Patents
Literature
40results about How to "Strong immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Special fodder for piglets
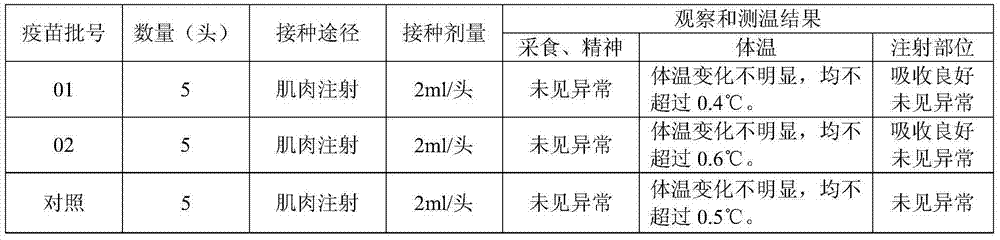
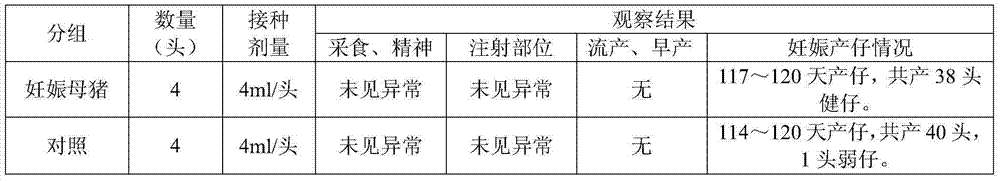
InactiveCN106260633AIncrease daily feed intakeStrong immunityFood processingLactobacillusChemistryPeanut meal
The invention discloses a special fodder for piglets. The fodder is characterized by containing the following raw materials: extruded corns, soybean meal, fermented soybean meal, soybean flour, peanut meal, rapeseed dregs, bran, corn alcohol lees, plasma protein powder, silkworm chrysalis meal, cane sugar, oligosaccharide, salt, natural glycine betaine, calcium hydrophosphate, whey mist, phosphatidylcholine, hawthorn powder, codonopsis pilosula powder, pine needle meal, gamino acid, decavitamin, compound microelement, forage-used probiotics, cellulase, alpha-amylase, xylanase, acid proteinase, phytase and premix for piglets. The premix for the piglets is prepared through the following steps: adding water into attapulgite powder and rectorite powder, then adding citric acid, tartaric acid, maslinic acid, fumaric acid and food phosphate, conducting reaction after high pressure homogenization, performing filter pressing after the temperature drops to indoor temperature and calcining, thus obtaining the premix for the piglets. By changing the fodder structure, the special fodder for the piglets reinforces the digestive ability of the piglets and improves the appetite and the immunity of the piglets.
Owner:宿松县春润食品有限公司
Monoclonal antibody of anti-SARS-CoV-2 nucleocapsid protein and application
ActiveCN112225797AStrong immunityGood stabilityImmunoglobulins against virusesFermentationMolecular biologyFully automated
The invention discloses a monoclonal antibody of an anti-SARS-CoV-2 nucleocapsid protein and an application. The monoclonal antibody comprises a light chain and a heavy chain, and is characterized inthat the amino acid sequences of variable regions CDR1, CDR2 and CDR3 of the light chain are respectively RASENIYSSLA, VATDLA and QHFWGTPWT; and the amino acid sequences of the variable regions CDR1,CDR2 and CDR3 of the heavy chain are respectively TDHYII, EIYPGNGHTYYNERFKG and SRYYGPFAYWG. The monoclonal antibody of the anti-SARS-CoV-2 nucleocapsid protein is an IgG1 subtype, the affinity of theantibody can reach 5.26 * 10 <11>, the immunocompetence is strong, the monoclonal antibody has the advantages of good stability, high activity, strong affinity and the like, the detection specificityis strong, the sensitivity is high, the accuracy is good, and the monoclonal antibody can be used in a kit of a full-automatic immunoassay analyzer. The kit is also applied to detection of the SARS-CoV-2 nucleocapsid protein by an immunochromatographic test strip, and can effectively promote popularization and application of SARS-CoV-2 detection.
Owner:ZHEJIANG MEDICAL COLLEGE
Preparation method for yellow rice wine
ActiveCN103695247AShorten the soaking timeSufficient soaking timeMicroorganism based processesAlcoholic beverage preparationFlavorFiltration
The invention discloses a preparation method for yellow rice wine and belongs to the technical field of yellow rice wine production processing. The preparation method comprises the following steps of (1) smashing, immersing, stewing and cooling coix lacryma-jobi and millets which serve as raw materials; (2) adding cooled rice into distilled yeast, uniformly mixing and stirring the materials for diastatic fermentation, adding yeast and purified water to implement constant-temperature fermentation, performing ultrasonic treatment after fermentation is executed, performing squeezed filtration, collecting filtrate, sterilizing the filtrate, and storing the filtrate in a sealed manner to obtain the yellow rice wine. The preparation method for the yellow rice wine is low in production cost and simple in technology; the yellow rice wine has good color, fragrance and flavor, tastes delicious and mellow and does not contain any additive and pigment; by virtue of long-time drinking, the yellow rice wine has good effects for resisting fatigue, enhancing the immunity of a human body, tonifying Yang and kidney and the like.
