESAT6. The recombinant BCG vaccine can express the human GM-CSF and Mycobacterium tuberculosis ESAT6 gene chimeric protein GMCSF-ESAT6 stably, and has an immunogenicity superior to that of the conventional BCG vaccine. The invention provides a process for preparing the recombinant BCG vaccine, researches the immunity thereof, and belongs to the field of gene engineering and the field of tuberculosis vaccine. The novel recombinant vaccine prevents the generation and the propagration of tuberculosis more effectively.
Owner:SICHUAN UNIV
Method for extracting live bacteria RNA in Mycobacterium tuberculosis and detection kit thereof
InactiveCN101760518AEasy to detectImprove featuresMicrobiological testing/measurementDNA preparationRNA extractionAntituberculous drugs
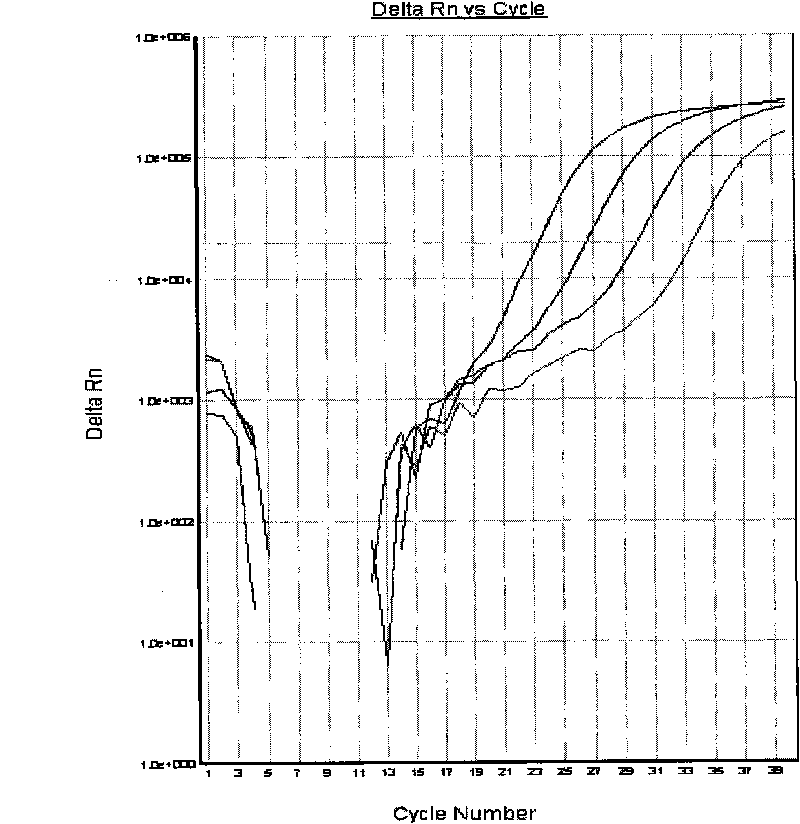
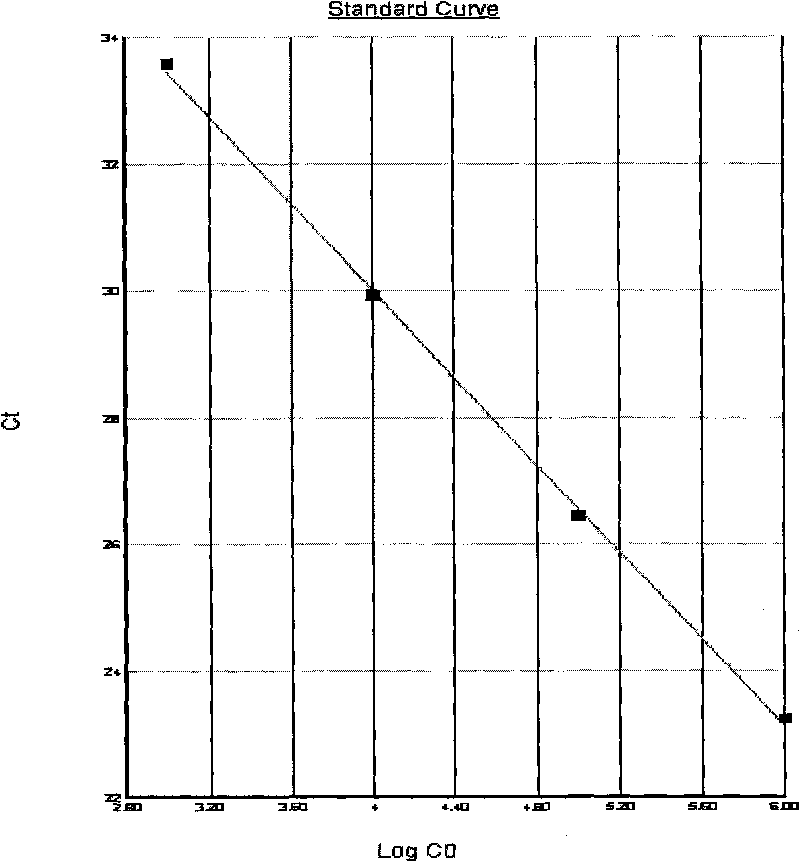
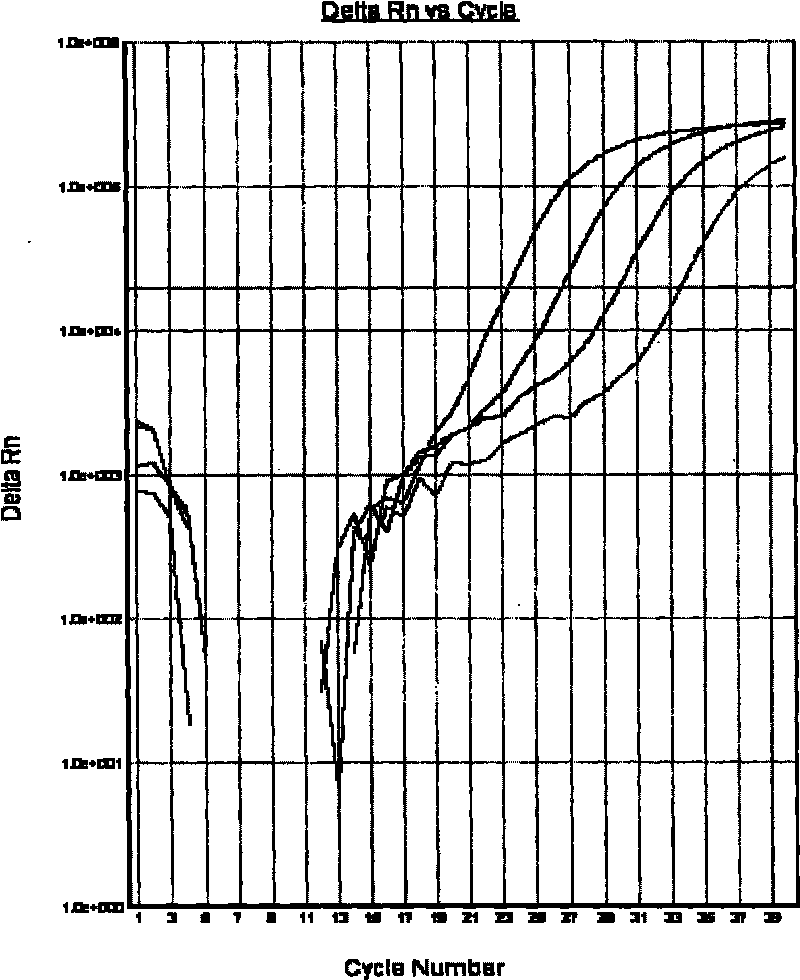
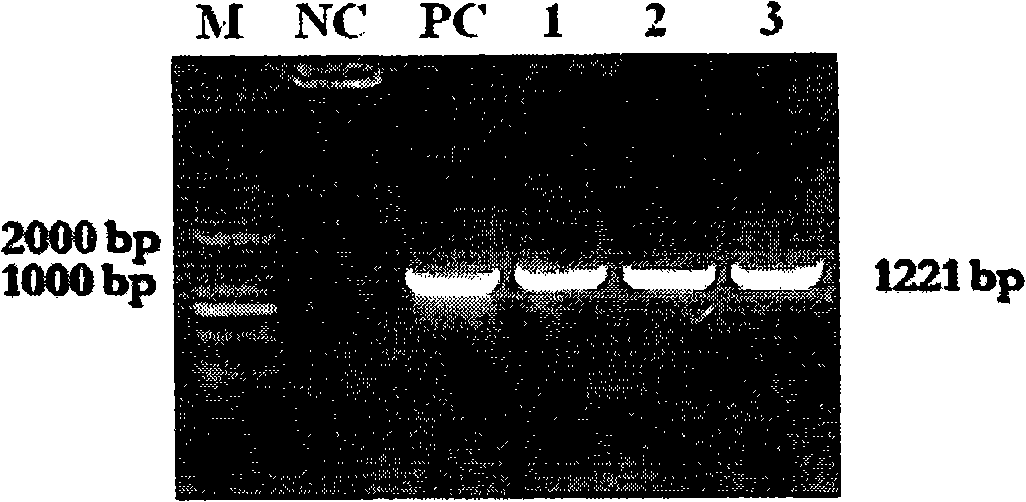
The invention relates to a method for extracting live bacteria RNA in Mycobacterium tuberculosis and a detection kit thereof, and particularly provides a method for extracting live bacteria RNA in Mycobacterium tuberculosis and a detection kit which is used for the live bacteria RNA in the Mycobacterium tuberculosis and is obtained by applying the method and combining fluorescent quantitative PCR technology. The kit can accurately, sensitively and quickly identify dead bacteria and the live bacteria of the Mycobacterium tuberculosis, reduce the cost and shorten the detection time, and more importantly, the kit is the basis of studies such as the diagnosis of tuberculosis, medicinal susceptibility experiments, the monitoring of chemotherapy response, the screening of new antitubercular medicaments, the prevention of the tuberculosis and the like.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Recombinant mycobacterium smegmatis strain capable of expressing mycobacterium tuberculosis Ag 85B and ESAT-6 fusion protein and application thereof
InactiveCN101875913AStrong immune responseGood immune protectionAntibacterial agentsBacterial antigen ingredientsMycobacterium smegmatisEukaryotic plasmids
The invention relates to a recombinant mycobacterium smegmatis strain capable of expressing mycobacterium tuberculosis Ag 85B and ESAT-6 fusion proteins and application thereof. Recombinant plasmids containing the Ag 85B and ESAT-6 fusion protein genes are turned into mycobacterium smegmatis (AE-MS for short) through electrotransformation, and the preservation serial number thereof is CCTCC M2010097. When used for immunizing mice, the recombinant mycobacterium smegmatis strain AE-MS obtained through screening can induce the immune response level which is stronger than that of the mycobacterium smegmatis. The invention also relates to the application of the structured mycobacterium smegmatis strain to the preparation of preparations or medicaments used for preventing and treating tuberculosis. The recombinant mycobacterium smegmatis expressing the Ag 85B and ESAT-6 fusion genes integrates the advantages of target antigens and live carriers; and after immunization, the recombinant mycobacterium smegmatis can increase the immune protection of an organism by simulating the stronger immune response of the organism, thereby having good prospect of application.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
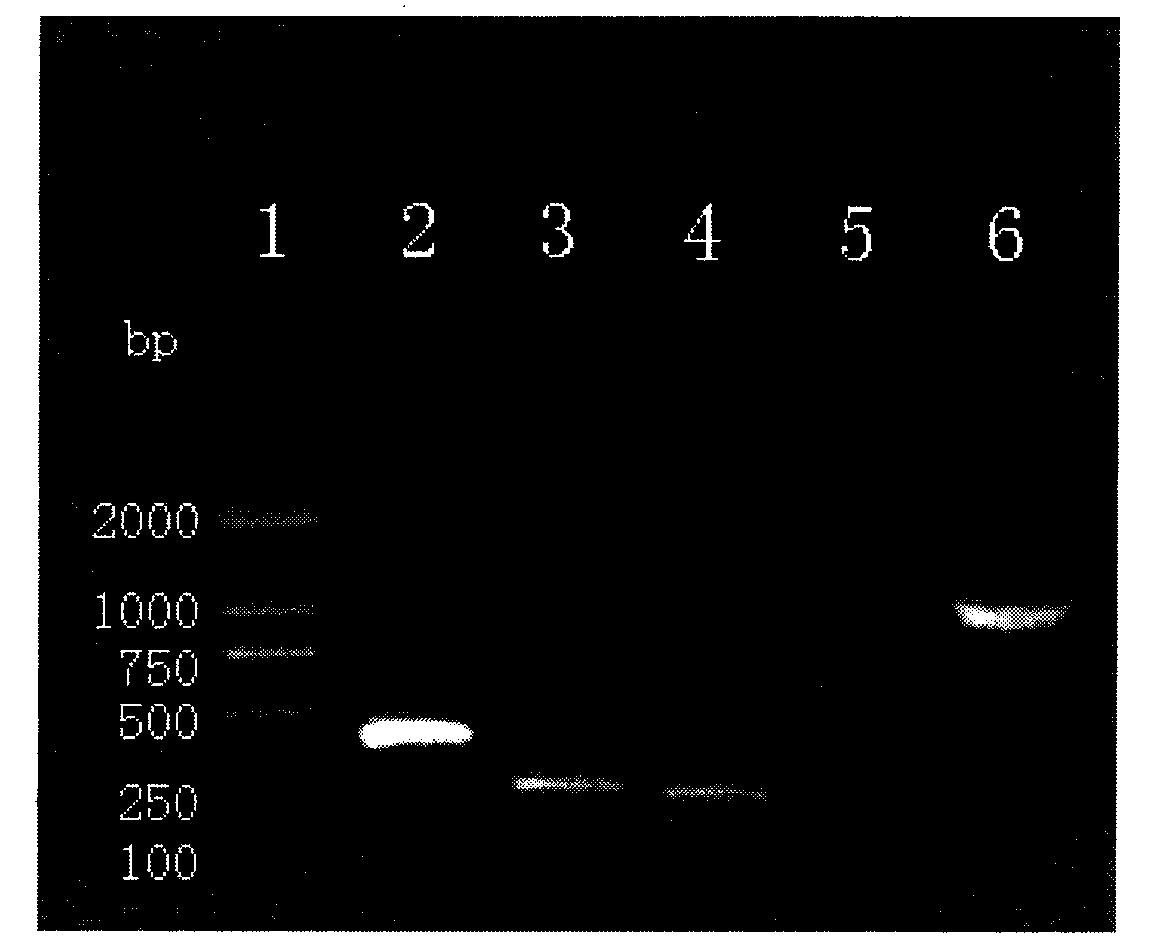
Mycobacterium tuberculosis drug-resistant mutant gene detection kit
ActiveCN102424859AReduce spreadGuaranteed therapeutic effectMicrobiological testing/measurementMicroorganism based processesIsoniazidNucleotide
The invention discloses a mycobacterium tuberculosis drug-resistant mutant gene detection kit comprising: (1) a gene chip which has (i) a nucleotide probe, wherein the probe is a sequence of SEQIDNos: 1-31 or a complementary sequence of SEQIDNos: 1-31, (ii) a DNA sequence labeled with biotin points, wherein the sequence is SEQIDNO. 32, (iii) an IS6110DNA sequence of a mycobacterium tuberculosis complex, wherein the sequence is SEQIDNo. 33; (2) various primers used for amplifying DNA sequences in clinical samples, wherein the DNA sequences of the primers are SEQIDNos. 34-41. The kit of the invention detects the drug resistance of mycobacterium tuberculosis to rifampin and isoniazid, provides reference basis for the prevention and treatment of tuberculosis, and has significant meaning.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
Research and application of Mycobacterium extract
InactiveCN101518549AStimulate immune responseHas immunogenic activityAntibacterial agentsPowder deliverySide effectMedicine
The invention relates to a method for extracting effective components of Mycobacterium, aims at removing lipid components, reducing side effects, remaining effective components thereof and leading to a function of immunological adjuvant, and comprises the strain of Mycobacterium and application of the extract thereof on the prevention and treatment of tuberculosis.
