Monoclonal antibody of anti-SARS-CoV-2 nucleocapsid protein and application
A monoclonal antibody, nucleocapsid protein technology, applied in anti-viral immunoglobulin, application, immunoglobulin and other directions, can solve the problem of not actually preparing antibody, not providing antibody, etc., to achieve strong affinity, high sensitivity and stability good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of SARS-CoV-2-N recombinant protein
[0025] The N protein gene sequence is shown in SEQ ID NO: 1, and the designed primers are as follows:
[0026] N-F: 5'-GCCGGATCCATGTCTGATAATGGACCCCAAAA-3' (with BamHI restriction site);
[0027] N-R: 5'-GCCGTCGACAGGCCTGAGTTGAGTCAGCAC-3' (with SalI restriction site),
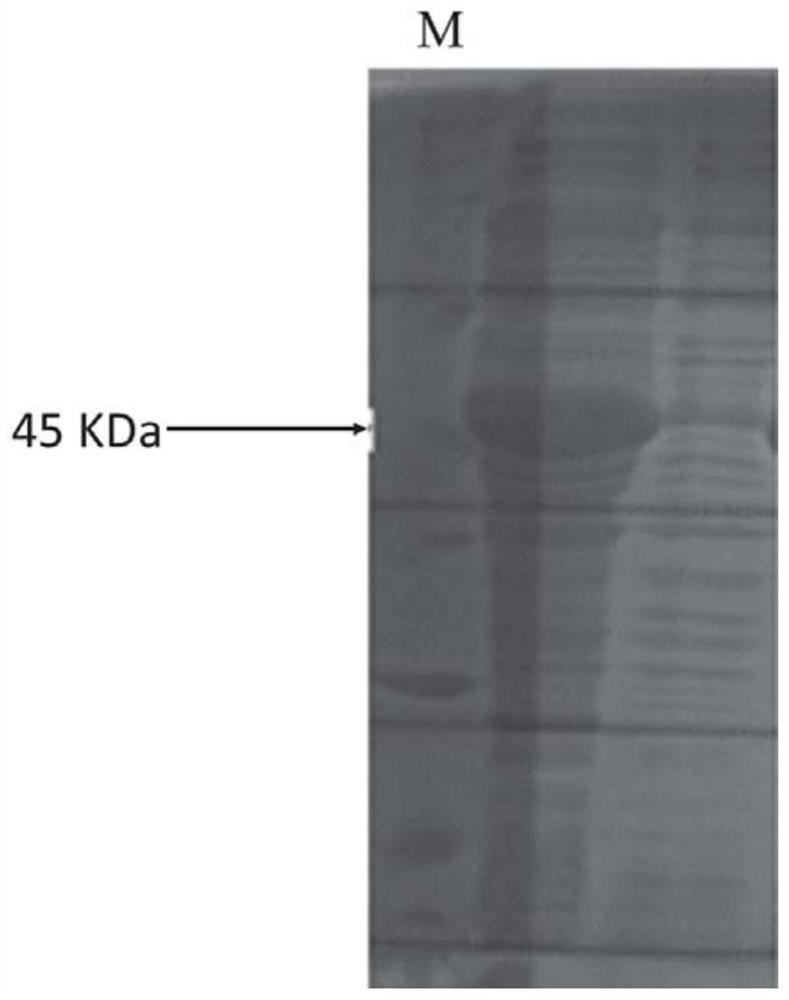
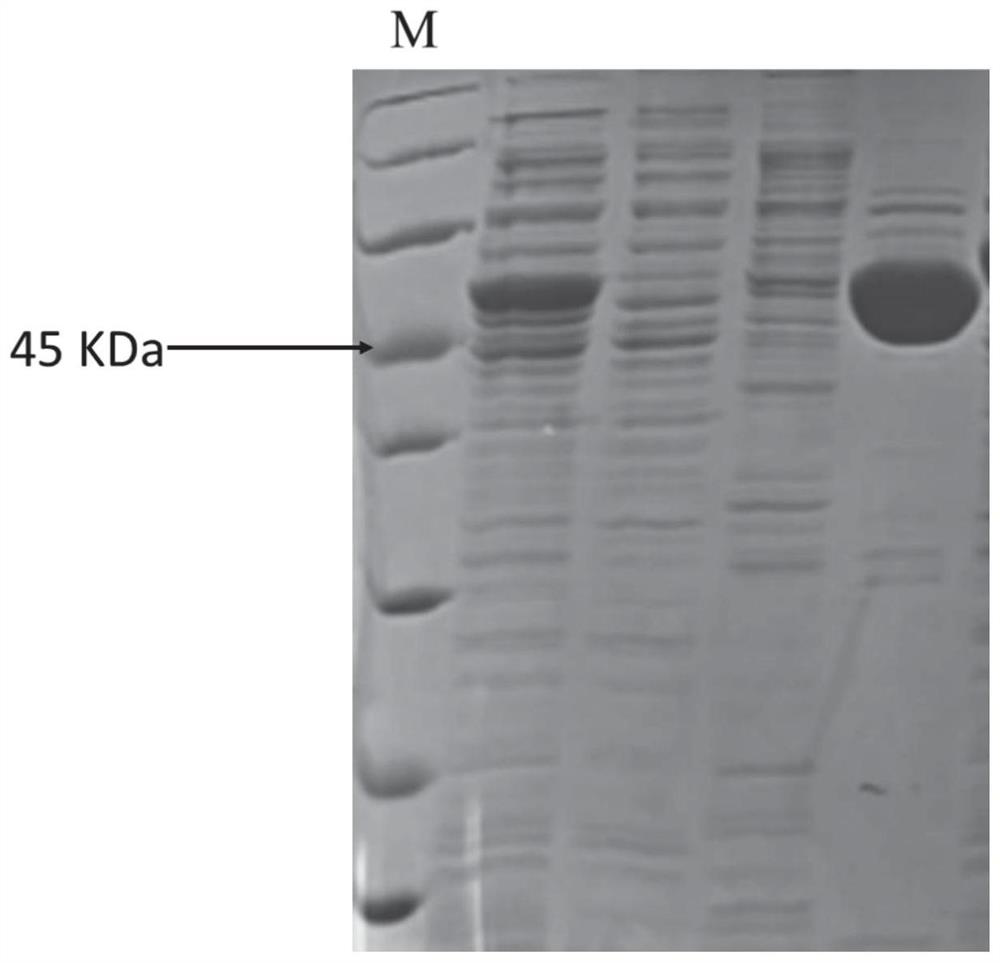