Owner:福建老福洲酒业股份有限公司
Additive premix for stereo cultivation of Tilapia mossambica as well as preparation method and use method thereof
InactiveCN104397429APromote growthGuaranteed appetiteAnimal feeding stuffVitamin K2Additive ingredient
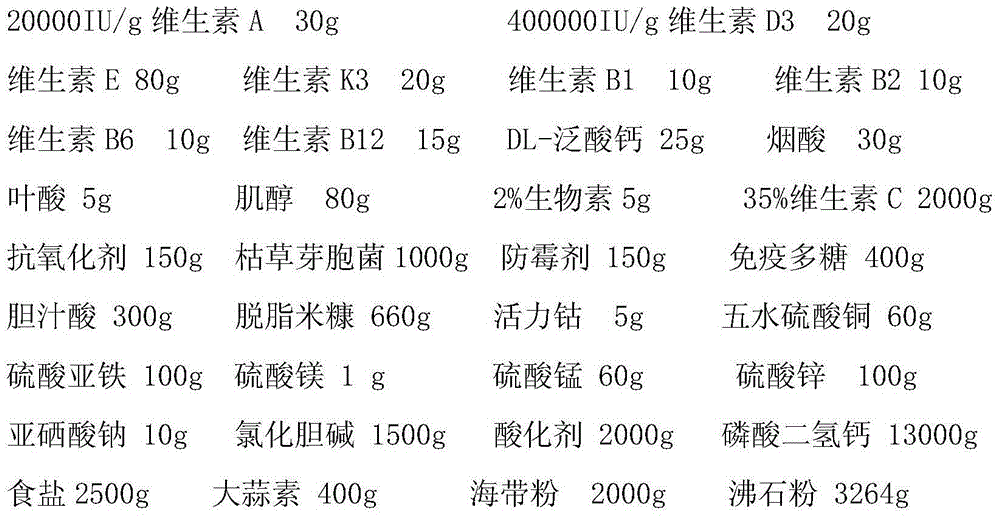
The invention discloses an additive premix for stereo cultivation of Tilapia mossambica as well as a preparation method and a use method thereof. The premix comprises a material A which is prepared from vitamin A, vitamin D3, vitamin E, vitamin K3, vitamin B1, vitamin B2, vitamin B6, vitamin B16, DL-calcium pantothenate, niacin, folic acid, inositol, 2% biotin, 35% vitamin C, an antioxidant, bacillus subtillis, a mildew preventive, immunopolysaccharide, bile acid and defatted rice bran; the premix which is prepared from the following components such as vital cobalt, copper sulfate pentahydrate, ferrous sulfate, magnesium sulfate, manganese sulfate, zinc sulfate, sodium selenite, choline chloride, an acidifier, calcium dihydrogenphosphate, table salt, garlicin, kelp powder, zeolite powder and the like is added into Tilapia mossambica feed in proportion. Various special components are added to satisfy the demand of Tilapia mossambica on components of vitamins or mineral elements under the stereo cultivation condition, so that the immunity of Tilapia mossambica can be effectively improved and the incidence of Tilapia mossambica under severe environments can be reduced.
Owner:ZHANJIANG HAIRONG FEED
Mixed virus-like particle (VLP) of avian influenza and Newcastle disease, preparation method thereof and application thereof
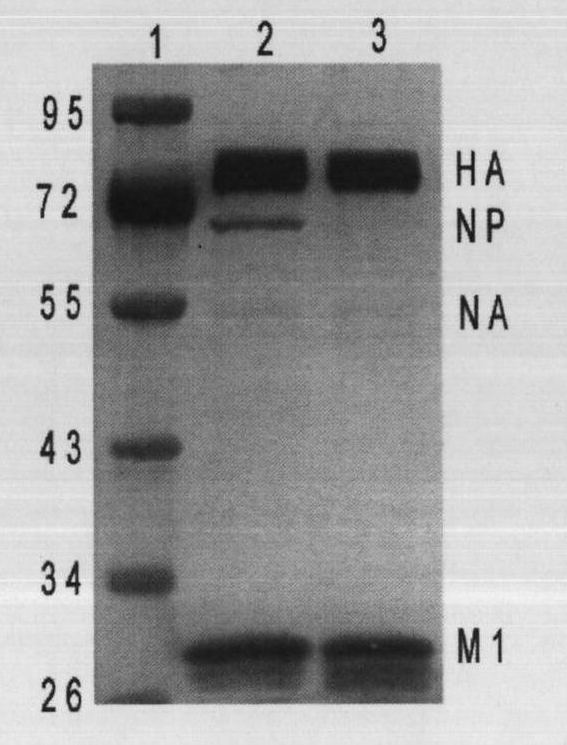
InactiveCN102373183AStrong immunityLong durationViral antigen ingredientsInactivation/attenuationAdjuvantHemagglutinin
The invention provides a mixed VLP. The mixed VLP comprises: a stromatin M1 of an influenza virus; a hemagglutinin HA of a surface antigen of the influenza virus; a nexus protein which comprises an extracellular domain of a main antigen epitope of a Newcastle disease virus (NDV) F protein, a transmembrane domain and an intracellular domain of the hemagglutinin HA of the influenza virus, wherein the extracellular domain of the main antigen epitope of the NDV-F protein replaces an extracellular domain which is at 5'-terminus of the hemagglutinin HA of the influenza virus and has a roughly same length; and the hemagglutinin HA of the influenza virus and the surface antigen F protein of the NDV which are simultaneously expressed on the surface of the mixed VLP. The invention also provides a divalent vaccine of avian influenza and the NDV and a method for preparing the mixed VLP, wherein the divalent vaccine contains the mixed VLP and an adjuvant.
Owner:SUN YAT SEN UNIV
Method for large-scale production of chicken Marek's virus
ActiveCN102260650AStrong immunityControllable conditionsMicroorganism based processesViruses/bacteriophagesNutrientSuspension culture
The invention discloses a large-scale production method of fowl Marek's disease virus, which uses a bioreactor to produce fowl Marek's disease virus in a cell microcarrier suspension culture system. The method comprises the following steps: inoculating chick embryo fibroblast for preparing virus into a carrier tank containing culture fluid and microcarrier, and evenly mixing the fibroblast and microcarrier, so that the fibroblast is attached to the microcarrier; in a proper culture environment, providing sufficient nutrients and a proper gas environment for the fibroblast, so that the fibroblast can grow to 5-10 times of the inoculation concentration on the microcarrier; changing for a cell maintenance liquid, preparing the fowl Marek's disease virus into a virus suspension, and enabling the virus suspension onto the fibroblast; culturing the virus in a proper culture environment; after continuously culturing for 3-4 days, harvesting the fibroblast to obtain a cell suspension; and filling the cell suspension into an ampoule bottle, sealing the ampoule bottle, putting in a programmed cooling instrument, and storing in liquid nitrogen. The method has the advantages of large production scale, high single-batch yield and lower production cost.