Owner:中国人民解放军总医院第二附属医院
Mycobacterium tuberculosis fusion protein (EAMMH) and constructing, expressing and purifying method and application thereof
ActiveCN104098700AImprove shortcomingsStrong immunityAntibacterial agentsBacterial antigen ingredientsAntigenInclusion bodies
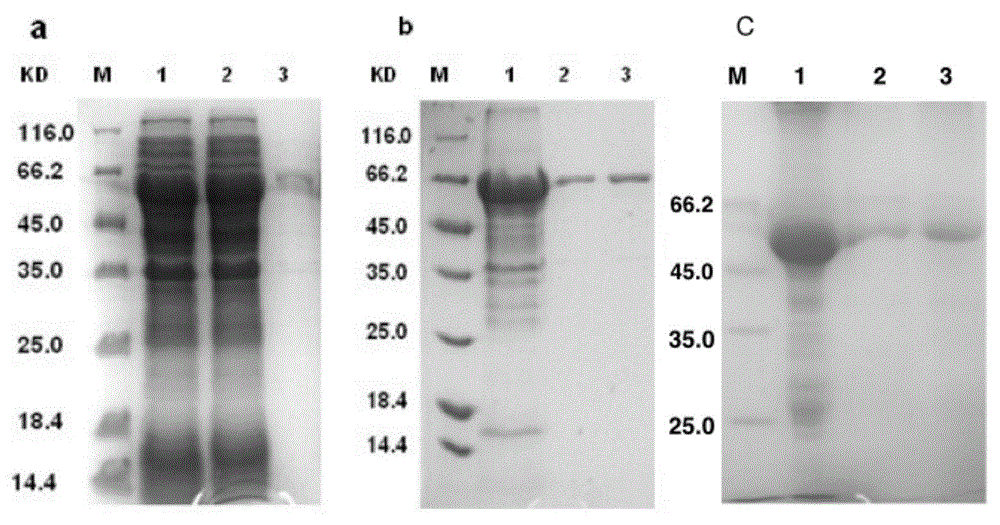
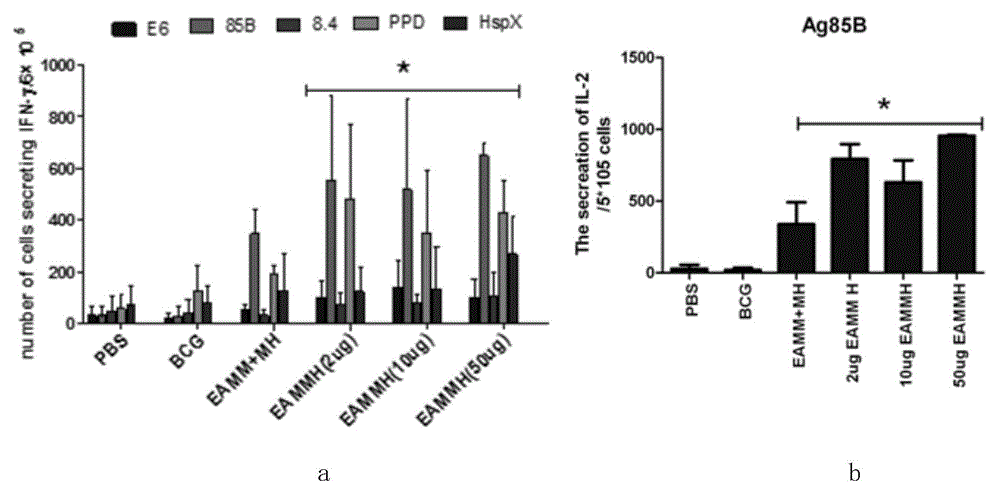
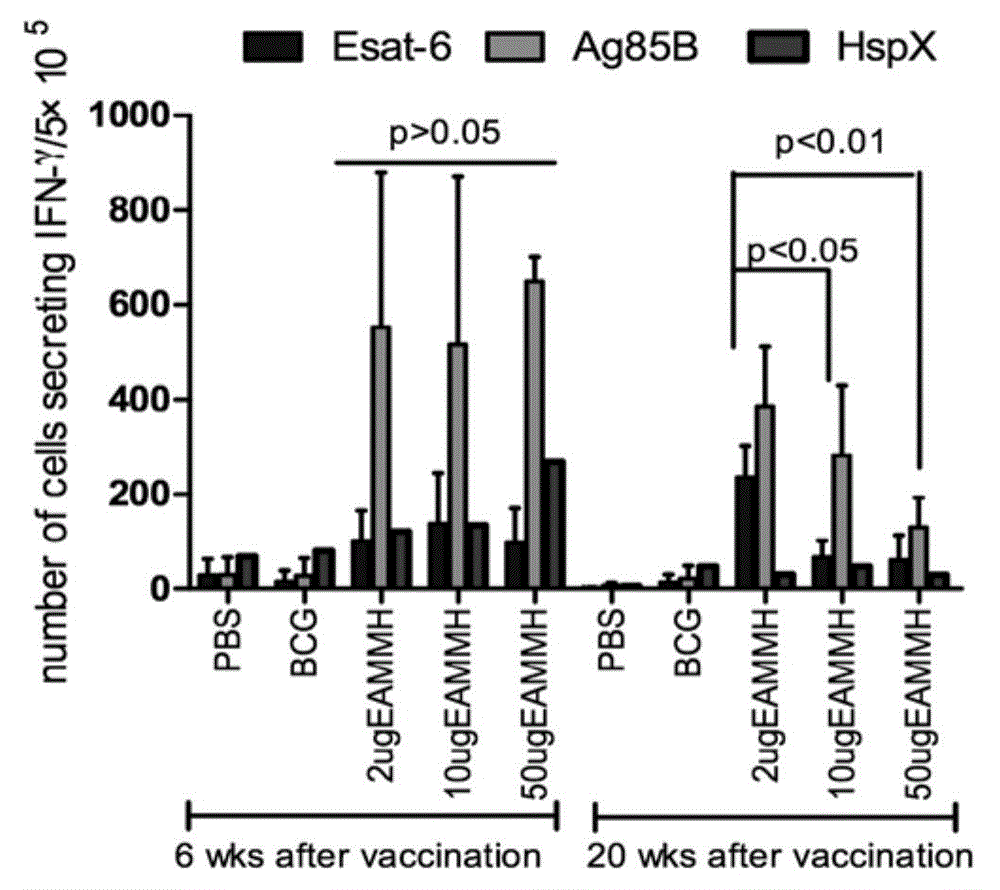
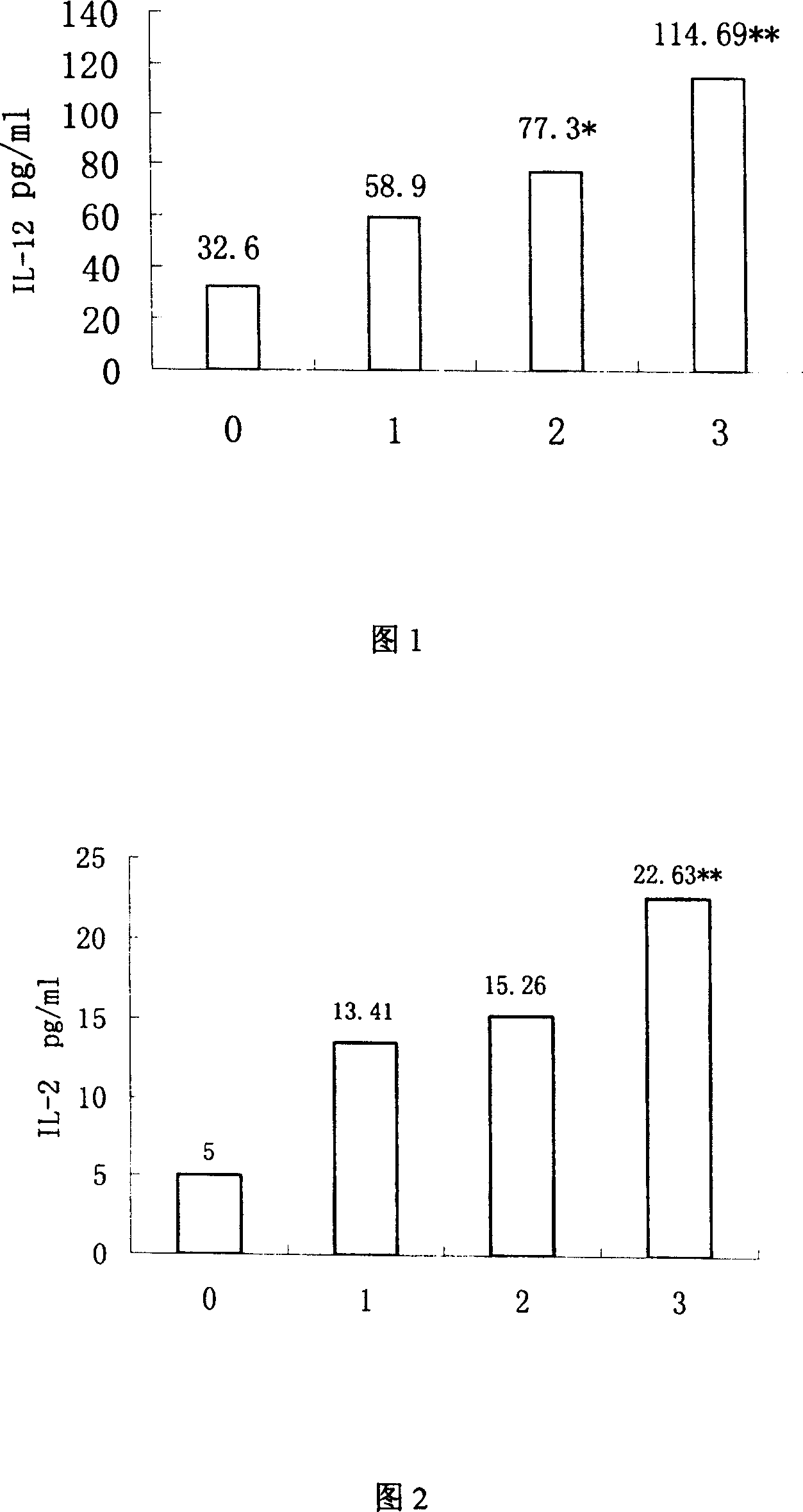
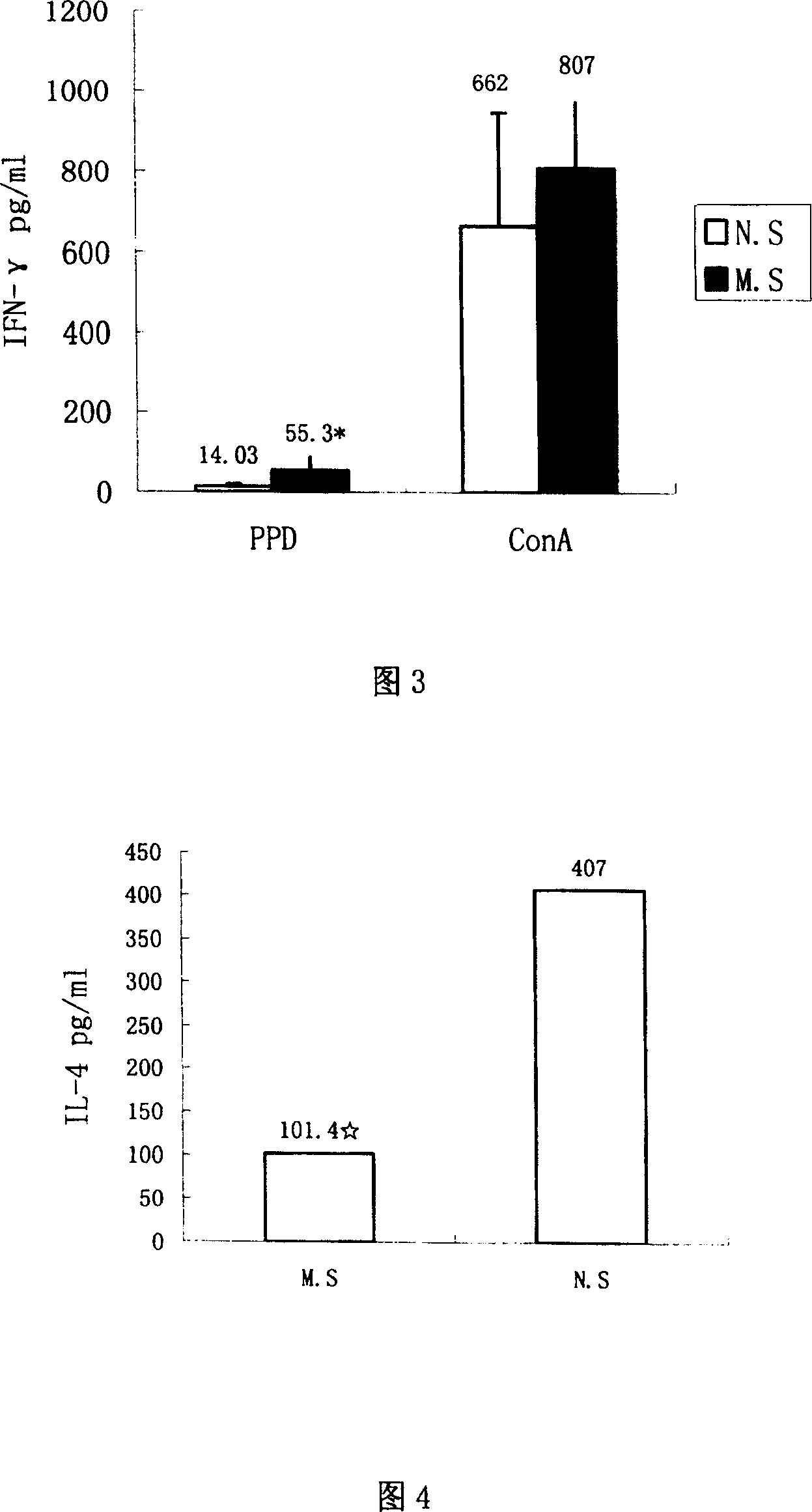
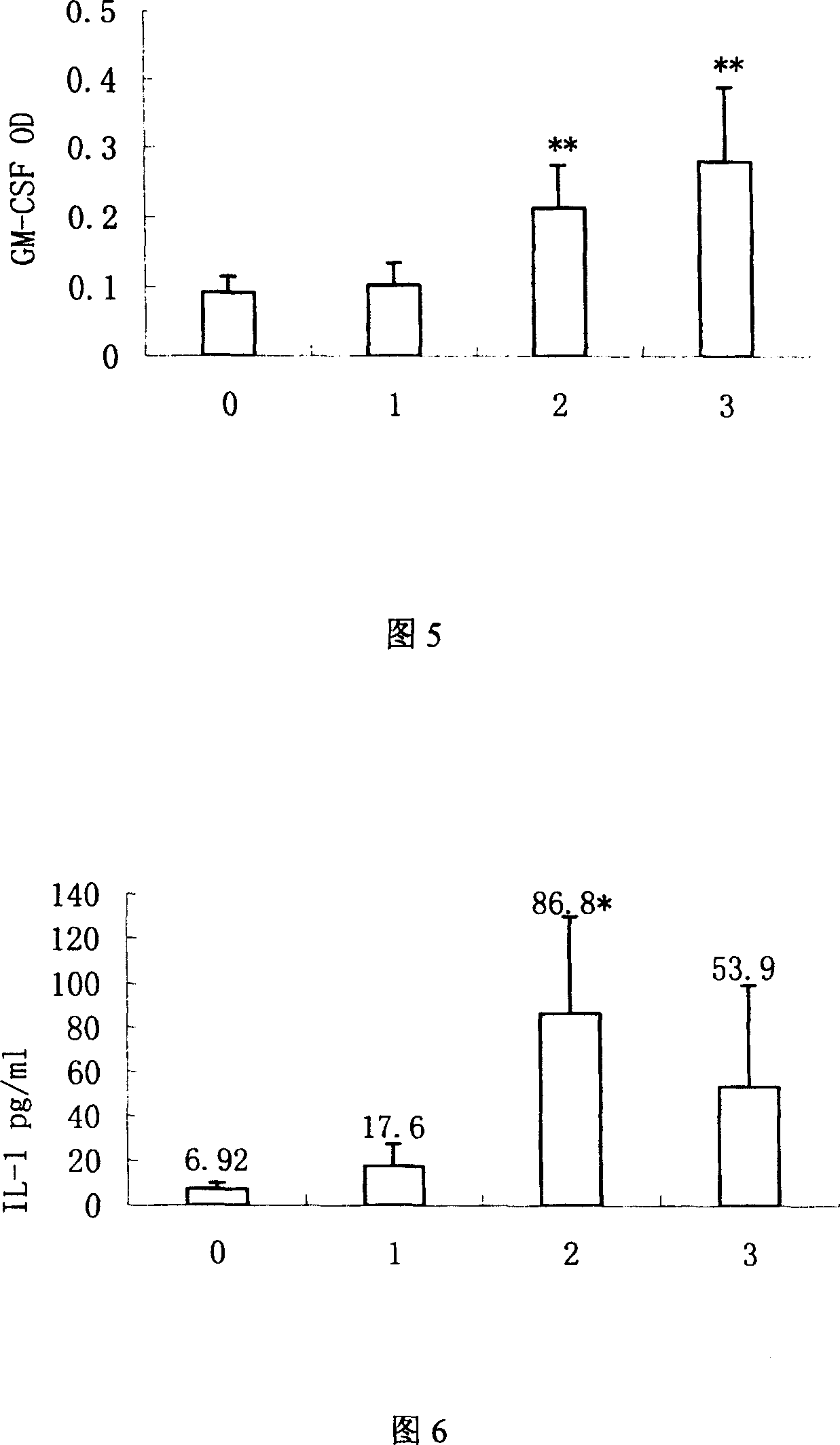
The invention discloses mycobacterium tuberculosis fusion protein EAMMH and a constructing, expressing and purifying method and application thereof. The fusion protein is expressed in a soluble form, and greatly improves EAMM inclusion body form expression weakness. Tuberculosis subunit vaccine (LT69) constructed by combination of the fusion protein and an adjuvant has strong protective immunity, is superior to traditional BCG (Bacillus Calmette-Guerin) vaccine and EAMM+MH combined vaccine; the vaccine as an enhanced vaccine can significantly enhance the BCG initial immune immunity and protection effect of anti tuberculosis, and to a certain extent, reduces the pathological injury of the lung; in addition, the subunit vaccine contains a wide variety of antigens of growth period and latency period of mycobacterium tuberculosis, can induce strong specific cellular immune and humoral immune response aiming at each period of tubercle bacillus antigen, has protective effect on the tubercle bacillus in different metabolic states, is long in protection time, and is expected to become an effective vaccine for clinical tuberculosis prevention.
