Method for producing marek disease live vaccine of chicken by using cell line
A technology of chicken Marek's disease and cell lines, which is applied in the field of production of chicken Marek's disease live vaccines with cell lines, can solve problems affecting vaccine production and quality, contamination of cells with exogenous viruses, and low vaccine potency, and achieve good economy Benefits and application prospects, high virus content, good immune efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
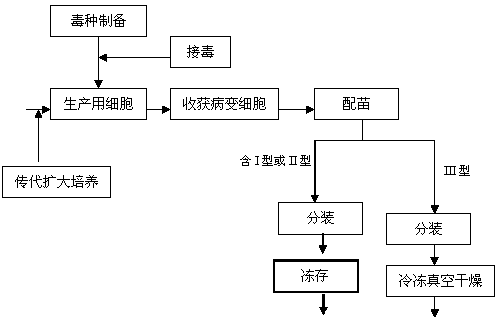
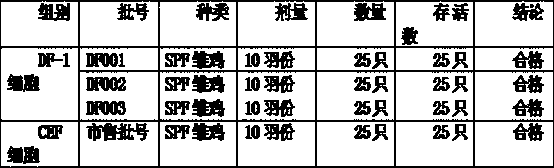
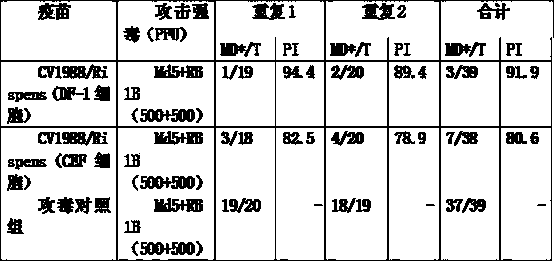
Image
Examples
Embodiment 1
[0030] Preparation of virus seeds: Take out the preserved CVI988 / Rispens virus seeds from liquid nitrogen, melt them quickly (within 1 minute) in warm water at 37°C, immediately dilute them in DF-1 cell growth medium at 1:5, and centrifuge at 1000r / min After 10 minutes, discard the supernatant, dilute with cell growth medium to contain 200,000 PFU / ml, inoculate 100ml square flask with DF-1 single layer, inoculate 0.5ml in each bottle, absorb at 36.5-37.5°C for 1 hour, add maintenance solution , cultured at 36.5-37.5°C in 5% CO2 for 2-4 days, when 70% of the monolayer showed cytopathic changes, and a large number of confluent cells and refraction round cells were produced, add 0.25% trypsin-0.02%EDTA (1:4) Digest and disperse the cells with the cell dispersion solution. After the harvested cell suspension is centrifuged at 1000r / min for 10 minutes, the supernatant is discarded, and the precipitated cells are suspended and inoculated with DF-1 monolayer with an appropriate am...
Embodiment 2
[0042] Propagation of virus seeds: the chicken Marek's disease type III turkey herpes virus Fc-126 basic seeds are diluted with seed virus (equal mixture of 2 times 199 and 2 times hydrolyzed milk protein solution of 5% newborn bovine serum, containing 100 Unit / ml double antibody, pH adjusted to 7.2) diluted to 400,000 PFU / ml, inoculated on well-grown DF-1 cells in a 0.5ml / 100ml square bottle, adsorbed at 36.5-37.5°C for 1 hour, and then added an appropriate amount of maintenance solution , continue to cultivate, when 70% and above monolayer cells have typical Marek's disease cell lesions, digest and disperse the cells with EDTA-trypsin cell dispersion solution, and the harvested cell suspension is used as a virus seed for seedling production;
[0043] Passage and culture of cells for seedling production: DF-1 cell line is digested and passaged with EDTA-trypsin cell dispersion liquid, and then cultured with cell growth medium at 36.5-37.5°C. When a good monolayer is formed, it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com