Owner:PU LIKE BIO ENG
Mycobacterium tuberculosis fusion protein (EAMMH) and constructing, expressing and purifying method and application thereof
ActiveCN104098700AImprove shortcomingsStrong immunityAntibacterial agentsBacterial antigen ingredientsAntigenInclusion bodies
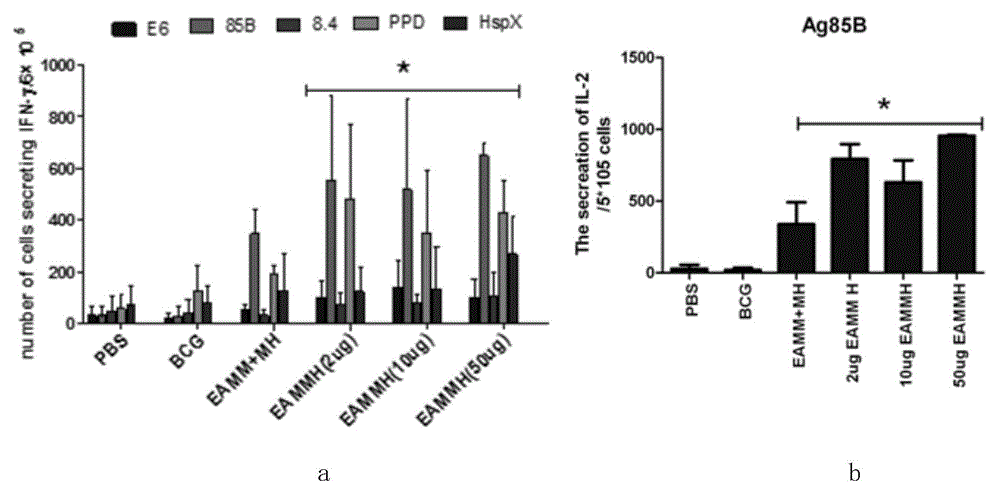
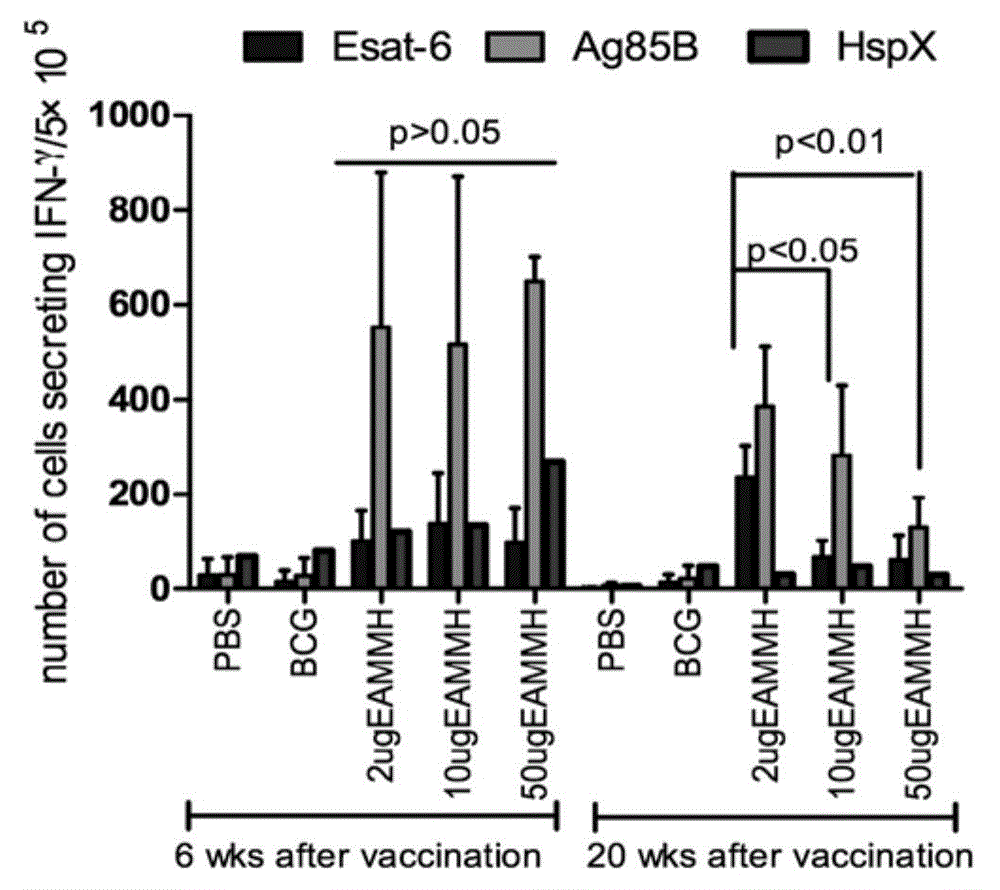
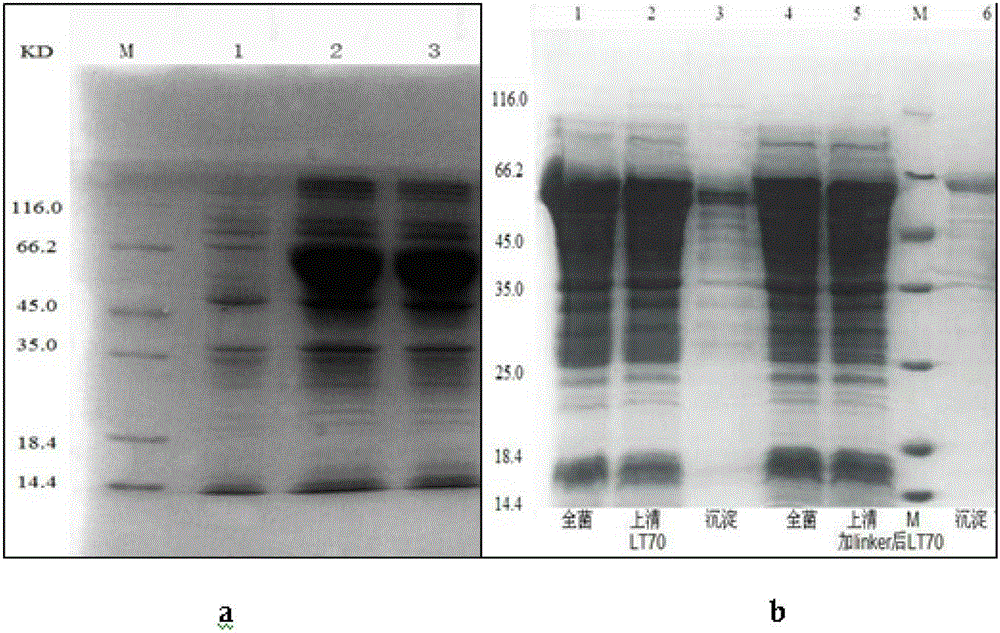
The invention discloses mycobacterium tuberculosis fusion protein EAMMH and a constructing, expressing and purifying method and application thereof. The fusion protein is expressed in a soluble form, and greatly improves EAMM inclusion body form expression weakness. Tuberculosis subunit vaccine (LT69) constructed by combination of the fusion protein and an adjuvant has strong protective immunity, is superior to traditional BCG (Bacillus Calmette-Guerin) vaccine and EAMM+MH combined vaccine; the vaccine as an enhanced vaccine can significantly enhance the BCG initial immune immunity and protection effect of anti tuberculosis, and to a certain extent, reduces the pathological injury of the lung; in addition, the subunit vaccine contains a wide variety of antigens of growth period and latency period of mycobacterium tuberculosis, can induce strong specific cellular immune and humoral immune response aiming at each period of tubercle bacillus antigen, has protective effect on the tubercle bacillus in different metabolic states, is long in protection time, and is expected to become an effective vaccine for clinical tuberculosis prevention.