Owner:LANZHOU UNIVERSITY
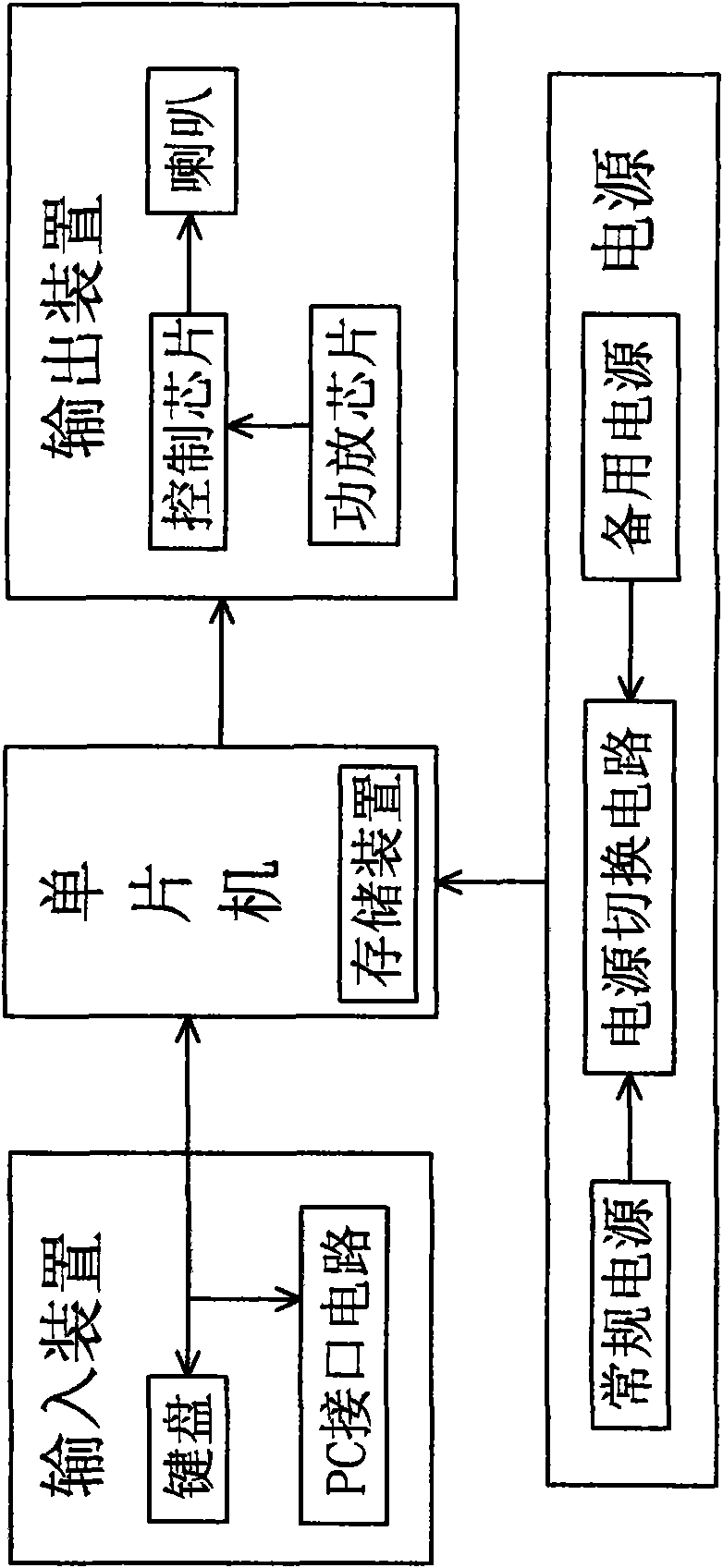
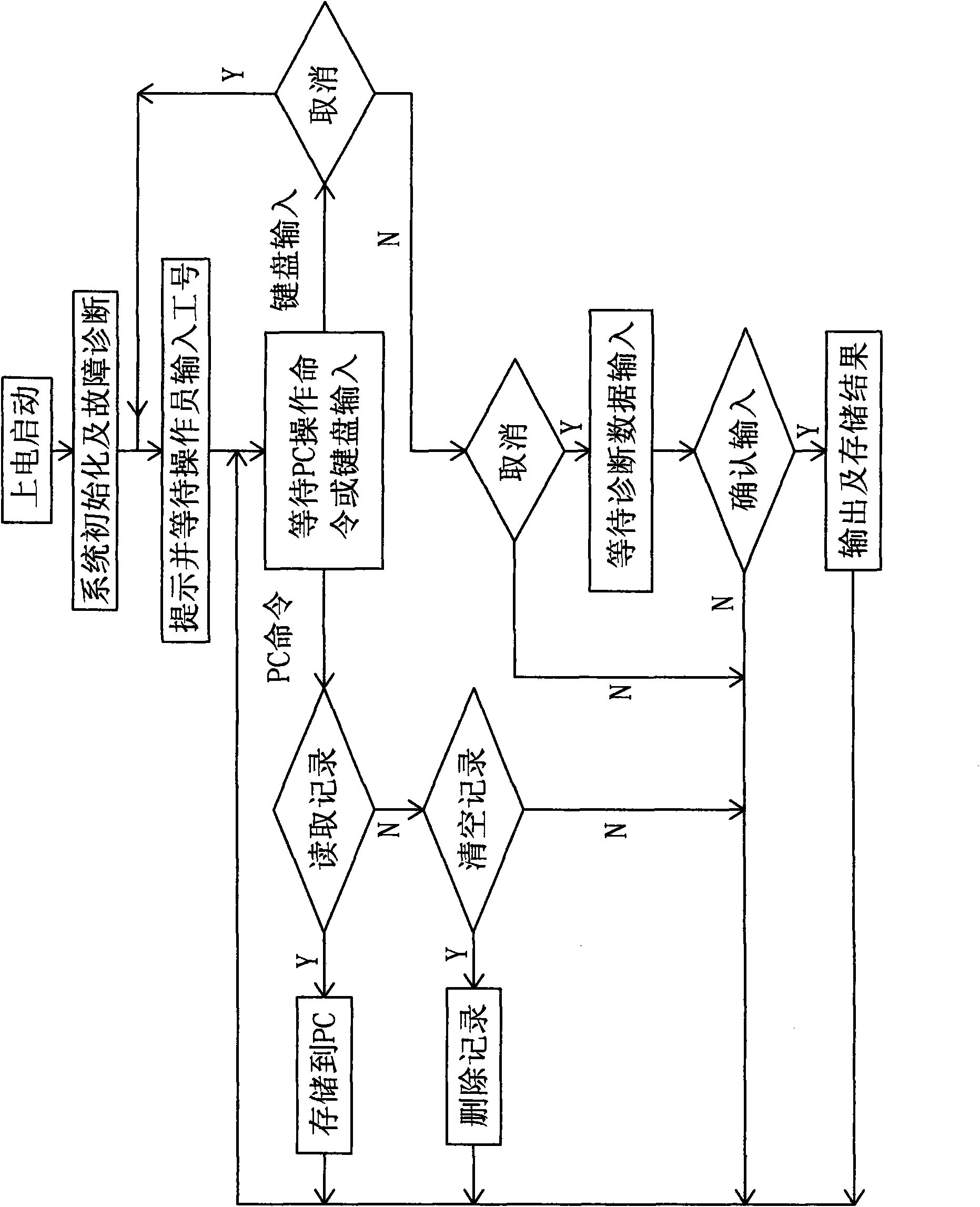
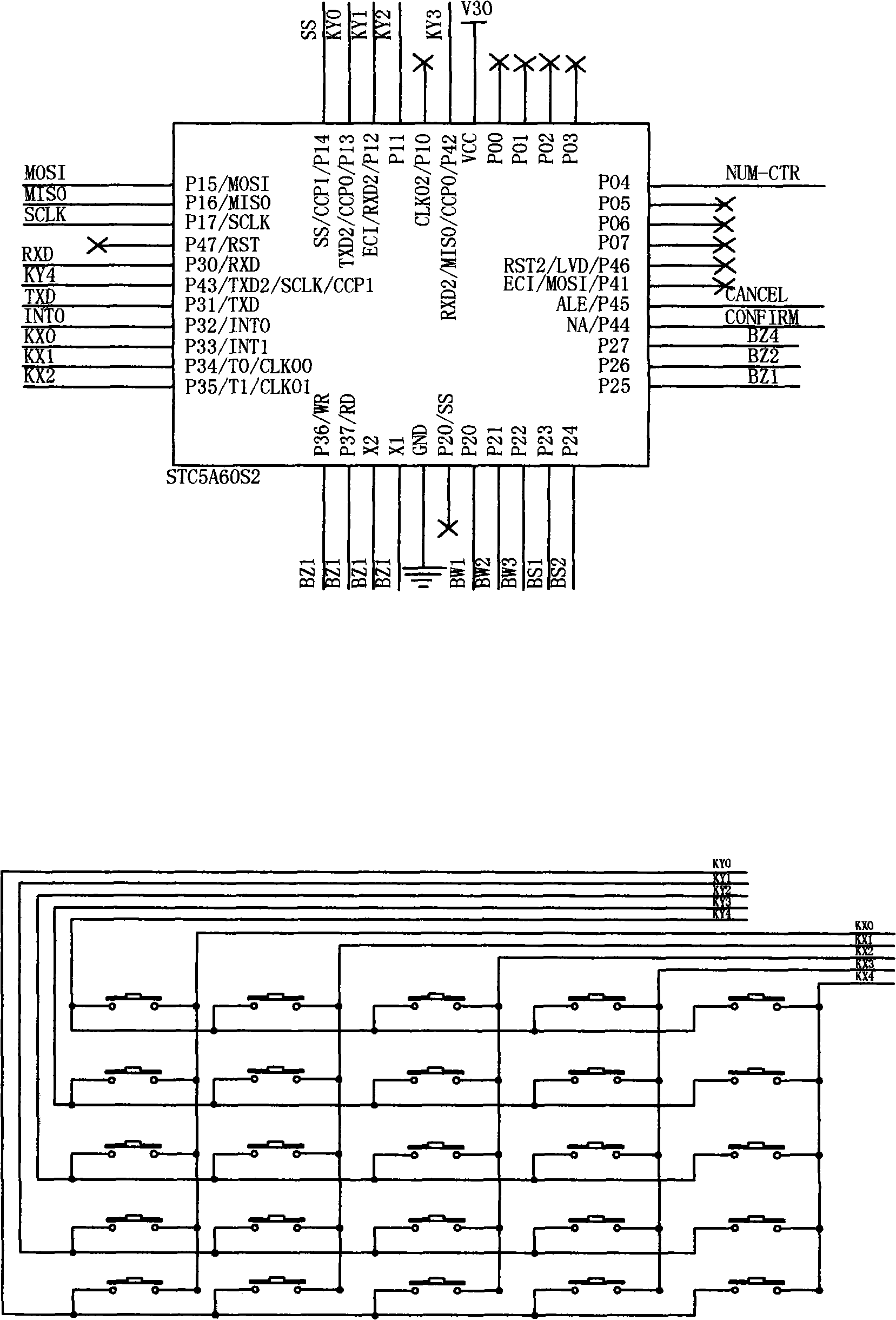
Diagnostic equipment for suspected tuberculosis and image processing method thereof
The invention discloses diagnostic equipment for suspected tuberculosis. The diagnostic equipment comprises a control device and an input device, an output device and a power supply device which are connected with the control device in series respectively, wherein the control device is an STC12C5A60S2 series singlechip microcomputer, the input device is a keyboard and a PC interface circuit, and the output device is a speech output device; and by the image processing method, the image information is divided into three categories, totally eight types of characteristics, a value corresponding to each type of characteristics is set, the values corresponding to the three categories of characteristics are summed to give a result value, the result value is divided into three value intervals, and one image processing result corresponding to each interval is outputted. When the diagnostic equipment is used for non-professional tuberculosis prevention and control organizations, a finding rate of patients suffering tuberculosis is improved, the spread of tuberculosis among people is avoided, a misdiagnosis rate and a rate of missed diagnosis are both greatly lowered, the work load of medical staff in the X-ray department, the working efficiency is improved, and the information on the medical staff making the diagnosis is also recorded in order to apply a responsibility system and strengthening the sense of responsibility of the medical staff.
Owner:陈卫
Construction and expression purification method of mycobacterium tuberculosis fusion protein LT29 and application of mycobacterium tuberculosis fusion protein LT29
InactiveCN112979825AEfficient purificationNo obvious side effectsAntibacterial agentsBacterial antigen ingredientsEscherichia coliAdjuvant
The invention discloses an expression purification method of a mycobacterium tuberculosis fusion protein LT29. An active-period antigen EAST6 of mycobacterium tuberculosis and an incubation-period antigen Rv2626c of the mycobacterium tuberculosis are fused and connected by a flexible peptide fragment so as to construct the fusion protein ESAT6-linker-Rv2626c. The fusion protein is subjected to expression purification in escherichia coli B21 engineering bacteria, and is combined with an adjuvant DP to construct a tuberculosis subunit vaccine. The fusion protein is successfully expressed and purified in escherichia coli; strong specific cellular immunoreaction aiming at the specific antigens (ESAT6 and Rv2626c) of the mycobacterium tuberculosis can be induced to be generated in a mouse body, the generation of long-term memory T cells is promoted, and the fusion protein has strong immunogenicity. According to the invention, the novel fusion protein LT29 without a label is successfully constructed, expressed and purified, the protein fuses the active-period antigen and the incubation-period antigen of tubercle bacillus, antigen-specific cellular immunity and humoral immunity can be induced to be generated, and the novel fusion protein LT29 is expected to become an effective candidate vaccine for preventing and treating tuberculosis.
