Mycobacterium tuberculosis fusion protein (EAMMH) and constructing, expressing and purifying method and application thereof
A technology of Mycobacterium tuberculosis and fusion protein, which is applied in chemical instruments and methods, biochemical equipment and methods, peptide preparation methods, etc., can solve problems such as inability to effectively prevent adult pulmonary tuberculosis, and no new anti-tuberculosis vaccines have been developed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
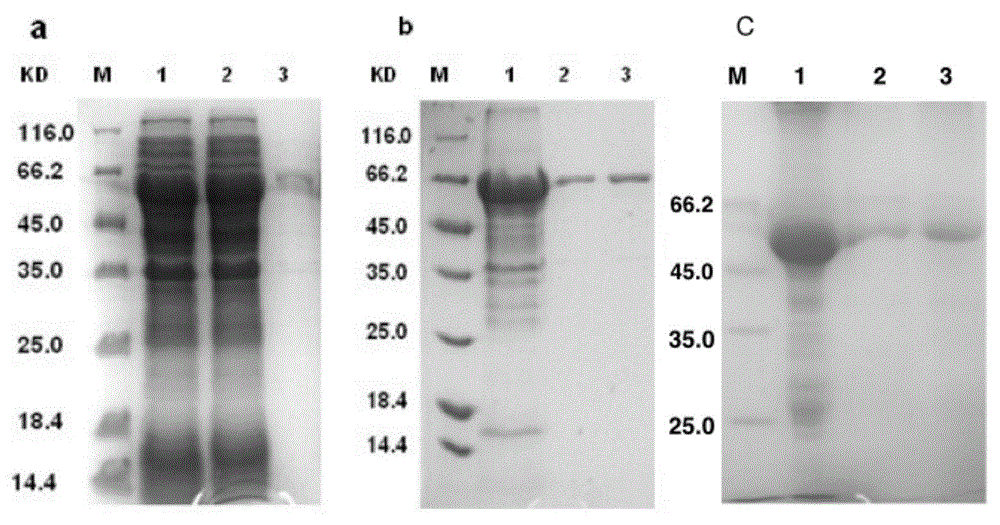
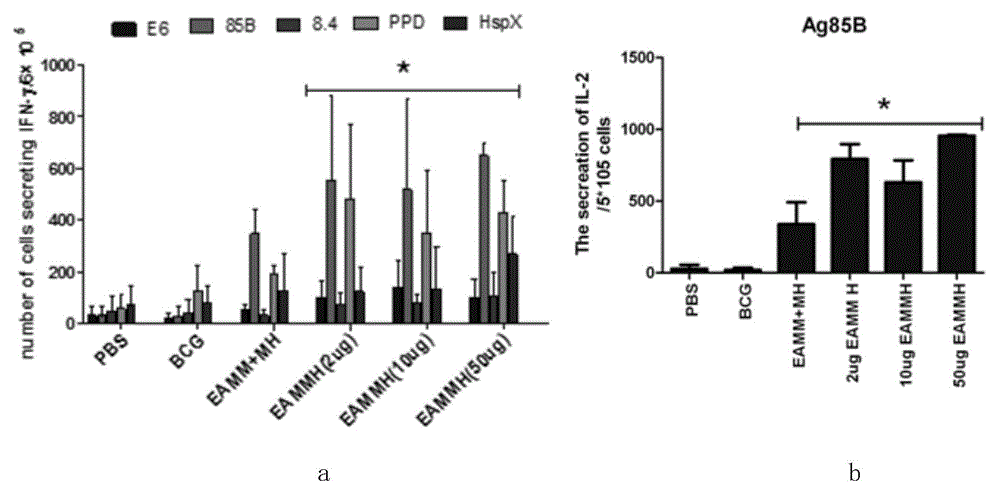
[0096] Construction, expression and purification of Mycobacterium tuberculosis fusion protein EAMMH:
[0097] The fusion antigen EAMM and the single antigen HspX were fused at the gene level to construct the recombinant vector pET30a(+)-EAMMH. Since the terminal antigen M gene fused in the existing pET30a(+)-EAMM plasmid vector (ie Mtb8.4) has a base sequence corresponding to the stop codon UAA, HspX cannot be directly fused behind EAMM.
[0098] First construct pET30a(+)-MPT64 190-198 -Mtb8.4-HspX (MMH) vector, then PCR amplify the ESAT6-Ag85B (EA) gene fragment, and insert the EA fragment into the pET30a (+)-MMH vector by restriction endonuclease digestion and ligase connection The front end of the MMH fragment was successfully constructed into the pET30a(+)-EAMMH plasmid vector. Then, the pET30a(+)-EAMMH plasmid vector was transformed into Escherichia coli E.coli to express the fusion protein EAMMH. Finally, according to the protein properties of the protein, the fusion ...