Owner:LANZHOU UNIVERSITY
Compound feed for loach
ActiveCN104171570AImprove the survival rate of seedlingsStrong immunityClimate change adaptationAnimal feeding stuffSOYBEAN SEED OILSoybean oil
Owner:HUZHOU HAIHUANG BIOTECH
Application of pyroptosis-associated protein GSDMD (Gasdermin-D) for preparing bacterial ghost vaccine
ActiveCN109303916AAvoid damageInfection controlAntibacterial agentsPeptide/protein ingredientsHumoral immune reactionOrganism
The invention discloses application of pyroptosis-associated protein GSDMD (Gasdermin-D) for preparing a bacterial ghost vaccine. A bacteriolysis plasmid containing a pyroptosis-associated protein GSDMD coding gene is constructed and is converted into salmonella enteritidis; under the induction of gum sugar, bacteria are split to successfully obtain a salmonella novel bacterial ghost vaccine. An experiment proves that GSDMD mediated bacteriolysis has an extremely long persistent period, and a splitting rate is 99.9985% or more. After a novel bacterial ghost immune mouse prepared by the invention is adopted, an organism can be stimulated to generate powerful humoral immunity and cellular immunity; protective immunity response is induced; an experiment animal can be protected so as to resistsalmonella infection. The salmonella bacterial ghost vaccine prepared by the invention has a good immune protection effect.
Owner:HARBIN WEIKE BIOTECH DEV
Genetic engineering subunit oral bicombined inactivated vaccine
InactiveCN107158371AStrong immunityReduce morbidity and mortalityBacteriaVirus peptidesGenetic engineeringDisease
The invention provides a genetic engineering subunit oral bicombined inactivated vaccine, and in particular relates to a porcine epidemic diarrhea virus and porcine transmissible gastroenteritis virus genetic engineering subunit oral bicombined inactivated vaccine, which is prepared by using lactococcus lactis capable of carrying out recombinant expression on S protein of porcine epidemic diarrhea virus and SLN protein of porcine transmissible gastroenteritis virus, wherein an amino acid sequence of the S protein of the porcine epidemic diarrhea virus is SEQ ID No. 1, a nucleotide sequence of a coding gene of the S protein of the porcine epidemic diarrhea virus is SEQ ID No. 2, and the SLN protein is formed by connecting genes of A and D antigenic sites with a gene of an N321 antigenic site of the porcine transmissible gastroenteritis virus. The prepared inactivated vaccine can provide effective homologous attacking protection; after being used for immunization, the inactivated vaccine can generate stronger immunity; after a pig group is inoculated with the vaccine, the morbidity and mortality rates of the pig group are obviously reduced; the genetic engineering subunit oral bicombined inactivated vaccine can be used for effectively preventing the prevalence and spread of porcine epidemic diarrhea and porcine transmissible gastroenteritis and reducing the economic loss, caused by the diseases, of pig husbandry, thus having wide application prospect.
Owner:QINGDAO AGRI UNIV
Whole body moxibustion bed
InactiveCN104997627AImproved Qi and blood circulation throughout the bodyQi and blood circulation around the body is acceleratedDevices for heating/cooling reflex pointsCombustion chamberWhole body
The invention discloses a whole body moxibustion bed which is an closed box body, wherein the interior of the box body comprises a smoke accumulating chamber, a moxibustion chamber and a moxibustion combustion chamber; a tray is arranged in the smoke accumulating chamber; the bottom of the tray is a two-layer screen; base holes used for allowing moxa sticks to be inserted are formed in the inner screen; sieve holes are formed in the bottom of each of the base holes; the smoke heat in the moxibustion combustion chamber goes upwards through a porous barrier to act on human bodies, and the smoke heat in the smoke accumulating chamber goes downwards through the screen to act on human bodies, so that hot gas in the box body circulates around the whole human bodies, and the purpose of moxibustion for the whole human bodies is achieved. According to the whole body moxibustion bed, time is saved; pollution is avoided; patients can receive moxibustion treatment in a clean, warm and comfortable state; the whole body moxibustion effect is 50-70 times of that of manual whole body moxibustion, and 2-3 times of that of a one-side moxibustion bed; the functions of disease treatment and health maintenance are multiply increased.