Owner:LANZHOU UNIVERSITY
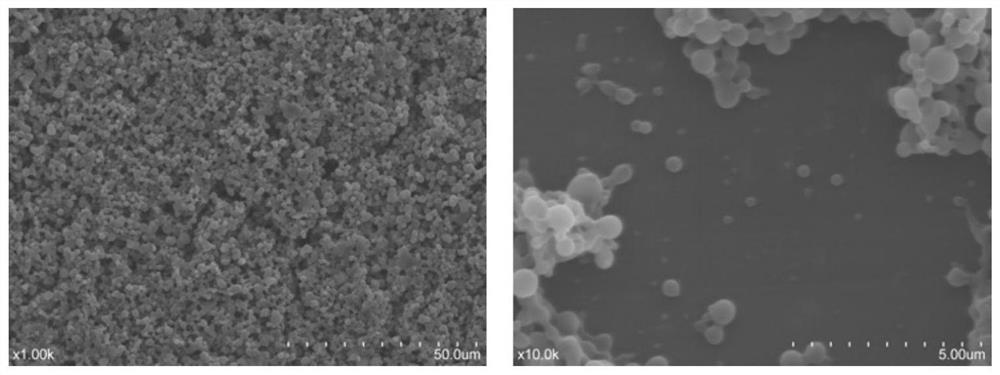
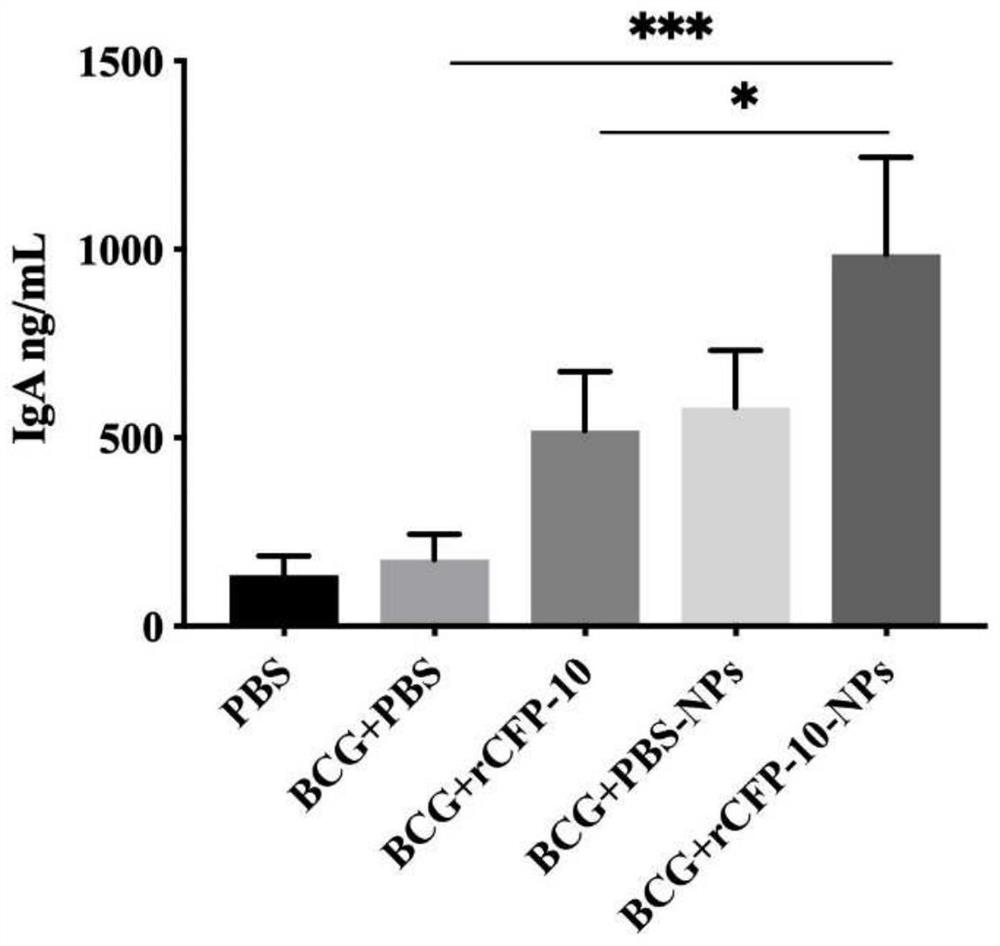
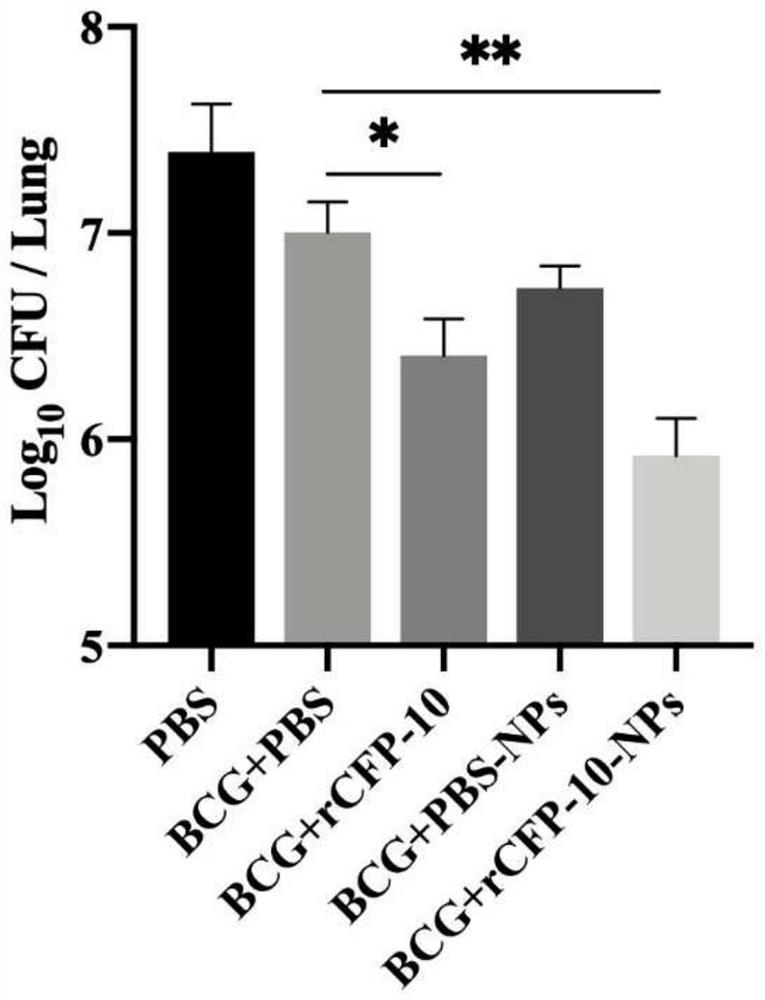
Preparation and application of recombinant protein CFP-10 nanoparticles
ActiveCN110354098AImprove immunityReduce bacterial loadAntibacterial agentsBacterial antigen ingredientsBCG immunizationEmulsion
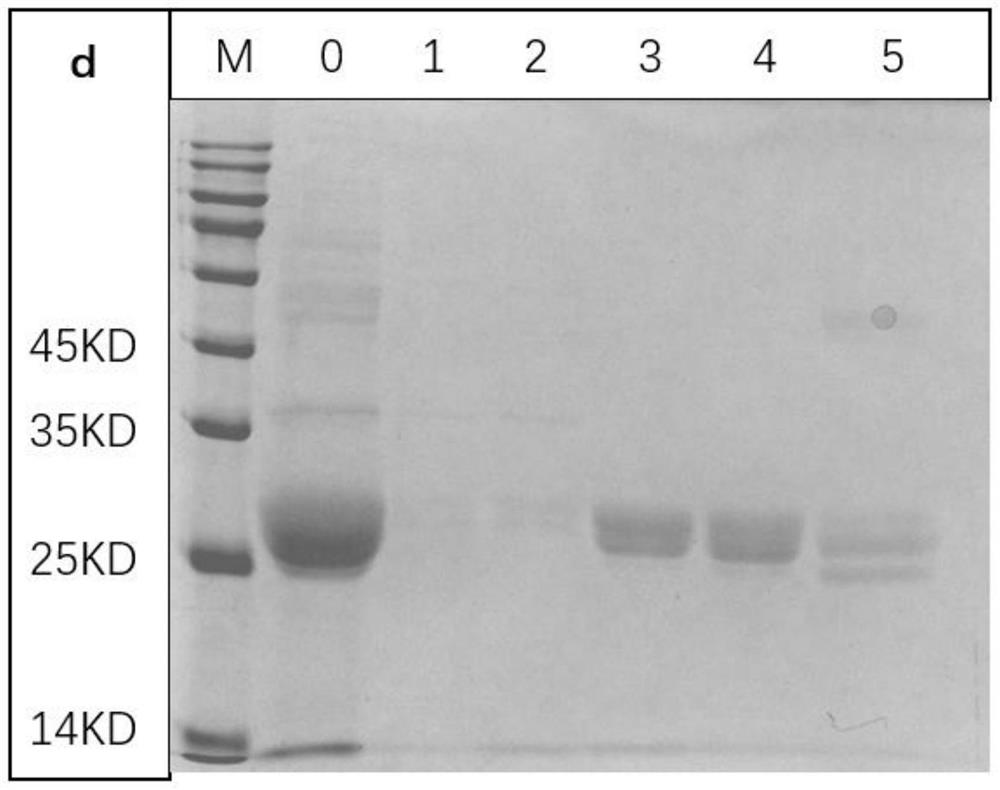
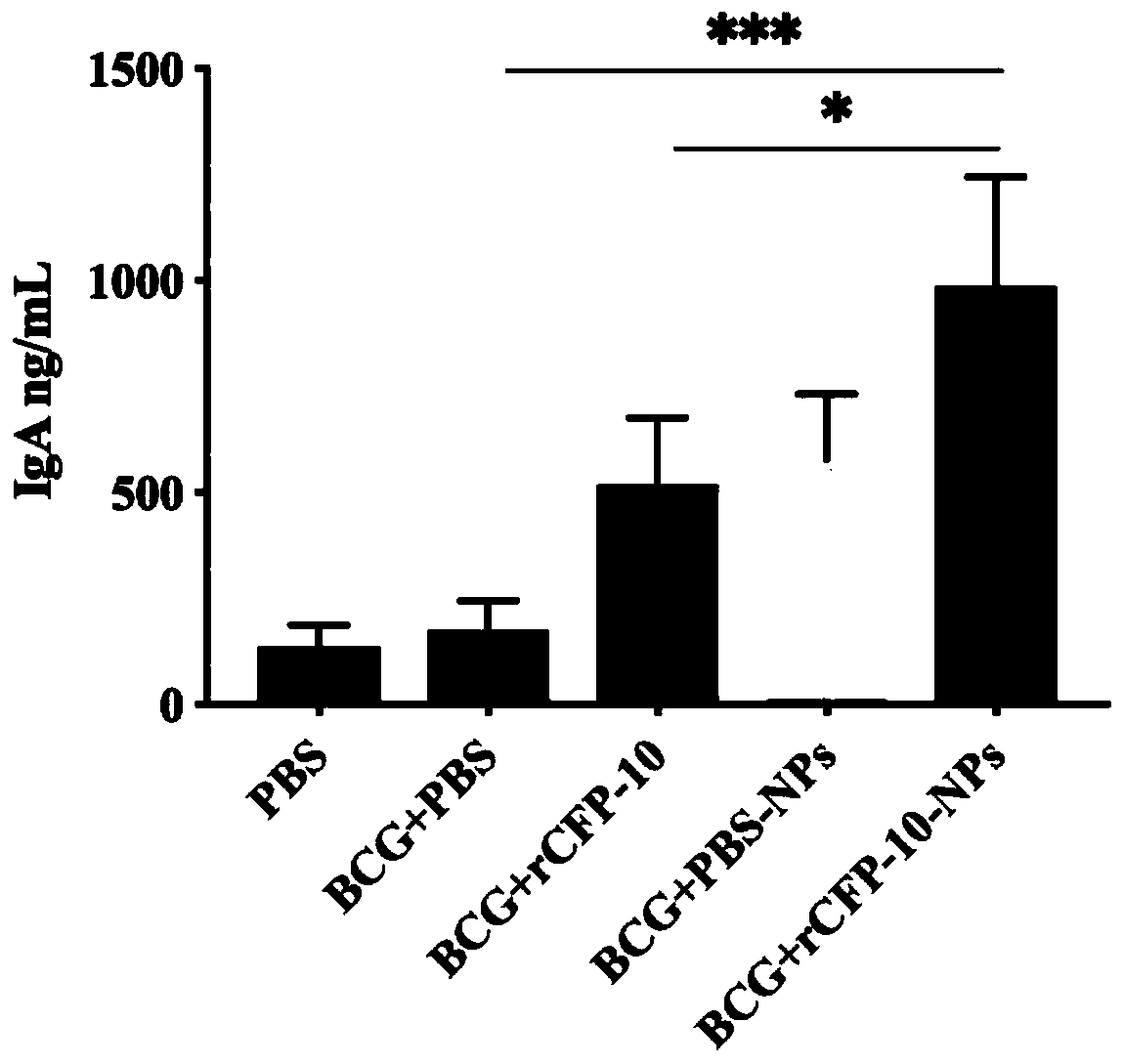
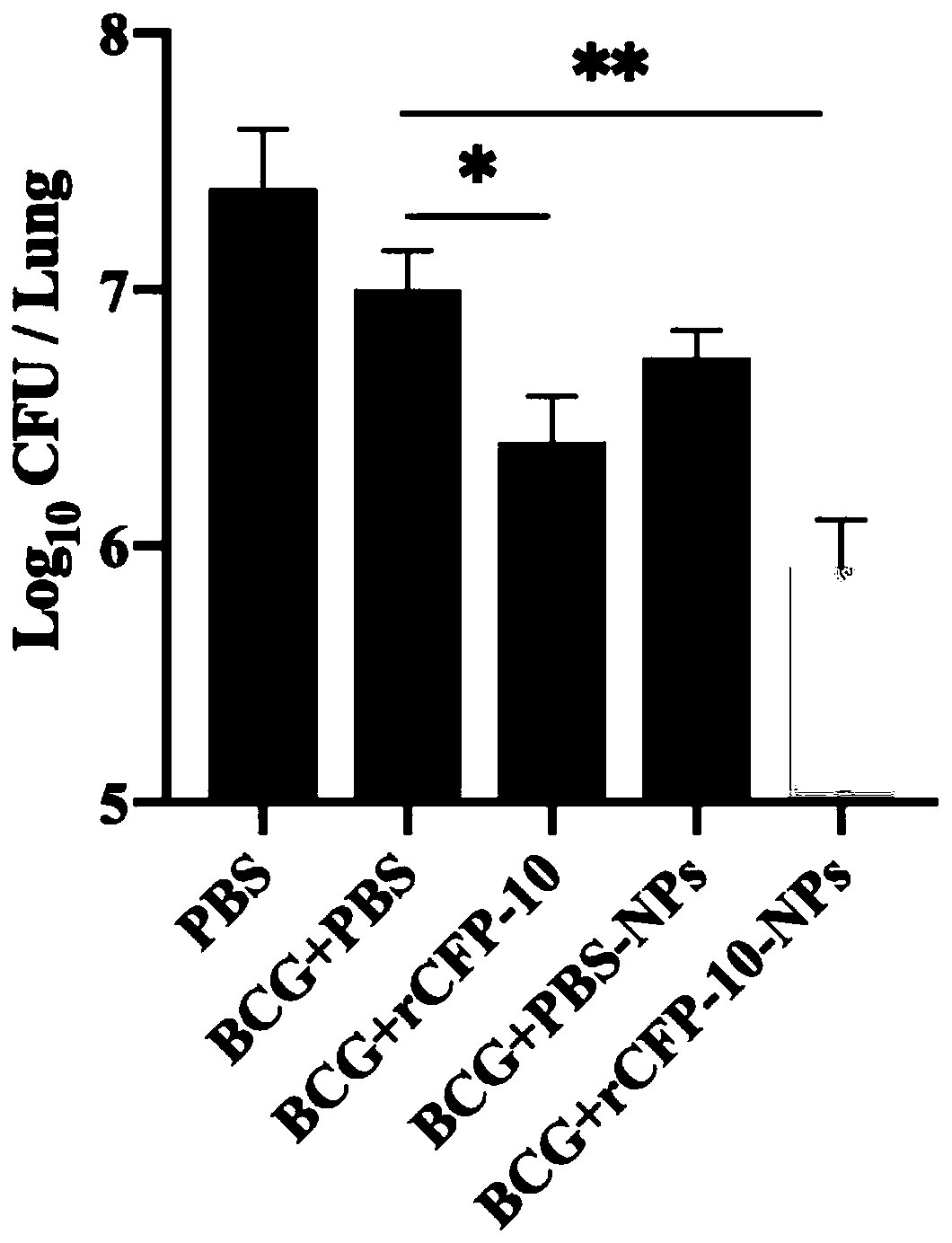
The invention provides a preparation method of recombinant protein CFP-10 nanoparticles and the application thereof in preparing tuberculosis prevention vaccines. The preparation method includes the steps of (1) configuration of a polylactic acid-glycolic acid copolymer PLGA solution; (2) configuration of a recombinant protein CFP-10 solution; (3) preparation of primary emulsion; (4) preparation of compound emulsion; (5) formation of the nanoparticles. The recombinant protein CFP-10 nanoparticles prepared by the preparation method are used for enhancing the immunity of BCG-immunized mice through intranasal drip, and it is confirmed that the recombinant protein CFP 10 nanoparticles have the effects of improving mucosal immunity level and promoting BCG immunity.
Owner:CHINA AGRI UNIV
Functional mycobacterium tuberculosis antigen polypeptide and application thereof
ActiveCN102653554APromote proliferationAntibacterial agentsBacterial antigen ingredientsLymphocyteAntigen-antibody reactions
The invention discloses a functional mycobacterium tuberculosis antigen polypeptide and an application thereof. According to the polypeptide disclosed by the invention, an amino acid sequence is represented by a sequence 1 in a sequence table. The polypeptide can stimulate H37Rv infected mice spleen cells and lymphocytes to be obviously multiplied so as to be subjected to an antigen-antibody reaction with infected mice blood serum; and therefore, the polypeptide has the antigen property and can be used for developing novel tuberculosis preventing and treating vaccines.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
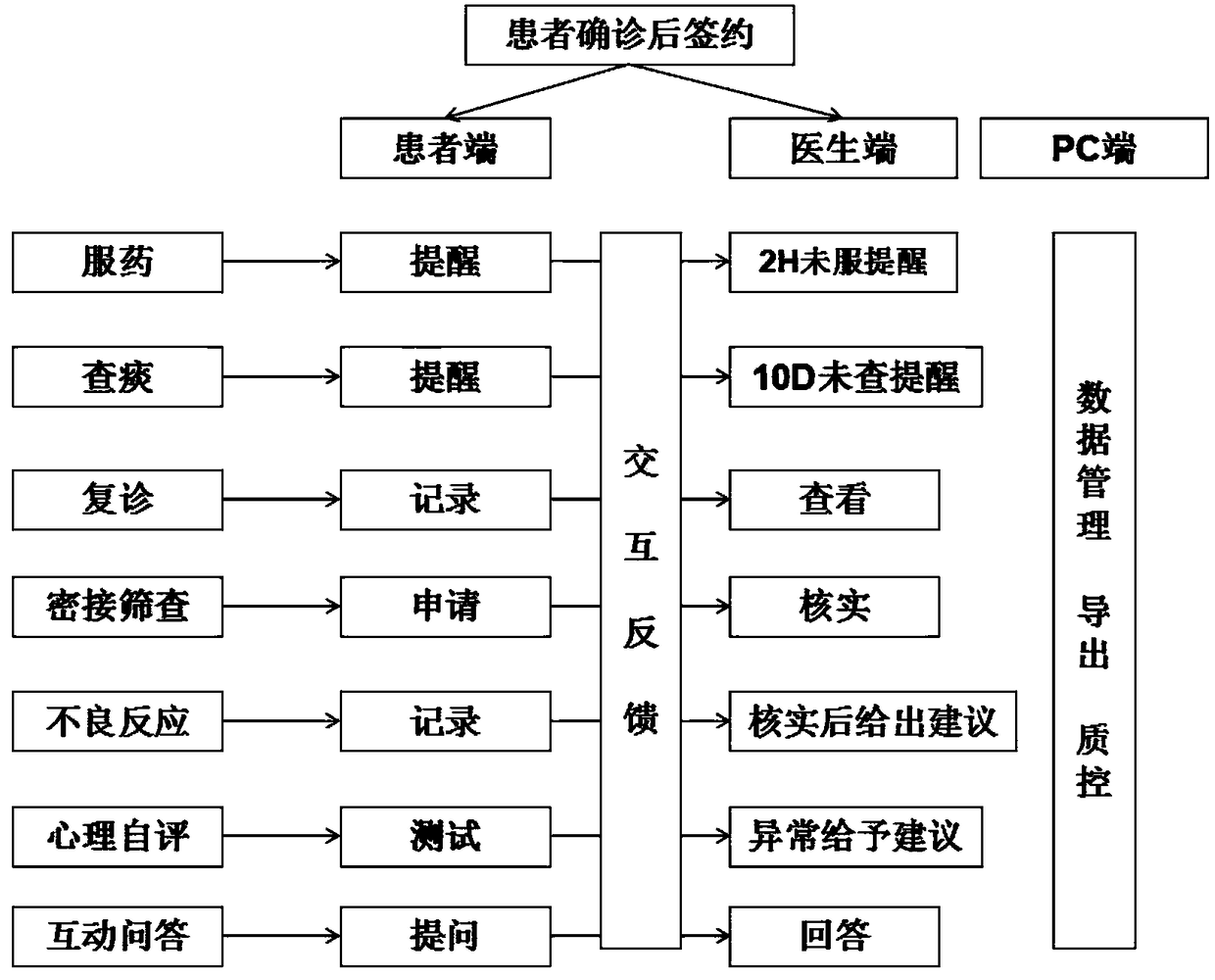
Integrated tuberculosis management system and mobile APP project
The invention provides an integrated tuberculosis management system and a mobile APP project. Specifically, the integrated tuberculosis management system provided by the invention not only can reminda tuberculosis patient to take medicine, but also can monitor the psychological condition of the tuberculosis patient by means of psychological evaluation, comprehensively analyzes the data of the obtained medication records and the psychological evaluation, and outputs the data to a community tuberculosis prevention and treatment management doctor or a disease control center. The community doctoror the disease control center monitors the medication compliance and psychological status of the tuberculosis patient according to an analysis result.