Embodiment 2
[0121] Preparation of fusion protein EAMMH subunit vaccine:
[0122] Dilute the fusion protein EAMMH with PBS (phosphate buffered saline) to 0.2mg / ml or 0.04mg / ml; PolyI:C (acting on the agonist of Toll-like receptor 3) is dissolved in PBS to 0.5mg / ml; cationic lipid Plasmid—dimo-thylidioctyl ammonium bromide (DDA) was prepared with sterile distilled water to a concentration of 2.5 mg / ml, placed in a water bath at 80°C for 10 minutes, and cooled to room temperature. Take 50 μl of PolyI:C solution and mix well with an equal amount of EAMMH protein solution, and let stand at room temperature for 1 min. Add 100 μL of DDA solution dropwise to the mixed solution, and then fully emulsify to make the vaccine in the form of a uniform cream to obtain the subunit vaccine EAMMH-DDA / PolyI:C. When the vaccine is used for immunization, the dosage is 200 μL / mouse.
Embodiment 3
[0124] Detection of immunological activity of fusion protein EAMMH subunit vaccine:
[0125] 1. Experimental materials: subunit vaccine EAMMH-DDA / PolyI:C; BCG (BCG); phosphate buffered saline (PBS);
[0126] 2. Experimental animals: C57BL / 6 mice;
[0127] 3. Grouping of experimental animals (four groups in total):
[0128] (1) PBS; (2) BCG; (3) (EAMM+MH)-DDA / PolyI:C; (4) 2 μg EAMMH-DDA / PolyI:C; (5) 10 μg EAMMH-DDA / PolyI:C; ( 6) 50 μg EAMMH-DDA / PolyI:C;
[0129] 4. Immunized animals:
[0130] In the 0th week, subcutaneously immunize the animals in the experimental group once with the prepared subunit vaccine inguinal (200 μl / animal), and at the same time immunize the control group PBS and BCG groups (5×10 6 CFU / only), the animals in the experimental group were immunized twice with the same dose in the 2nd and 4th weeks after immunization in the 0th week;
[0131] 5. Determination method of immune index:
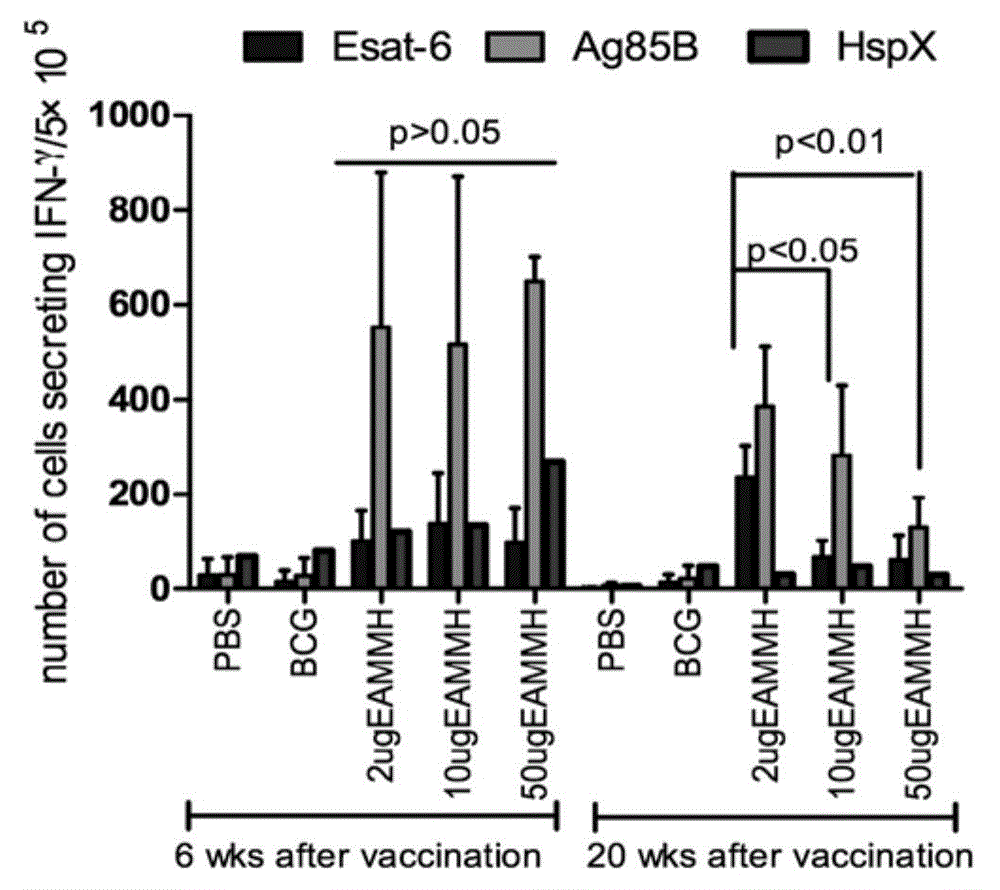
[0132] (1) ELISPOT method to detect the level of antigen-specific I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com