Owner:冯瑞金
Method for producing marek disease live vaccine of chicken by using cell line
ActiveCN103977400AEliminate potential contaminationEnsure safetyAntiviralsAntibody medical ingredientsVenomMarek Disease
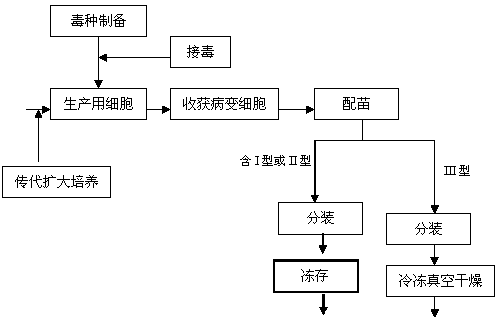
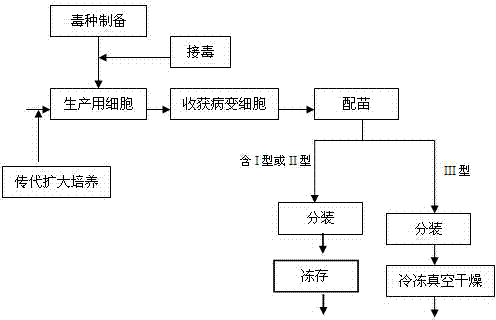
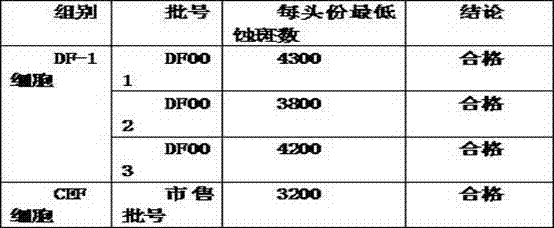
The invention discloses a method for producing a marek disease live vaccine of chicken by using a cell line. The method comprises the following steps: A, selecting the cell line as a vaccine-preparing cell; B, passaging and cultivating the vaccine-preparing cell; C, breeding cytotoxic species; D, breeding vaccine-preparing venom; E, preparing vaccine, carrying out split charging, freeze-drying or cryopreserving in liquid nitrogen. By adopting the method, the defects of the prior art can be solved, the method has the advantages of being stable in production process, easy to operate, high in virus content, small in difference between batches, and easy in quality control, and the yield and quality of the vaccine can be significantly improved. The marek disease live vaccine of chicken produced by the method is good in safety, and high in immunity, and has complete immune protection action on attack of a virulent strain or a very virulent strain of chicken marek's disease.
Owner:南京创启生物科技有限公司
Mixed virus-like particles of swine influenza virus and foot and mouth disease virus, preparation method and application thereof
ActiveCN102370976AStrong immunityLong durationMicroorganism based processesAntiviralsAdjuvantFoot-and-mouth disease virus
The invention provides a mixed virus-like particles, containing stromatin M1 of influenza virus, surface antigen erythrohemagglutinin HA of influenza virus and a nexus protein containing main antigenic epitope contained capsid protein of foot and mouth disease virus and a transmembrane zone and an inner membrane zone of the erythrohemagglutinin HA of influenza virus. The main antigenic epitope contained capsid protein of foot and mouth disease virus substitutes a basically same length outer membrane zone of a 5' terminal of the erythrohemagglutinin HA of influenza virus. Surface antigen erythrohemagglutinin HA of influenza virus and the main antigenic epitope contained capsid protein of foot and mouth disease virus are expressed simultaneously on surfaces of the mixed virus-like particles. The invention also provides a divalent vaccine of swine influenza and foot and mouth disease; and the vaccine contains the mixed virus-like particles and adjuvants. The invention also provides a method for preparing the mixed virus-like particles.
Owner:SUN YAT SEN UNIV
Mycobacterium tuberculosis fusion proteins and their preparation method and use
InactiveCN104151433APurification succeededComprehensive evaluation of immune protection effectAntibacterial agentsBacterial antigen ingredientsAdjuvantMouse virulence
The invention provides mycobacterium tuberculosis fusion proteins. The mycobacterium tuberculosis fusion proteins are shown in sequences 2 and 4 in the sequence table. The invention provides a preparation method and a use of the mycobacterium tuberculosis fusion proteins. The fusion protein LT70 and adjuvants of DDA and PolyI: C are combined and form a tuberculosis sigmasubunit vaccine. An experiment result shows that the vaccine can effectively induce immune response of tuberculosis antigen-specific cells and body fluid in mouse body and has a strong immune protection capacity, lasting immune protection time, immune protection effects better than that of BCG and BCG improvement effects in a mouse virulent strain attack protection efficiency test. The tuberculosis sigmasubunit vaccine is an effective tuberculosis vaccine candidate.
Owner:LANZHOU UNIVERSITY
Bivalent inactivated vaccine for porcine reproductive and respiratory syndrome and preparation method thereof
PendingCN113521271AEasy to prepareSimple and fast operationSsRNA viruses positive-senseViral antigen ingredientsPig farmsNeutralising antibody
The invention discloses a bivalent inactivated vaccine for porcine reproductive and respiratory syndrome and a preparation method thereof. The bivalent inactivated vaccine for the porcine reproductive and respiratory syndrome comprises the following two inactivated viruses: a porcine reproductive and respiratory syndrome virus with the strain number of PRRSV-ZJ / H strain and the preservation number of CCTCC NO: V202130, and a porcine reproductive and respiratory syndrome virus with the strain number of PRRSV-ZJ / NB strain and the preservation number of CCTCC NO: V202140. The bivalent inactivated vaccine prepared by the invention can effectively prevent and control two major prevalent PRRS virus types in the current field, and a needle of the PRRS inactivated vaccine is immunized in a commercial pig field which is naturally infected or is immunized by using a PRRS attenuated live vaccine, so that the virus specificity neutralizing antibody can be obviously improved, the virus load in the infected pig body can be reduced, the PRRS epidemic situation can be quickly and effectively controlled, the daily gain of pigs can be obviously improved, and the economic benefits of pig farms are increased.