Owner:上海市普陀区疾病预防控制中心
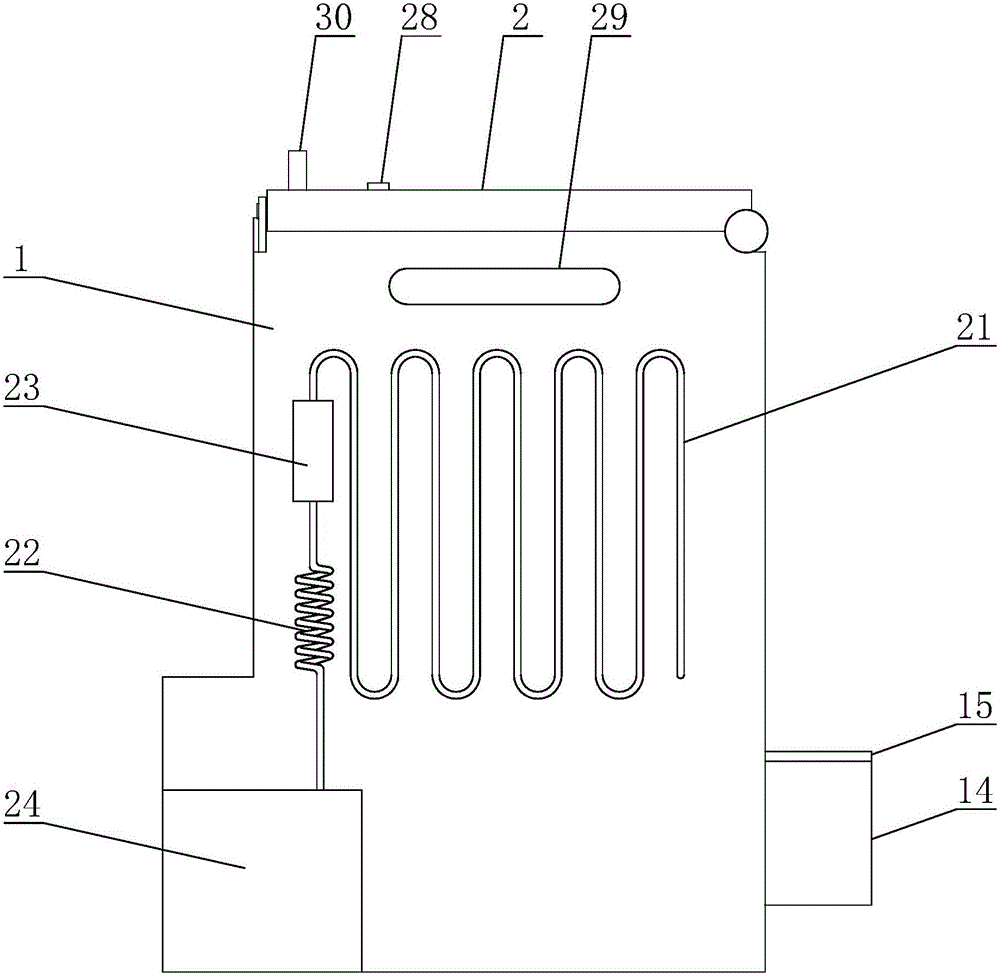
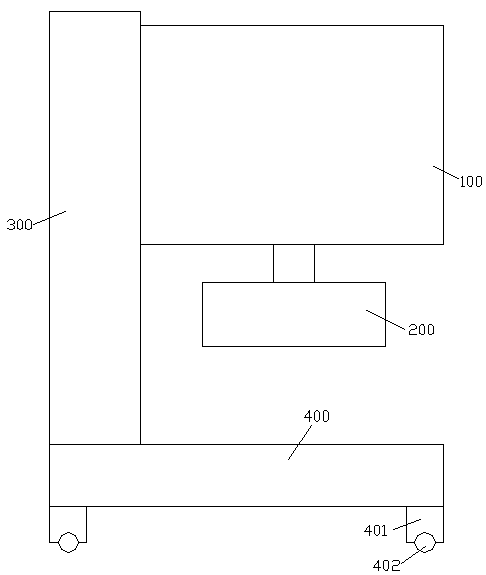
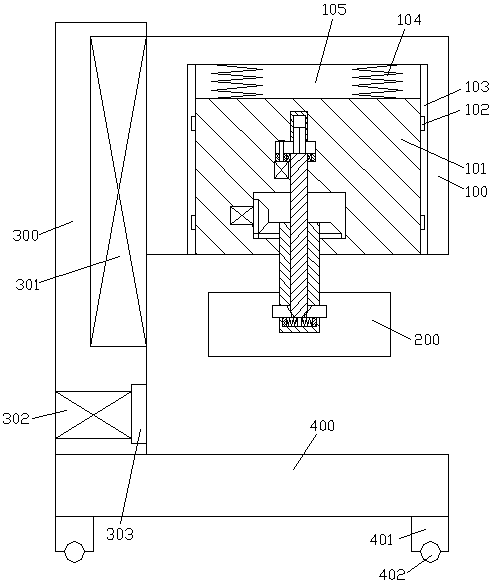
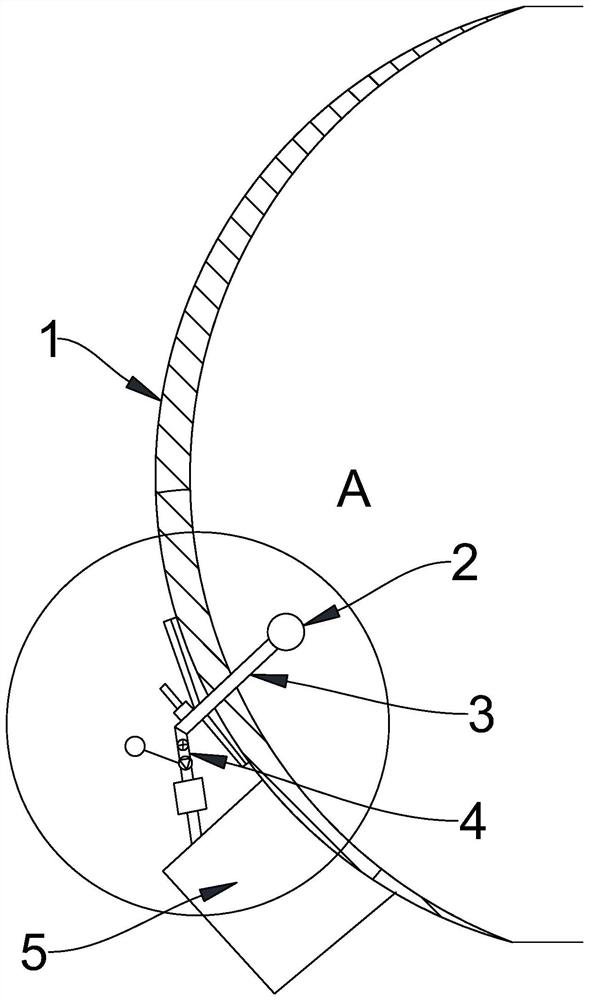
A preparation device for tuberculosis prevention and treatment drugs
InactiveCN108465544BSimple structureEasy to operateGrain treatmentsSteering columnDrugs preparations
Owner:王承莲
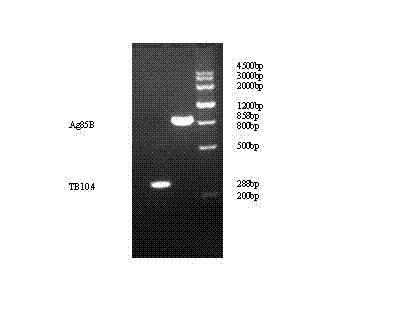
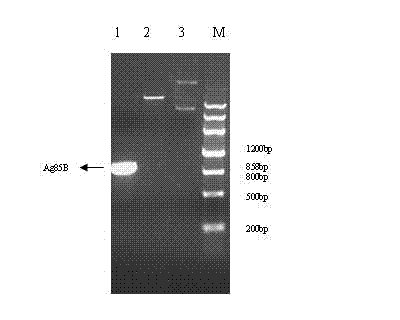
Tag-free tuberculosis fusion protein TB10-Ag85B
InactiveCN102174112AEfficient purificationSolve the experimentAntibacterial agentsBacterial antigen ingredientsGenetic engineeringTuberculosis prevention
The invention relates to a tag-free tuberculosis fusion protein TB10-Ag85B. A method comprises the following steps of: recombining and fusing the genes TB10.4 and Ag85B of mycobacterium tuberculosis by a gene engineering technology to construct a recombinant plasmid, performing inducible expression to obtain the expression product of a recombinant gene, and purifying the expression product of the recombinant gene to clone and construct a tag-free mycobacterium tuberculosis fusion protein TB10.4-Ag85B. The invention has the advantages that: the subsequent problem that tags carried by the fusion protein can influence animal experiments and further clinical tests is solved, and the fusion protein is effectively purified by different chromatographic analysis methods and is expected to become a candidate vaccine for preventing tuberculosis.
Owner:LANZHOU UNIVERSITY
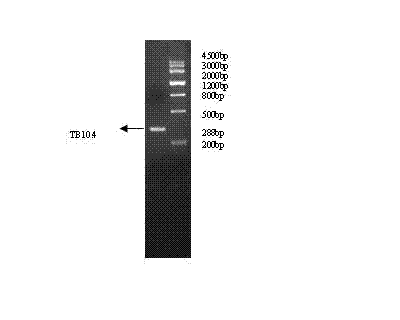
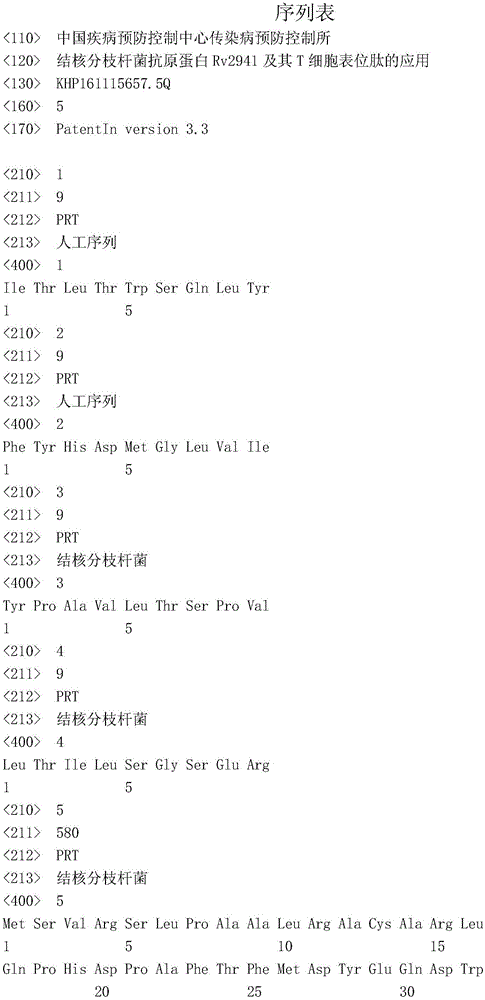
Use of mycobacterium tuberculosis antigen protein Rv2941 and its T cell epitope peptide
ActiveCN106405107AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsAntituberculous drugs
The invention relates to a use of mycobacterium tuberculosis antigen protein Rv2941 and its T cell epitope peptide in preparation of a tuberculosis detection agent, vaccine and drug. The antigen protein Rv2941 and its T cell epitope peptide have amino acid sequences respectively shown in the formulas of SEQ ID NO: 1 to 5. The mycobacterium tuberculosis Rv2941 protein antigen and its T cell epitope peptide as irritants are used for mycobacterium tuberculosis infection-caused specific T cell and B cell immunoreaction. Compared with the existing complete antigen, the mycobacterium tuberculosis antigen protein Rv2941 and its T cell epitope peptide can reduce a false positive result caused by antigen impurities. A detection reagent prepared from the Rv2941 protein antigen and its epitope peptide can be widely used in the related area of tuberculosis auxiliary diagnosis and epidemiological surveillance. A tuberculosis vaccine and an anti-tuberculosis drug prepared from the Rv2941 protein antigen and its epitope peptide can be used for tuberculosis prevention and treatment.
Owner:ICDC CHINA CDC
Mycobacterium Smegmatis preparation and use thereof
The invention provides a mycobacterium Smegmatis preparation, which is prepared from Mycobacterium Smegmatis through fragmenting decomposition, such as heavy pressure fragmenting method or ultrasonication method. The Mycobacterium Smegmatis is a safe and effective immuno-modulatory agent for enhancing normal body immune function, accelerating the restoration of the low body immune functions and inhibiting over-strong immune response of the immune sthenic body, i.e. bidirectional immunological control response.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
Liposome formulation suitable for treating or preventing tuberculosis
ActiveUS20150050327A1Suitable for preparationAntibacterial agentsBacterial antigen ingredientsSucroseMedicine
The invention provides liposome formulations comprising fragments from a Mycobacterium tuberculosis-complex strain, it also provides a Mycobacterium tuberculosis-complex strain, fragments of which may be incorporated into selected embodiments of the liposome formulation. The invention further provides suspensions and pharmaceutical compositions comprising the liposome formulations. Furthermore, it discloses the use of the liposome formulations for use in a method of treatment of the human or animal body by therapy, in particular for use in a method of treating or preventing tuberculosis, such as in preventing latent tuberculosis or in tuberculosis prophylaxis, optionally in combination therapy. The formulation of this invention contains sucrose and / or has a lower average particle size than conventional liposome-based agents of tuberculosis therapy, resulting in higher bioavailability and efficiency.
Owner:ARCHIVEL FARMA SL
Unlabeled tuberculosis fusion protein ESAT6-Ag85B
InactiveCN102453096AEfficient purificationSolve the experimentAntibacterial agentsBacterial antigen ingredientsGenetic engineeringTGE VACCINE
The invention relates to an unlabeled tuberculosis fusion protein ESAT6-Ag85B. The genetic engineering technique is used to recombine and fuse genes ESAT6 and Ag85B of tuberculosis mycobacteria to build recombinant plasmids; the induced expression is performed to obtain expression products of recombined genes; finally, the expression products of the recombined genes are purified and are cloned to build a tuberculosis mycobacteria fusion protein ESAT6-Ag85B without any labels. The invention has advantages of solving the subsequent problems that the labels brought by the fusion proteins can affect animal experiments and further affect clinical tests, and effectively purifying the fusion proteins by different chromatographic analysis methods. The unlabeled tuberculosis fusion protein can hopefully be candidate vaccine for preventing tuberculosis.
Owner:LANZHOU UNIVERSITY
A kind of vaccine for preventing tuberculosis and combination drug and preparation method and application
ActiveCN109078177BSide effects are rareMinor side effectsAntibacterial agentsBacterial antigen ingredientsAdjuvantLatent tuberculosis
The invention discloses a vaccine for preventing tuberculosis, a combined medicine, a preparation method and an application, and belongs to the field of medicines for treating latent infection of tuberculosis. Aiming at the problems that the existing subunit vaccines are ineffective or poor in treating people with latent tuberculosis infection, many types of adjuvants are used, and there are risks of hypersensitivity reactions and Koch reactions, the present invention provides a prevention method containing a mycobacterial microbial compound adjuvant Tuberculosis vaccine, which comprises Ag85b protein, ESAT6-CFP10 protein and Mycobacterium vaccae cell extract. The tuberculosis prevention vaccine containing the mycobacterial microbial compound adjuvant of the present invention is used for treating latent tuberculosis infection, and the effect is remarkable.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +3
Vaccine containing codon-optimized mycobacterium tuberculosis fusion protein AH
ActiveCN112852848AAntibacterial agentsBacterial antigen ingredientsEscherichia coliSecreted antigens
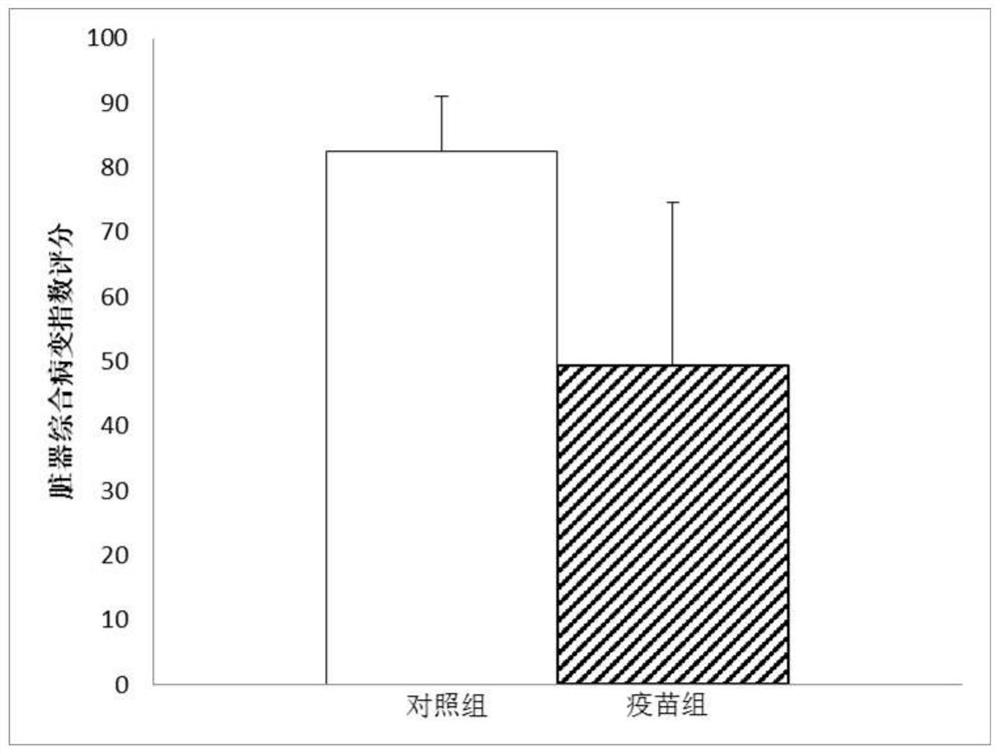
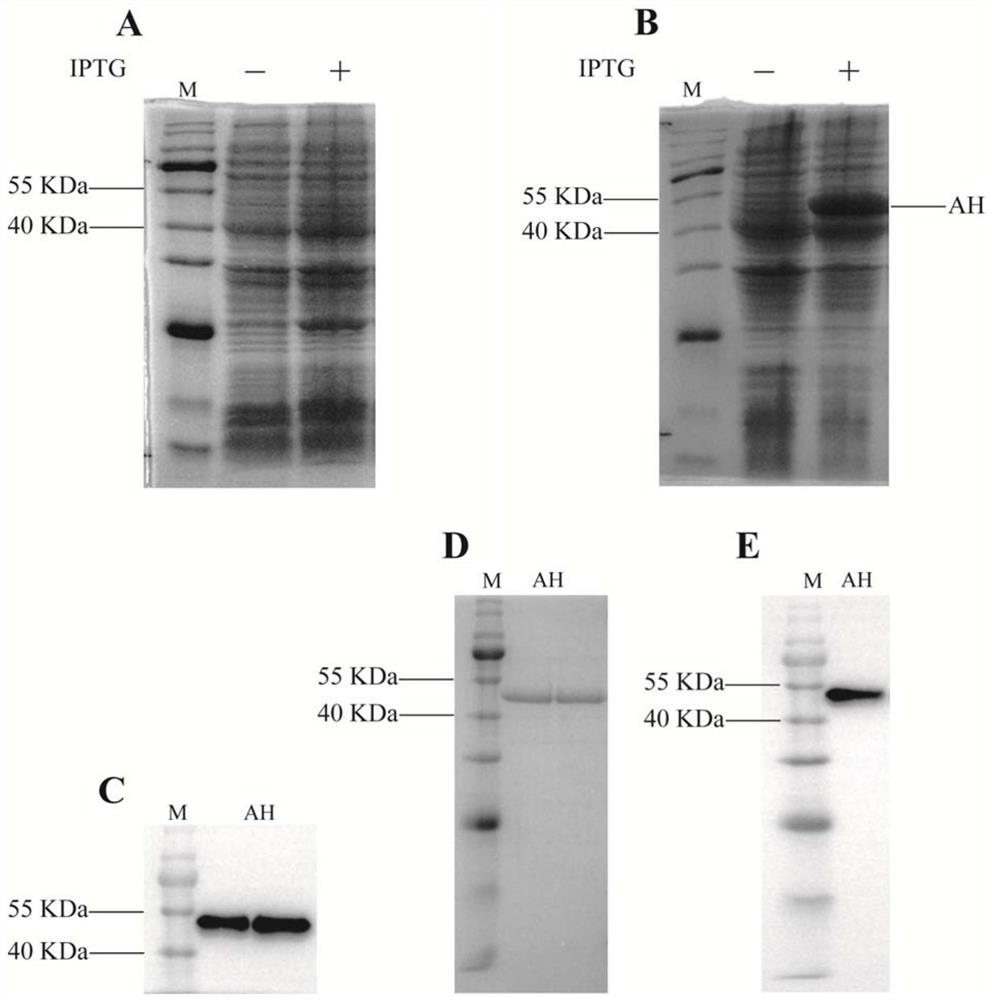
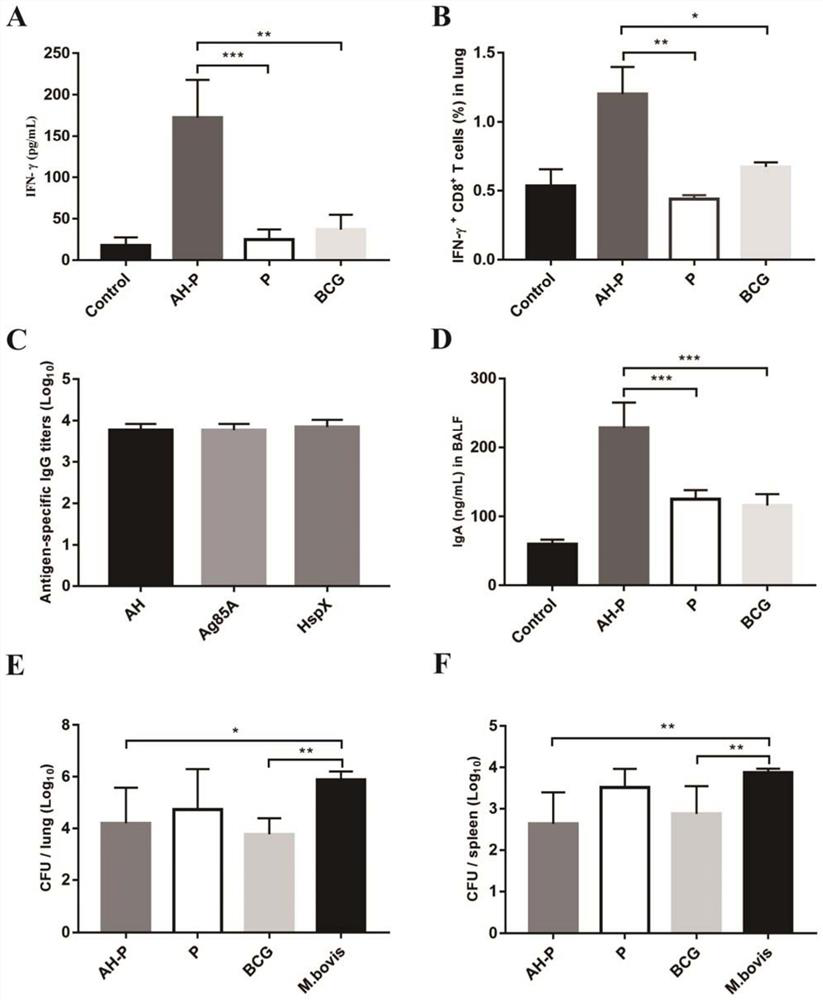
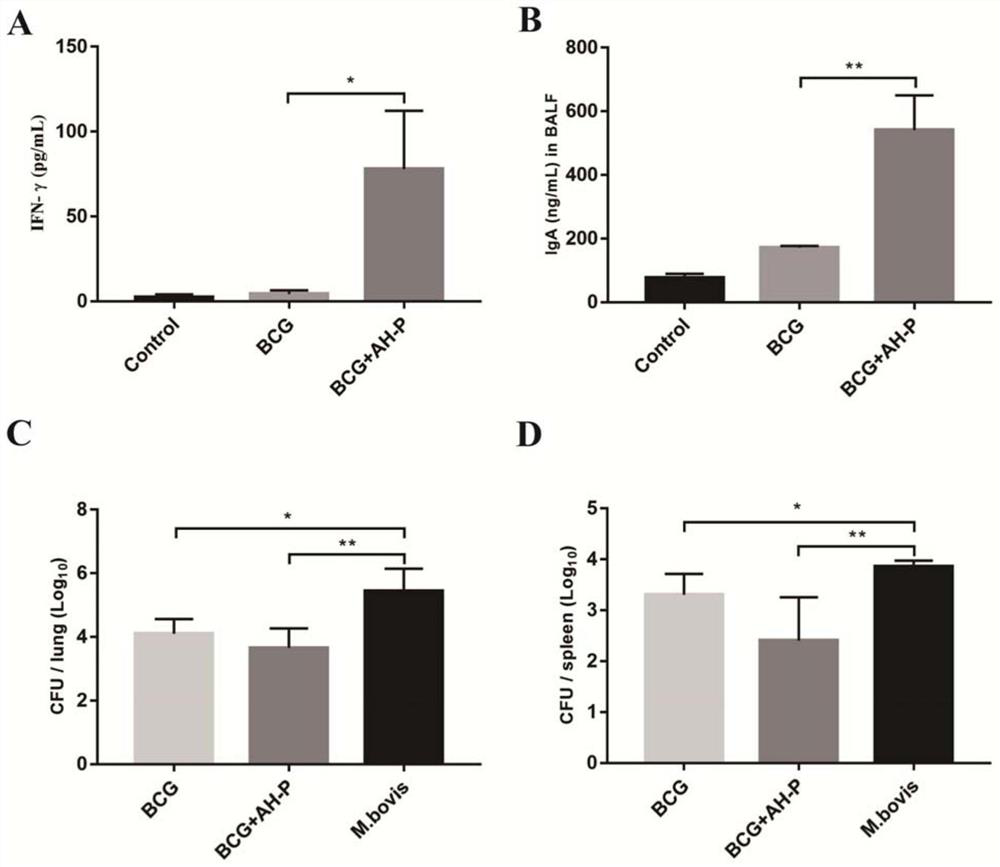
The invention provides a codon-optimized mycobacterium tuberculosis fusion gene, a corresponding fusion protein, a vaccine, application in tuberculosis prevention and a preparation method. The method comprises the following steps: carrying out fusion expression on a mycobacterium tuberculosis infection early secretion antigen Ag85A and a latency expression antigen HspX, connecting the two antigen genes by using flexible Linker (GGGGS)3, carrying out codon optimization on a fusion gene sequence according to the DNA codon bias of escherichia coli, and then connecting the gene sequence to a vector pET-30a(+) for escherichia coli expression. The purified recombinant protein Ag85A-HspX (AH for short) and polyinosinic cell (PolyI:C) are used for intranasal immunization of mice, and the result proves that the recombinant protein has good immunogenicity and a protective effect of resisting mycobacterium bovis infection.
Owner:CHINA AGRI UNIV
Breathing mask for preventing and treating tuberculosis
PendingCN114366976APromote expectorationExpectoration aidRespiratory masksSuction devicesRespiratory maskSurgery
The invention provides a breathing mask for preventing and treating tuberculosis, and relates to the technical field of breathing masks. A tuberculosis prevention and treatment breathing mask comprises a mask body and a sputum treatment assembly. The sputum treatment assembly comprises a sputum suction pipeline, the sputum suction pipeline is arranged on the mask body, and one end of the sputum suction pipeline is located on the inner side of the mask body and connected with a suction nozzle; the other end of the liquid suction pipeline is located on the outer side of the cover body and connected with a waste liquid treatment assembly. A pressurizing assembly is connected to the liquid suction pipeline in series and comprises a negative pressure assembly connected to the liquid suction pipe in series, the negative pressure assembly is used for generating negative pressure at the suction nozzle, and the negative pressure assembly is connected with a control switch. According to the breathing mask for preventing and treating tuberculosis, sputum and other substances which are easily coughed by a tuberculosis patient can be discharged without taking off the mask, and the breathing mask can facilitate sputum excretion of the patient and assist the patient in sputum excretion.
Owner:义乌市中心医院
Recombinant BCG vaccine for tuberculosis prevention
InactiveCN101822829BImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to construction of a BCG vaccine through chimeric expression of GMCSF-CFP10-ESAT6 protein with a human GM-CSF gene, and a CFP10 gene and an ESAT6 gene of mycobacterium tuberculosis and research on immunogenicity of the BCG vaccine. In the construction method, a human GM-CSF gene sequence and a CFP10 gene sequence and an ESAT6 gene sequence of the mycobacterium tuberculosis are inserted into one escherichia coli-mycobacterium tuberculosis shuttle plasmid pMV361 sequence through the genetic engineering technology to construct a recombinant shuttle plasmid rpMV361GMCSF-CFP10-ESAT6. Then the carrier is guided into a BCG vaccine through electroporation to construct a recombinant BCG vaccine rBCG: GMCSF-CFP10-ESAT6. The recombinant BCG vaccine constructed in the invention can stably express the GMCSF-CFP10-ESAT6 chimeric protein, and the immunogenicity of the recombinant BCG vaccine is superior to that of the immunogenicity of the traditional BCG vaccine. The inventionalso provides a preparation process of the recombinant BCG vaccine and research on the immunity of the recombinant BCG vaccine, which belong to the genetic engineering field and the tuberculosis vaccine field. Moreover, the invention can prevent the occurrence and spread of the tuberculosis more effectively.
Owner:SICHUAN UNIV
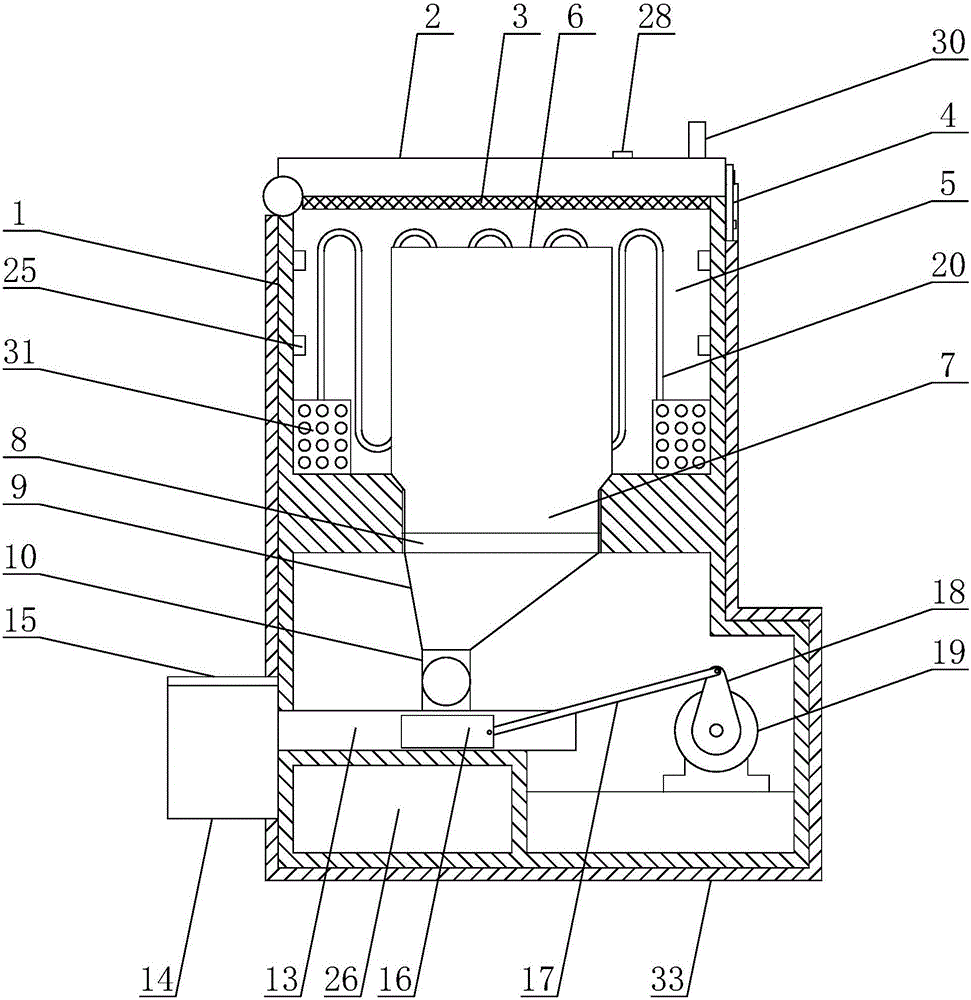
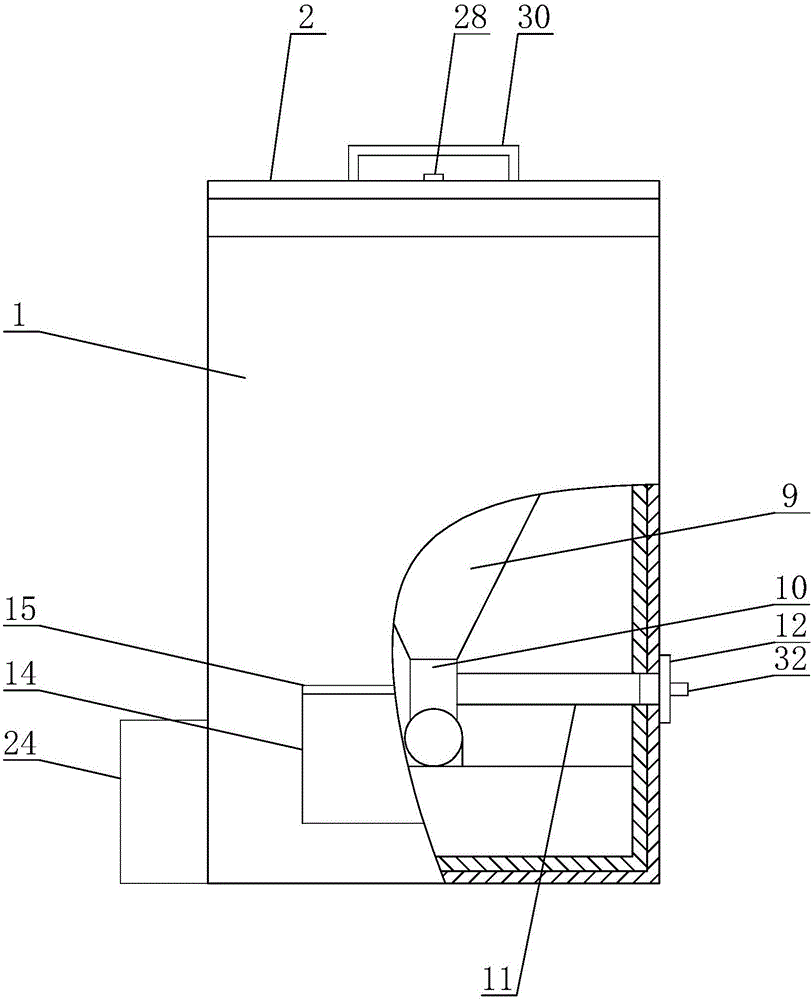
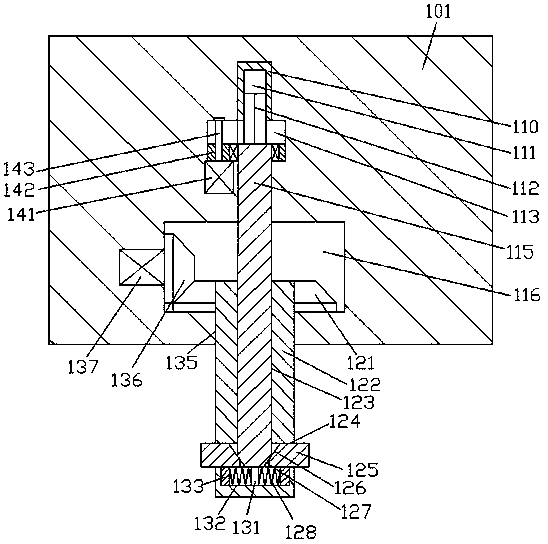
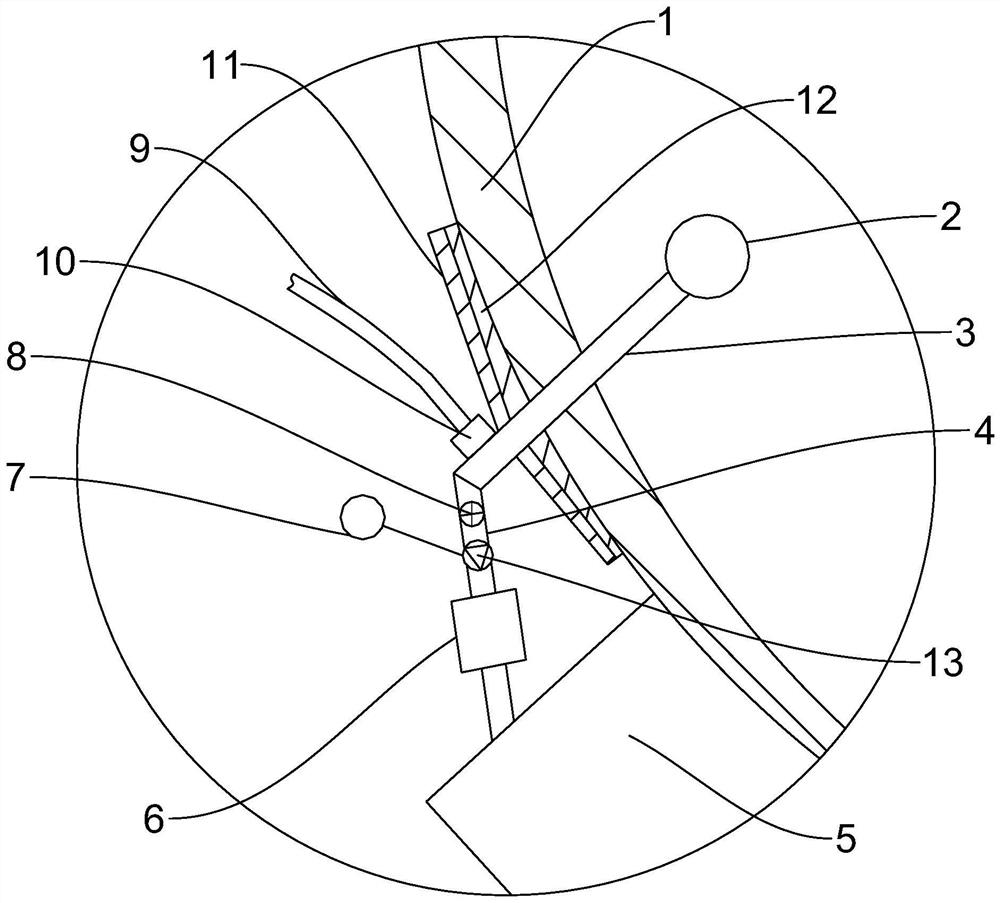
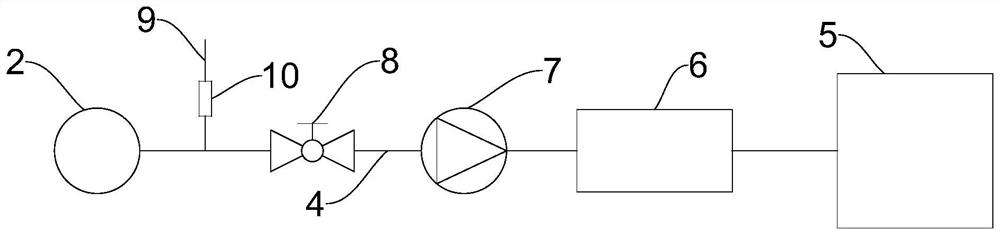
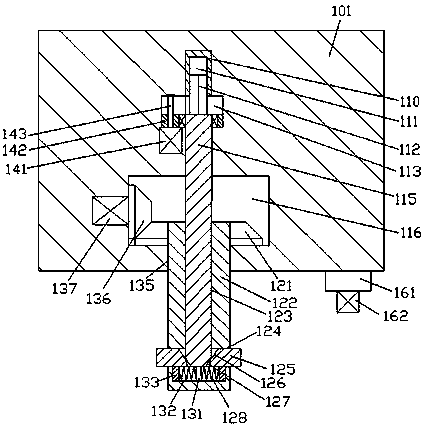
Improved tuberculosis prevention and treatment drug preparation device
InactiveCN109126979ASimple structureEasy to operateDirt cleaningCocoaSteering columnDrugs preparations
The invention discloses an improved tuberculosis prevention and treatment drug preparation device. The device comprises a support frame which is fixedly mounted on a baseplate and an embedding base body which is mounted on the right end face of the support frame through a lifting device; foot columns are fixed to the periphery of the bottom end face of the baseplate, rolling wheels are cooperatively mounted in the bottom end faces of the foot columns in a rolling mode, a buffer cavity is formed in the bottom end face of the embedding base body, a preparation base body is slidingly and cooperatively mounted in the buffer cavity, elastic pressing members are symmetrically and fixedly arranged on the left and right sides of the top of the preparation base body, and the tops of the elastic pressing members are fixedly connected to the inner bottom wall of the buffer cavity; a transfer cavity is formed in an inner wall body of the preparation base body, a guiding cavity is formed in the upper side of the transfer cavity, and a steering cavity is communicated with the inner bottom wall of the transfer cavity and runs through the bottom end face of the preparation base body. A steering column is rotatably and cooperatively disposed in the steering cavity, and an extension segment of the bottom of the steering column extends out of the bottom end face of the preparation base body and is cooperatively provided with a grinding wheel.