Owner:HANGZHOU JIANLIANG VETERINARY BIOLOGICAL PREPARATIONS CO LTD
Dog type II adenovirus live vector recombinant vaccine for displaying protective antigen of rabies virus
InactiveCN101406698AResist attackStrong immunityAntiviralsAntibody medical ingredientsDiseaseProtective antigen
The invention discloses a recombinant vaccine with canine adenovirus type-2 as a live vector for displaying a protective antigen of rabies virus, which relates to recombinant vaccines displaying the protective antigen or an antigenic epitope of the rabies virus respectively by utilizing the characteristics of different structural proteins of the canine adenovirus type-2. The protective antigen of the rabies virus or a ribonucleotide sequence expressing the antigenic epitope are inserted at a hydrophobic locus of a structural protein gene to ensure that an exogenous antigen or an antigenic epitope is subject to fusion expression with structural protein of the exogenous antigen or the antigenic epitope on the surfaces of adenoviruses. After the recombinant vaccines challenge, test animals are subject to closed and isolated rearing, feeding and disease conditions of the test animals are observed and recorded; and the results show that dogs taking any kind of recombinant vaccine by oral administration and intramuscular injection all can resist the attack of strong viruses, and the survival rate is more than 90 percent.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
SVCV (Spring viraemia of carp) resisting VLPs (virus-like particles), vaccine and preparation method thereof
ActiveCN104152418AStrong immunityLong durationViral antigen ingredientsInactivation/attenuationNucleic acid sequenceRecombinant baculovirus
The invention relates to SVCV (Spring viraemia of carp) resisting VLPs (virus-like particles), a vaccine and a preparation method thereof. The SVCV resisting VLPs are composed of influenza virus matrix protein M1 and fusion protein HA-G which are expressed at the same time; the fusion protein HA-G is built through influenza virus HA protein and SVCV G protein, and the nucleotide sequence of the fusion protein HA-G is shown in SEQ ID NO.3, 5, 7, 9, 11 or 13. The preparation method of the SVCV resisting VLPs comprises the following steps: preparing a fusion gene HA-G; preparing a recombinant baculovirus shuttle vector rBacmid; preparing recombinant insect baculovirus; obtaining VLPs which can express the influenza virus matrix protein M1 and the fusion protein HA-G simultaneously. The vaccine comprises any one virus-like particle. An organism immunity system can cause strong response by blending VLPs and the vaccine, the immunity is strong, the time of duration is long, the SVCV can be prevented, and high safety is achieved.
Owner:SUN YAT SEN UNIV
Pickering emulsion, preparation method thereof and application of pickering emulsion as vaccine immunologic adjuvant
ActiveCN113368231AImprove immunityEnhances immune protection and safetyAntibacterial agentsChlamydiaceae ingredientsImmunologic TechniqueImmunologic Reactions
The invention relates to the technical field of immunology, in particular to a pickering emulsion, a preparation method thereof and application of the pickering emulsion as a vaccine immunologic adjuvant. The GO-LP Pickering emulsion prepared by the preparation method disclosed by the invention shows good stability and an obviously-enhanced adjuvant effect through a stability test and in-vivo and in-vitro evaluation; a mouse genital tract anti-Ct infection model is established by taking a CtpORF5 recombinant protein vaccine as a model antigen, the immune protection capability and the safety of the GO-LPPickering adjuvant for the organism are evaluated, and a result shows that the GO-LPPickering emulsion provided by the invention can remarkably enhance the humoral immunity of a mouse and is relatively high in safety. Therefore, the pickering emulsion has potential values of enhancing immune response and improving the immune protection of the vaccine.
Owner:NANHUA UNIV
Maca Chinese wolfberry tablet and preparation method thereof
InactiveCN104256410AStrong ImmunityMildFood ingredient as taste affecting agentNatural extract food ingredientsMedicinal herbsOyster
The invention relates to a Maca Chinese wolfberry tablet and a preparation method thereof. The Maca Chinese wolfberry tablet is characterized by comprising the following raw materials in parts by weight: Maca 50-55, Chinese wolfberry 10-11, Gordon Euryale seed 3-4, Chinese yam 2-3, mulberry 4-5, Sharpleaf Galangal fruit 2-3, stem of Bengal Hiptage 1-2, Melica scabrosa Trin 1-2, oyster 4-5, donkey meat 5-6, sophora flower honey 7-8, rice wine 30-32, cashew nut 8-9, pureed pumpkin 15-18, mustard 1-1.2, cacao bean 10-11, maltodextrin 30-35, magnesium stearate 1.5-1.7, soybean oil 2-2.5, vinegar 40-45, flavor protease 0.1-0.12, and nutritional additive 10-11. The added traditional Chinese medicines are steamed with vinegar to kill the harmful materials such as harmful bacteria, pathogenic microorganisms and worm eggs which are adhered on the traditional Chinese medicines and make the medical speciality be milder. The Maca is used as the main ingredient, and the alkaloids component in Maca can enhance the human immunity and improve the sub-health state of people.
Owner:安徽阳光药业有限公司
Breeding method for native chicken
InactiveCN108157286AStrong immunityGrow wellFood processingAnimal feeding stuffWater drinkingAnimal science
The invention discloses a breeding method for a native chicken. The method comprises the following steps that (1) a breeding environment is set up, a breeding place is located in the slopes with sufficient lighting, no industry and villages, and a chicken house is built in the breeding place; (2) water drinking is managed, spring water is taken for the chicken to drink freely every day; (3) dailyfeeding is carried out, a first stage is that concentrated feed is fed from shell breaking to 12-day-old; a second stage is that special feed for chickens and green feed are fed from 13-day-old to 30-day-old; (4) daily management is carried out, after the chicken grows to 15-day-old, the chickens is driven out of the chicken house during a sunny day and is bred outside cages for 1.5-5 hours. The breeding method for the native chicken improves a traditional feeding method of the chicken according to the growth characteristics of the native chicken, the immunity and disease resistance of the native chicken are improved without antibiotics and other drugs, and the breeding rate of the native chicken is improved, thereby improving the breeding efficiency of the native chicken.