Owner:马洁
Mycobacterium tuberculosis drug-resistant mutant gene detection kit
ActiveCN102424859BReduce spreadGuaranteed therapeutic effectMicrobiological testing/measurementMicroorganism based processesIsoniazidNucleotide
The invention discloses a mycobacterium tuberculosis drug-resistant mutant gene detection kit comprising: (1) a gene chip which has (i) a nucleotide probe, wherein the probe is a sequence of SEQIDNos: 1-31 or a complementary sequence of SEQIDNos: 1-31, (ii) a DNA sequence labeled with biotin points, wherein the sequence is SEQIDNO. 32, (iii) an IS6110DNA sequence of a mycobacterium tuberculosis complex, wherein the sequence is SEQIDNo. 33; (2) various primers used for amplifying DNA sequences in clinical samples, wherein the DNA sequences of the primers are SEQIDNos. 34-41. The kit of the invention detects the drug resistance of mycobacterium tuberculosis to rifampin and isoniazid, provides reference basis for the prevention and treatment of tuberculosis, and has significant meaning.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
Preparation and application of recombinant protein cfp-10 nanoparticles
ActiveCN110354098BImprove immunityReduce bacterial loadAntibacterial agentsBacterial antigen ingredientsTGE VACCINECopolymer
The invention provides a preparation method of recombinant protein CFP-10 nanoparticles and the application thereof in preparing tuberculosis prevention vaccines. The preparation method includes the steps of (1) configuration of a polylactic acid-glycolic acid copolymer PLGA solution; (2) configuration of a recombinant protein CFP-10 solution; (3) preparation of primary emulsion; (4) preparation of compound emulsion; (5) formation of the nanoparticles. The recombinant protein CFP-10 nanoparticles prepared by the preparation method are used for enhancing the immunity of BCG-immunized mice through intranasal drip, and it is confirmed that the recombinant protein CFP 10 nanoparticles have the effects of improving mucosal immunity level and promoting BCG immunity.
Owner:CHINA AGRI UNIV
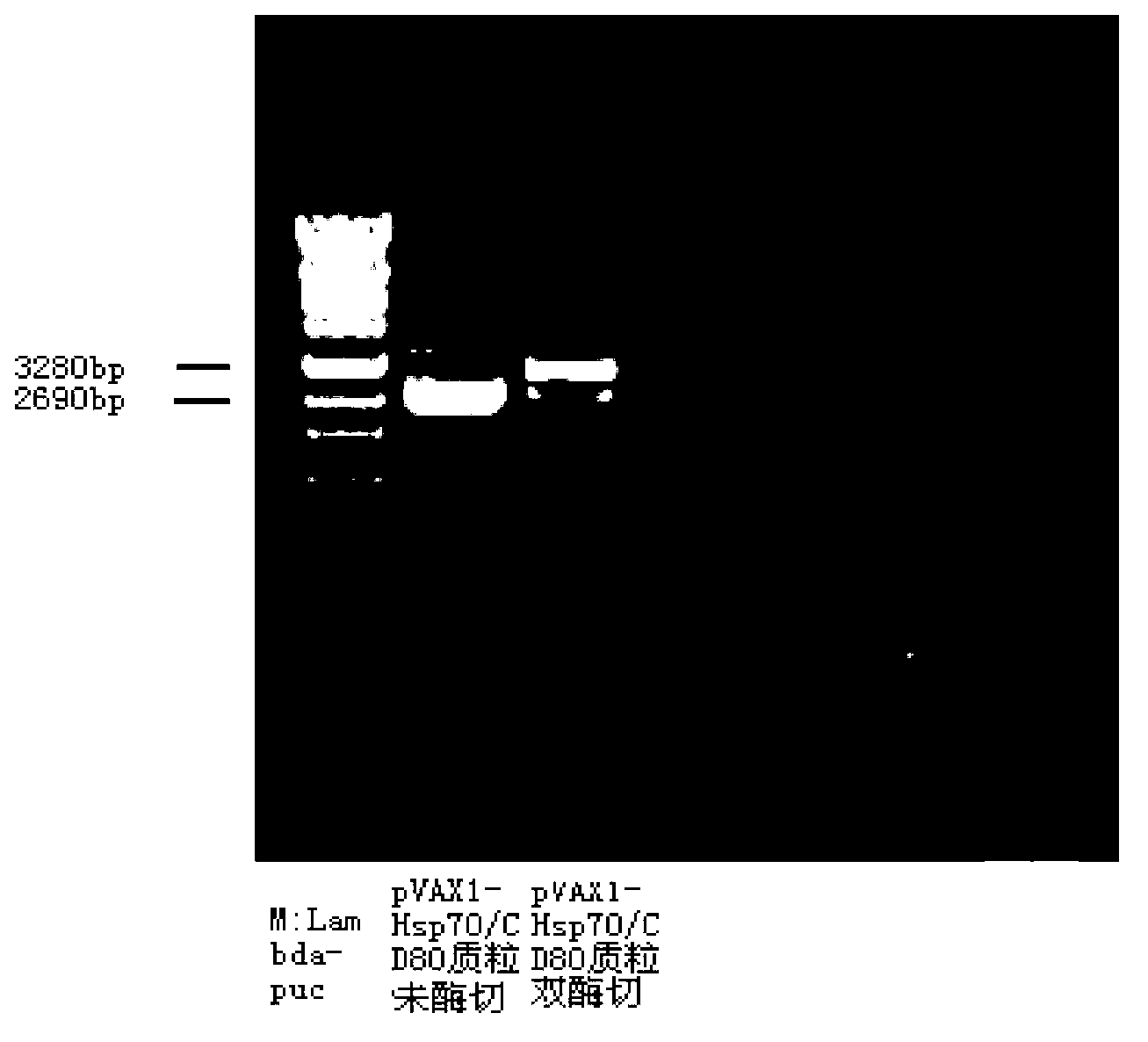
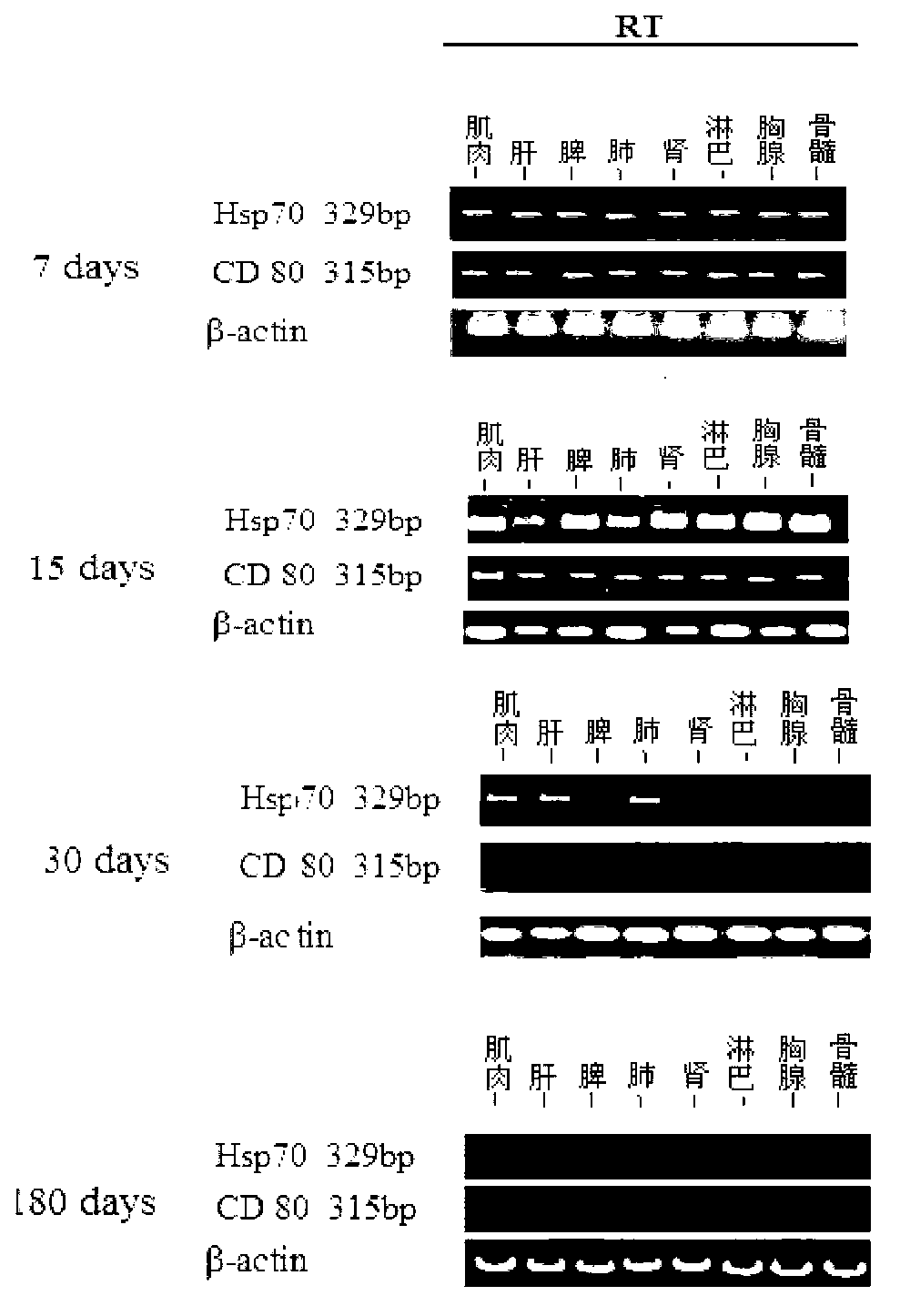
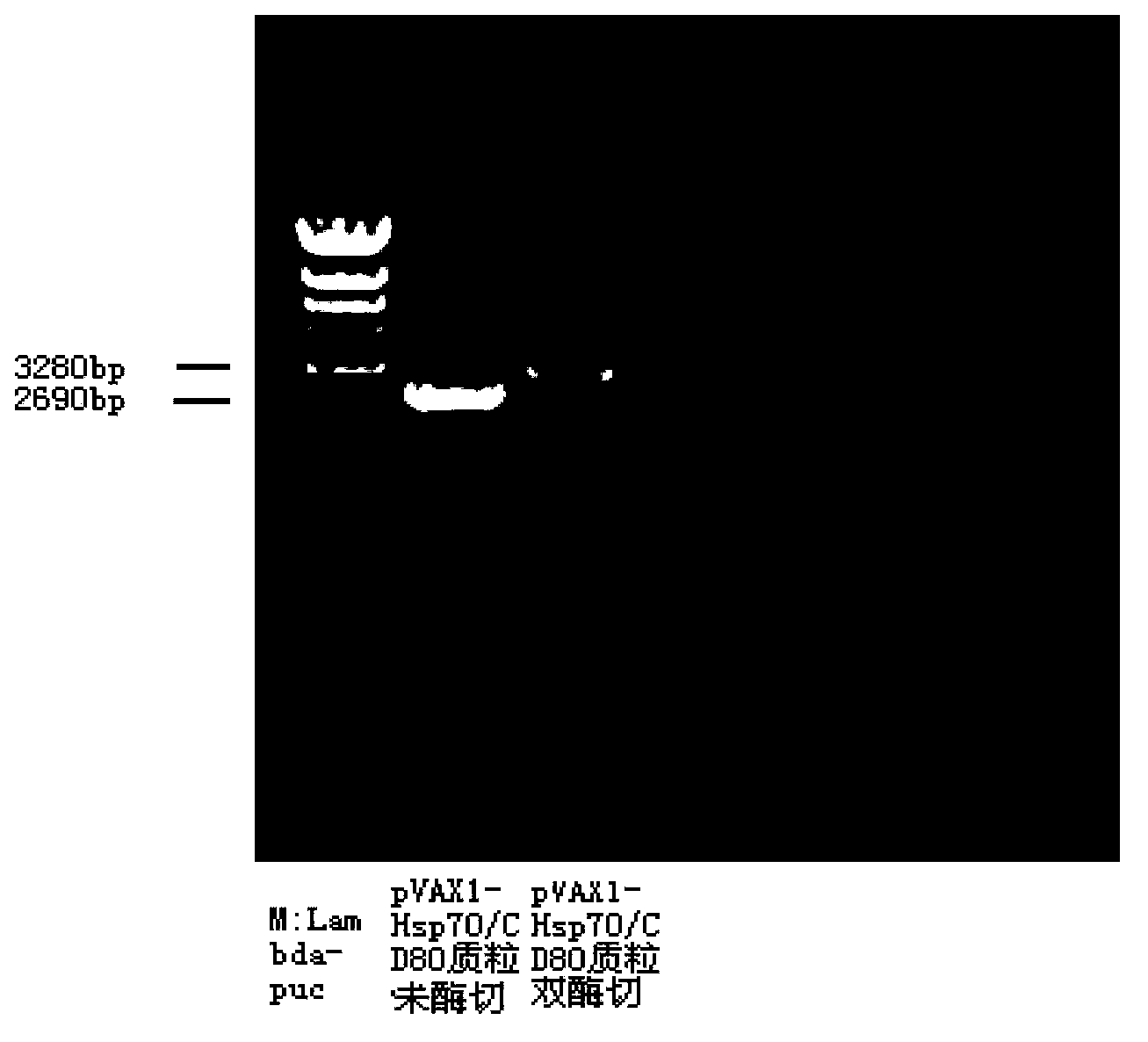
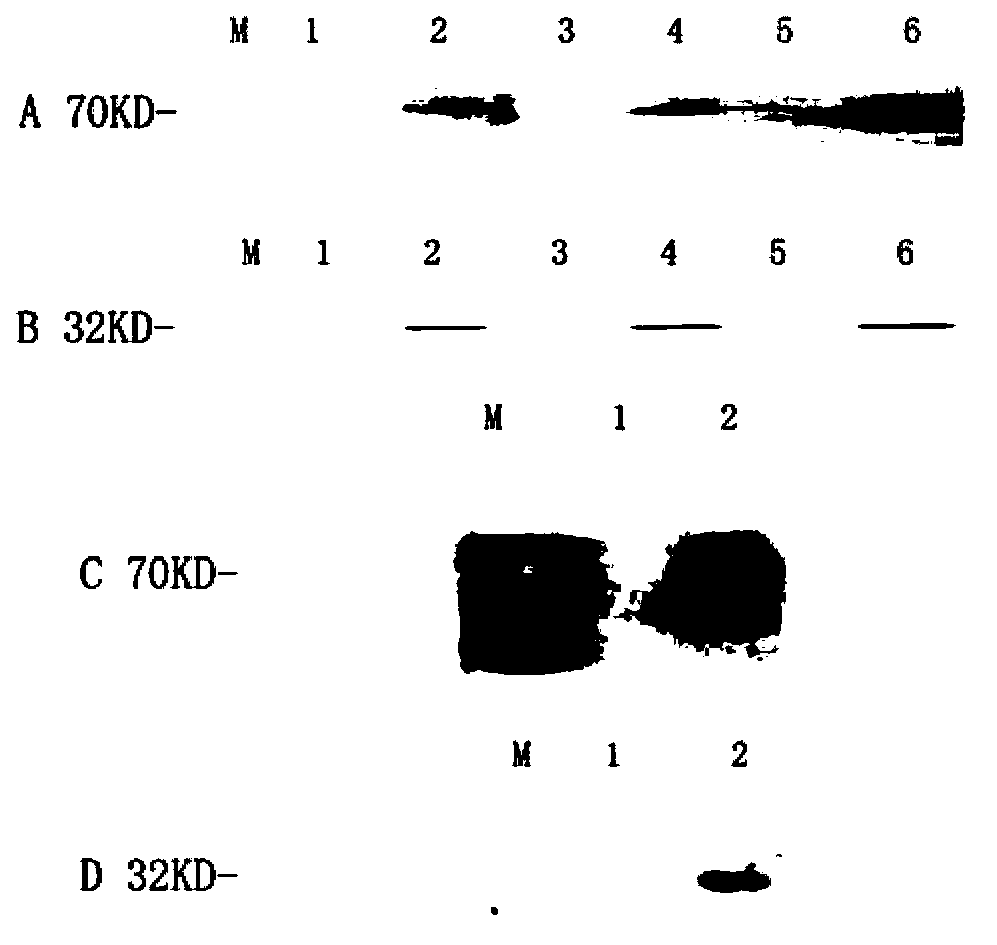
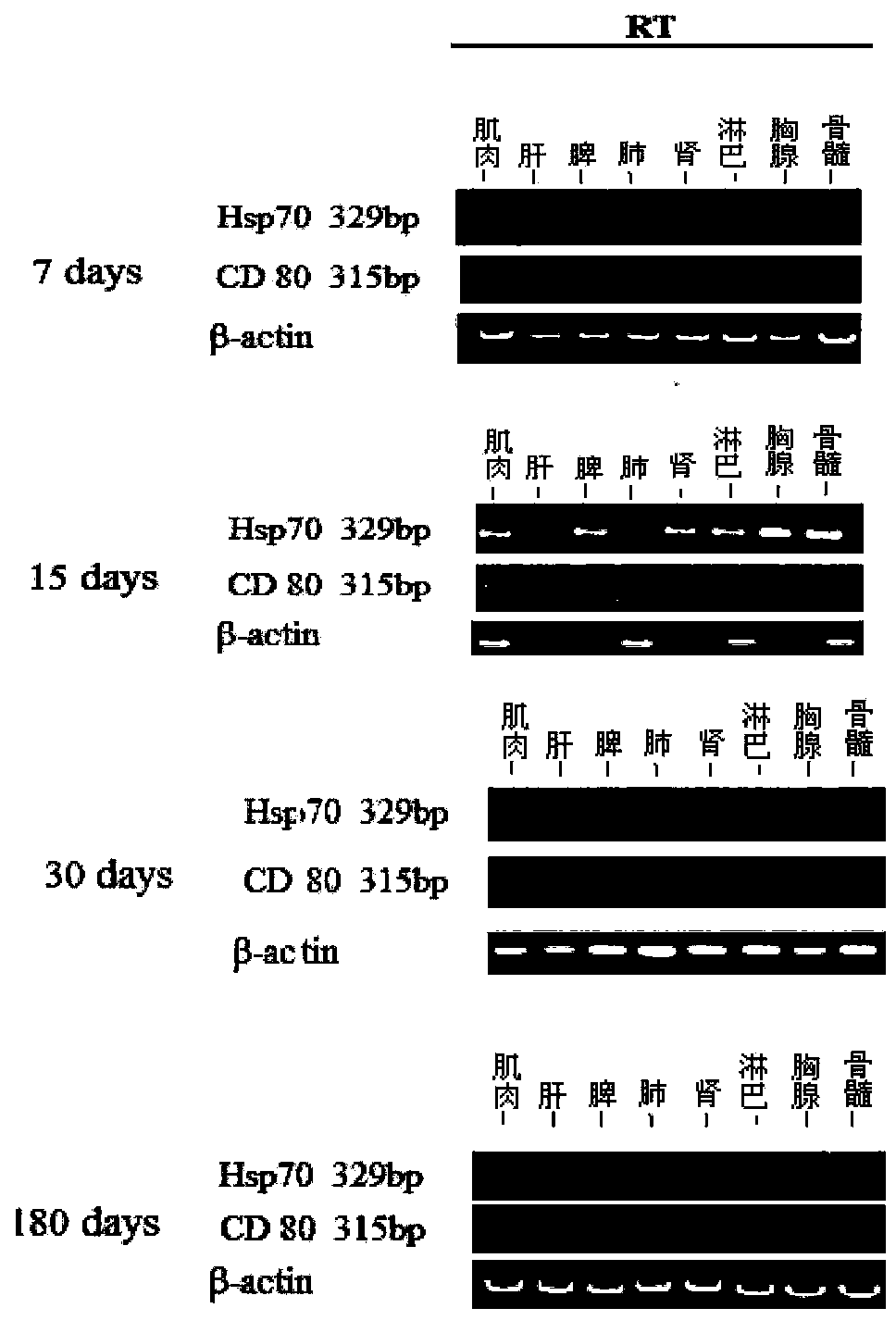
Mosaic type DNA (Deoxyribonucleic Acid) vaccine pVAXI-Hsp 70/CD 80 for preventing and immunologically treating tuberculosis
InactiveCN103169986AIncreased IFN-γGood effectAntibacterial agentsBacterial antigen ingredientsHypersensitive responseIn vivo
The invention belongs to the field of biological medicine, and particularly relates to a tubercular mosaic type DNA (Deoxyribonucleic Acid) vaccine. The tubercular mosaic type DNA vaccine solves the technical problems of nonideal medicine effect and bad safety of the traditional mosaic vaccine. The technical scheme is as follows: the mosaic type DNA vaccine contains the encoding gene of HSP 70 / CD 80 mosaic protein and can express the encoding gene in vivo. After using a pVAXI carrier, the tubercular mosaic type DNA vaccine disclosed by the invention has a better effect on the prevention and treatment of tuberculosis and can especially be used for inducing stronger Th1 type reaction and better prevent and treat tuberculosis; and besides, the pVAXI carrier has kanamycin resistance, cannot cause any anaphylactic reaction and can better prevent and immunologically treat tuberculosis.
Owner:钟森 +1
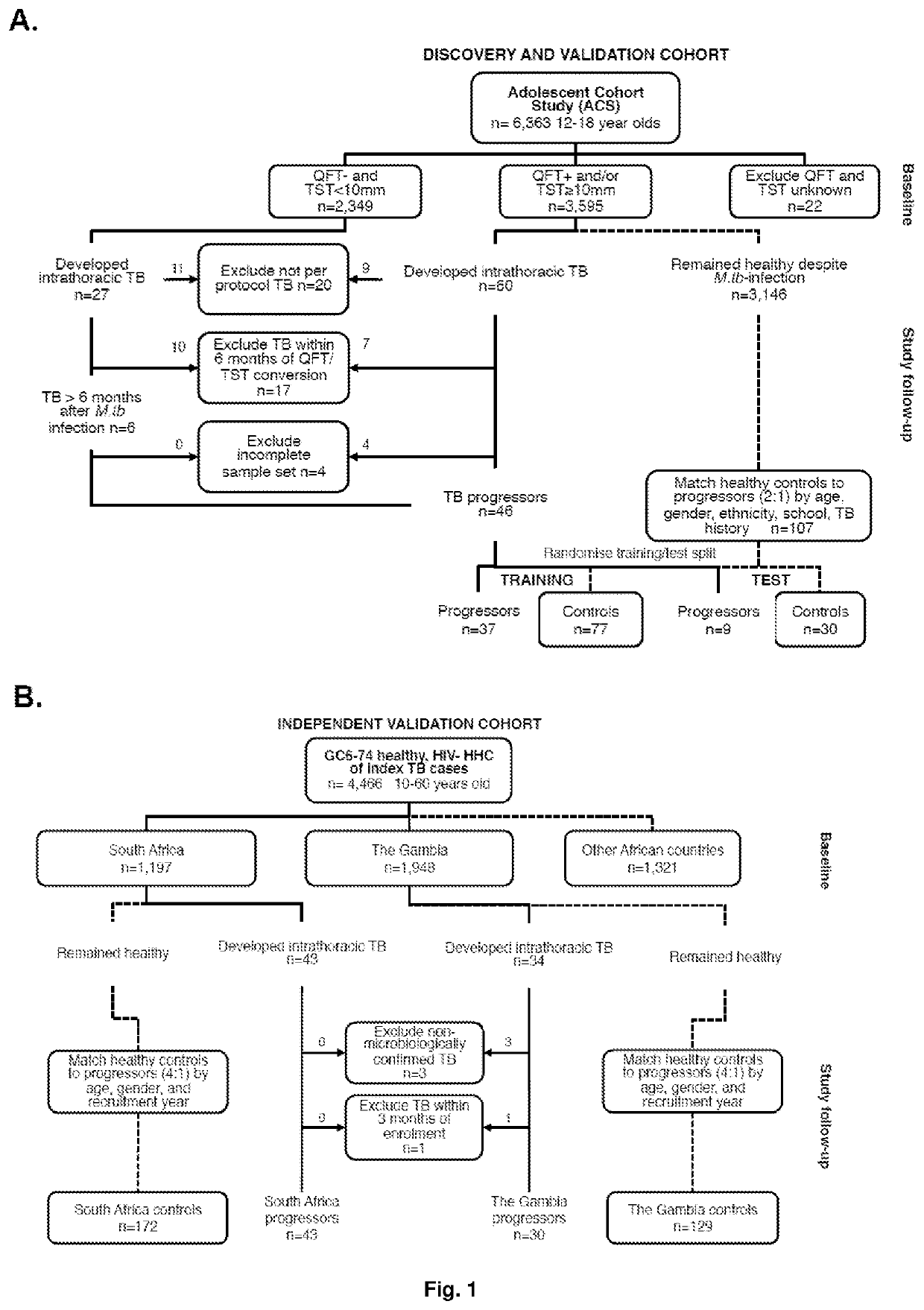
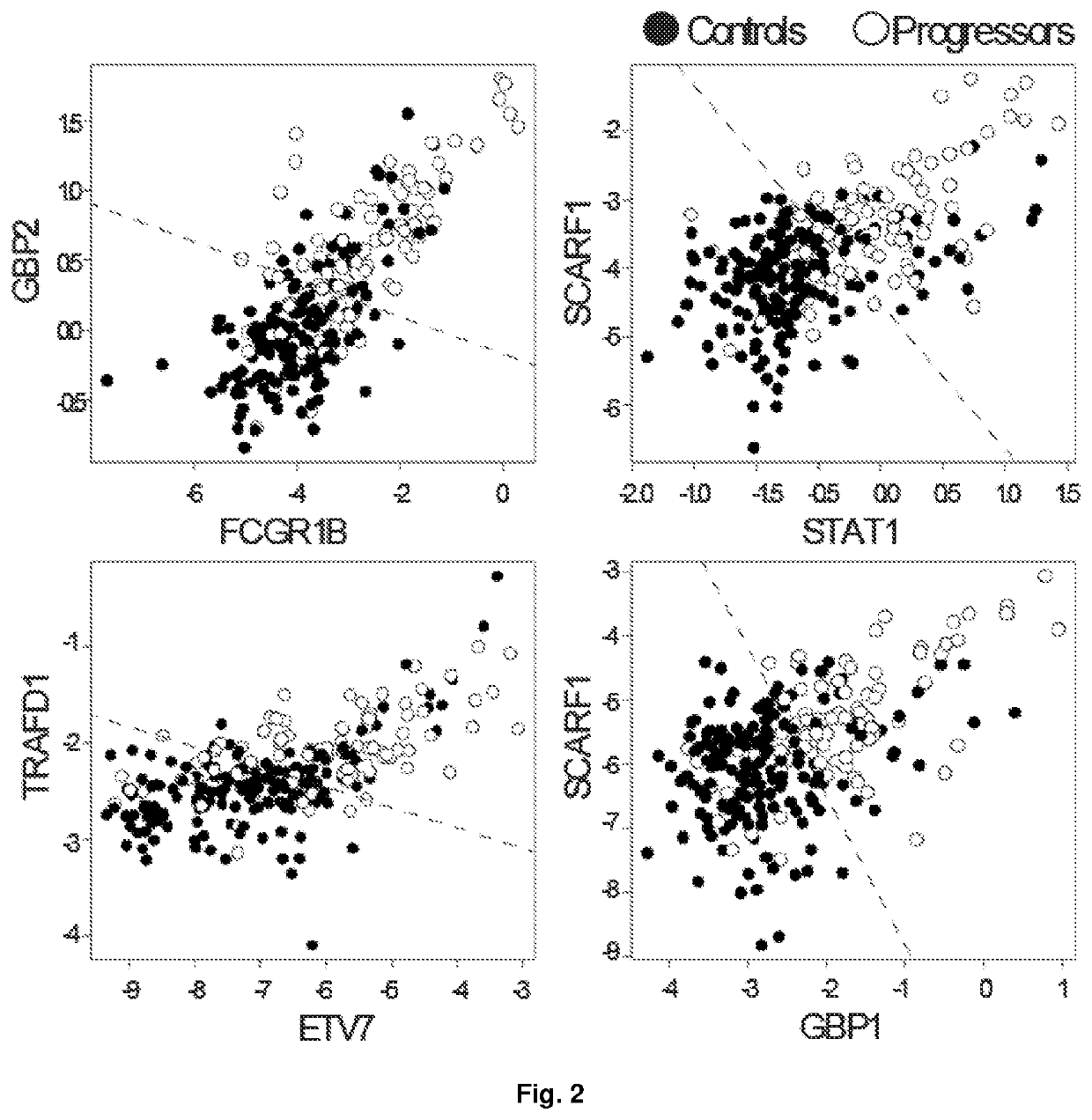
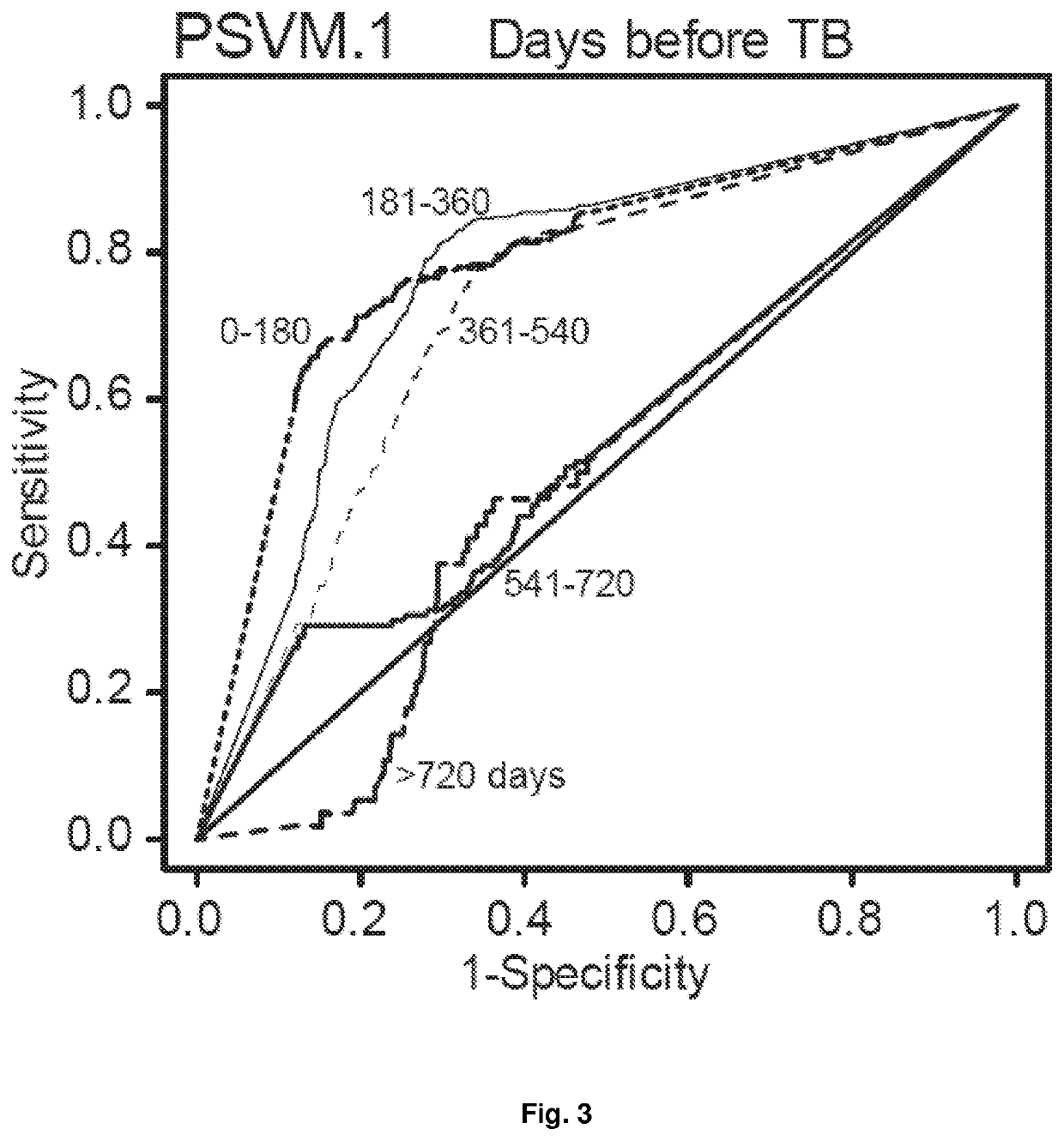
Biomarkers for prospective determination of risk for development of active tuberculosis
ActiveUS11220717B2Reduce rateMicrobiological testing/measurementICT adaptationDiseasePrognostic signature
This invention relates to a prognostic method for determining the risk of an asymptomatic human subject with latent tuberculosis (TB) infection or apparent latent TB infection and / or after suspected exposure to TB progressing to active tuberculosis disease comprising the steps of quantifying and computationally analysing relative abundances of a collection of pairs of gene products (“TB biomarkers”) derived from a sample obtained from the subject. The invention further relates to a collection of TB biomarkers that generates a transcriptomic signature of risk for prediction of the likelihood of an asymptomatic human subject with latent TB infection and / or after suspected exposure to TB progressing to active tuberculosis disease. Furthermore, a kit comprising gene-specific primers or oligonucleotide probes for the detection of pairs of TB biomarkers that generates a prognostic signature of risk for use with the method of the invention is described. In addition, the invention relates to a method of preventive treatment or prophylaxis for TB infection comprising the use of the prognostic method and / or the kit of the invention to select an appropriate or experimental treatment regime or intervention for the human subject and / or to monitor the response of the human subject to the TB prophylaxis.
Owner:SEATTLE CHILDRENS HOSPITAL +1
Mosaic type DNA (Deoxyribonucleic Acid) vaccine pVAXI-Hsp 70/CD 80 for preventing and immunologically treating tuberculosis
InactiveCN103169986BIncreased IFN-γGood effectAntibacterial agentsBacterial antigen ingredientsAnaphylactic reactionsChimera Protein
The invention belongs to the field of biological medicine, and particularly relates to a tubercular mosaic type DNA (Deoxyribonucleic Acid) vaccine. The tubercular mosaic type DNA vaccine solves the technical problems of nonideal medicine effect and bad safety of the traditional mosaic vaccine. The technical scheme is as follows: the mosaic type DNA vaccine contains the encoding gene of HSP 70 / CD 80 mosaic protein and can express the encoding gene in vivo. After using a pVAXI carrier, the tubercular mosaic type DNA vaccine disclosed by the invention has a better effect on the prevention and treatment of tuberculosis and can especially be used for inducing stronger Th1 type reaction and better prevent and treat tuberculosis; and besides, the pVAXI carrier has kanamycin resistance, cannot cause any anaphylactic reaction and can better prevent and immunologically treat tuberculosis.
Owner:钟森 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com