Owner:广西巴马泽成种养专业合作社
High-immunity crayfish breeding method
InactiveCN107372060AStrong immunityGood healthFood processingClimate change adaptationHerbCultural methods
The invention discloses a high-immunity crayfish breeding method. The high-immunity crayfish breeding method includes the following steps of pond selecting, fertilizing in water, crayfish seed putting, feeding and managing. According to the crayfish breeding method, algae and alligator alternanthera herbs are planted; nitrogen phosphorus fertilizer and farmyard manure are added into a pond; licorice root, houttuynia cordata and vitamin C are added into feed; later-period management is conducted. Though the reasonable measures, the bred crayfish are high in immunity and good in healthy state.
Owner:马家富
Method for producing marek disease live vaccine of chicken by using cell line
ActiveCN103977400BGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsFreeze-dryingQuality control
Owner:南京创启生物科技有限公司
Application of nocardia immunopotentiator in fish vaccine
The invention provides an application of a nocardia immunopotentiator in a fish vaccine, the nocardia immunopotentiator comprises nocardia, pachymaran and hyaluronic acid, and the invention also provides an application of the nocardia immunopotentiator in an oil emulsion inactivated vaccine for fish. According to the fish oil emulsion inactivated vaccine, vaccines aiming at different viruses or bacteria can be obtained according to different selected antigens; animal experiments prove that the nocardia immunopotentiator and the application thereof in fish vaccines enable the fish vaccines to be strong in immunity, and the oil emulsion vaccine added with the nocardia adjuvant has the advantages of high safety, no obvious residue in local vaccine injection, simple preparation process and wide application range, Tand the corresponding vaccine specificity is high.
Owner:广东渔跃生物技术有限公司 +1
A kind of mycoplasma hyopneumoniae and its application in the preparation of inactivated vaccine
ActiveCN109055283BFast growthHigh growth titerAntibacterial agentsBacterial antigen ingredientsMicroorganismMycoplasma hyopneumoniae
The invention discloses a mycoplasma hyopneumoniae and its application in the preparation of inactivated vaccines, belonging to the technical field of microorganisms and immunization. The classification of Mycoplasma hyopneumoniae of the present invention is named Mycoplasma hyopneumoniae ES-2 Mycoplasma hyopneumoniae ES-2, and the preservation number is CCTCC NO: M 2018570. Mycoplasma hyopneumoniae of the present invention has fast growth rate, high growth titer (37 ℃ cultivates 2 days and can reach 10 13 CCU / mL) and strong immune protection, it can be used to prepare inactivated vaccine against mycoplasma pneumoniae in swine. The use of the mycoplasma hyopneumoniae of the invention in the preparation of inactivated vaccines can greatly save time and cost, and reduce the production cost of vaccines.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Novel pestilence DNA vaccine and its construction and application
InactiveCN1241643CWeakened immunityStrong immunityViral antigen ingredientsGenetic material ingredientsTotal rnaFull length cdna
The invention relates to an animal epidemic DNA vaccine, which is consisted of NDV-Laso610taHN gene and eukayon expression vector. The preparing process of the DNA vaccine includes following steps: extracting and purifying NDV Lasota virus complete RNA, designing specific primer, and obtaining complete cDNA gene sequence by TDRT-PCR amplify cloning, recombining purified LasotaHN complete cDNA sequence to PCR 3.1 eukayon expression vector. The small dosage of the nucleic vaccine can leads to high immune competence, in addition its safe, stable, convenient and have longer acting time.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
A kind of avirulent c-type Clostridium botulinum genetic engineering subunit vaccine and its production method
ActiveCN110075288BEnsure safetyGuaranteed validityAntibacterial agentsBacterial antigen ingredientsAntigenHeavy chain
The invention relates to a non-toxic Clostridium botulinum type C genetically engineered subunit vaccine and a production method thereof. The non-toxic Clostridium botulinum type C subunit vaccine prepared by the invention adopts codon-optimized, maltose-containing protein ( MBP)-tagged botulinum toxins (BoNTs) heavy chain avirulent region H C (BoH C ) recombinant protein (rMBP‑BoH C ) production, that is, to ensure the safety of the vaccine to the greatest extent while maintaining its effectiveness. At the same time, the antigen of the vaccine can be expressed in a soluble form, so it has the advantages of simple preparation process, low immune dose, and good immune efficacy. It is an ideal candidate vaccine for upgrading the current Clostridium botulinum type C vaccine in my country.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Porcine circovivus2 strain and application thereof
ActiveCN103421748BStrong immunityExtended protection periodViral antigen ingredientsMicroorganism based processesDiseaseCircovirus
The invention provides a porcine circovivus2 strain and application thereof. The novel porcine circovivus2 strain is good in productivity and good in immunogenicity. A vaccine prepared through inactivation of the porcine circovivus2 strain can effectively prevent the porcine circovivus disease. The preservation serial number of the porcine circovivus2 strain is CGMCC NO. 7707. According to the porcine circovivus2 strain (PCV2SD), protective efficacy is good, MPR cells used for strain reproduction can be reproduced well with conventional methods, inoculation can be achieved synchronously as well as with conventional methods, and the virus titer obtained after inoculated culture can reach more than 105.25 TCID / 0.1ml; the vaccine prepared with the PCV2SD can well prevent the occurrence or prevalence of the porcine circovivus disease, and the protection rate can reach more than 90% if ELISA detection is carried out after immunity. Compared with other vaccines of the same kind in the domestic market in respect of protective efficacy, the vaccine prepared with the PCV2SD has the advantages of being fast in antibody production, long in protecting period, and capable of shortening the immunity blank area obviously, reducing immunity frequency, and improving economic performance.
Owner:YEBIO BIOENG OF QINGDAO
Chicken feed adding fluid capable of promoting digestion and preparation method of chicken feed adding fluid
InactiveCN107836566AStrong immunityExcellent digestive effectAnimal feeding stuffAccessory food factorsDigestionLycium chinense
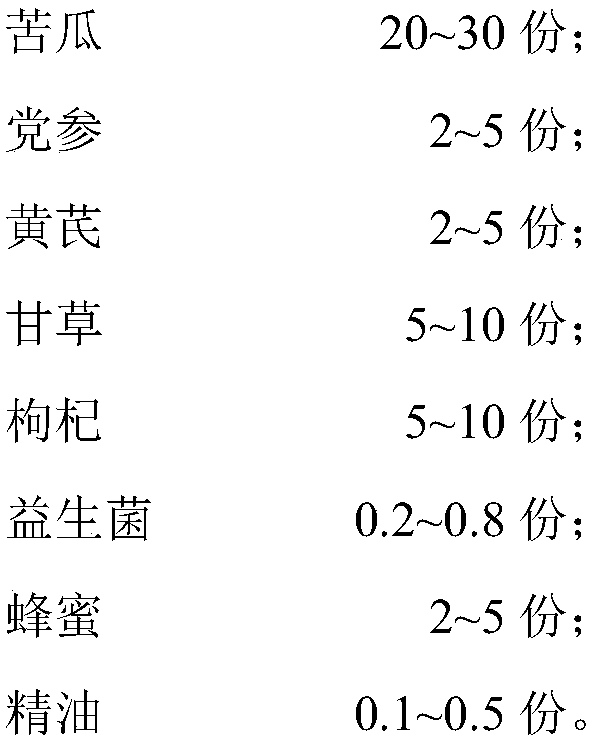
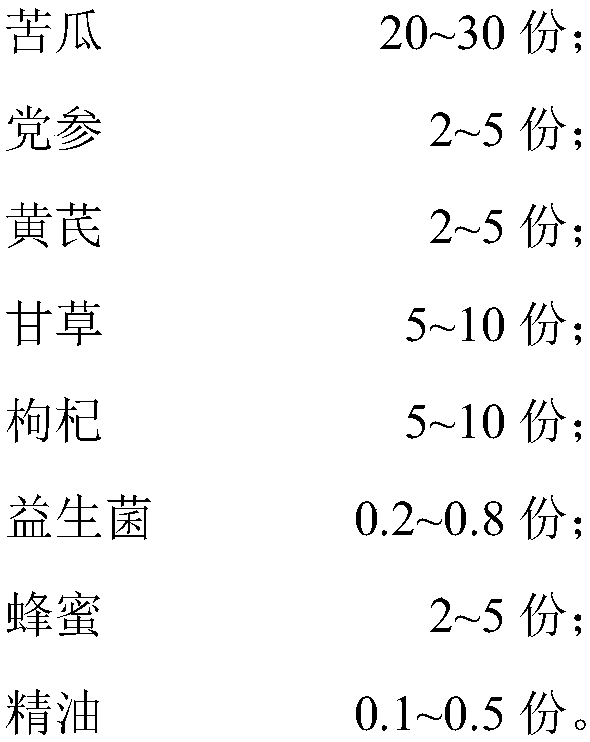
The invention discloses chicken feed adding fluid capable of promoting digestion. The chicken feed adding fluid comprises the following raw material components in parts by weight: 20-30 parts of bitter gourds, 2-5 parts of radix codonopsis, 2-5 parts of radix astragali, 5-10 parts of licorice roots, 5-10 parts of Chinese wolfberry fruits, 0.2-0.8 part of probiotics, 2-5 parts of honey and 0.1-0.5part of essential oil. According to the adding fluid disclosed by the invention, through the adjustment of the raw material components and the compounding ratio, and through the effects of purely natural medicated ingredients of Chinese herbal medicines and the essential oil for resisting bacteria, funguses and viruses, the long-lasting health-care effects of the honey and the digestion promotingeffect of the probiotics, the chicken feed additive namely the adding fluid has excellent efficacy of enhancing immunity and promoting digestion. The invention further discloses a preparation method of the chicken feed adding fluid. The preparation method is simple in technology, and is suitable for large-scale industrial production. Besides, a form of a liquid additive is adopted, and while in use, the adding fluid is added to an ordinary chicken ration in a sprinkling form.
Owner:FOSHAN UNIVERSITY
II type adenovirus live vector recombinant vaccine of dog for showing lyssa virus protective antigen by using spike
InactiveCN102335423AResist attackStrong immunityAntiviralsAntibody medical ingredientsToxic materialFood search
The invention discloses an II type adenovirus live vector recombinant vaccine of a dog for showing a lyssa virus protective antigen by using a spike and relates to a recombinant vaccine for respectively showing the lyssa virus protective antigen or an epitope by using characteristics of different structure proteins of the II type adenovirus of the dog. A lyssa virus protective antigen gene or a nucleotide sequence for expressing the epitope is inserted in a hydrophobic locus spike of the structure protein gene of the II type adenovirus of the dog, so that an exogenous antigen or epitope is subjected to fusion expression with the structure proteins of the II type adenovirus of the dog on the surface of the adenovirus. After toxic substances are eliminated from the recombinant vaccine, the closed isolated feeding for a test animal is carried out and the food searching and pathogenic conditions of the test animal are observed and recorded. The result shows that the dog subjected to oral administration and intramuscular injection of any recombinant vaccine can resist attack of virulent strain and has a survival rate of over 90 percent.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
KaKadu plum health preserving buccal tablet and preparation method thereof
The invention relates to a KaKadu plum health preserving buccal tablet and a preparation method thereof, and belongs to the technical field of health-care products. The KaKadu plum health preserving buccal tablet is prepared from the following raw materials in parts by weight: 80 to 120 parts of a KaKadu extract, 30 to 45 parts of a camu extract, 5 to 10 parts of a lycium ruthenicum extract, 3 to 5 parts of an astragalus extract, 2 to 5 parts of a rhodiola rosea extract, 1 to 3 parts of a gastrodia elata extract, 3 to 6 parts of gynostemma pentaphylla, 1 to 3 parts of cordyceps sinensis and 3 to 6 parts of pseudo-ginseng. The KaKadu plum health preserving buccal tablet disclosed by the invention is rich in antioxidant components such as vitamin C, has good effects of enhancing the immunity, resisting oxidization, resisting aging, resisting tumor and the like, further achieves the effects of eliminating chloasma, eliminating senile plague, expelling toxins, beautifying the face, protecting the skin, caring the skin and the like, and is a health-care medicine very suitable for old people and women to take.
Owner:王永妍
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com