[0028] Using the above primers, PCR was carried out using the artificially synthesized N protein gene as a template, and the product was double-digested with BamHI and SalI restriction endonucleases, and then inserted into pET-28a(+) prokaryotic expression treated with the same two restriction enzymes Vector, the recombinant plasmid pET28a-SARS-CoV-2-N inserted with the N protein gene sequence was obtained.
[0029] Transform the correct pET28a(+)-SARS-CoV-2-N expression vector plasmid verified by sequencing into Escherichia coli BL21, spread it on an LB plate containing 100 μg / ml kanamycin sulfate, culture overnight at 37°C, and pick Take ...
Embodiment 2
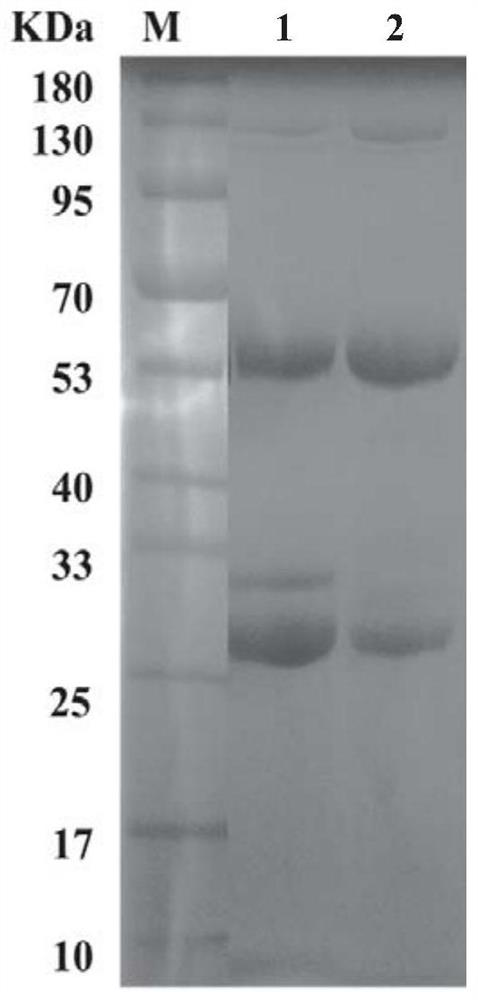
[0032]Embodiment 2: Preparation of SARS-CoV-2-N monoclonal antibody
[0033] Using the recombinant antigen SARS-CoV-2-N protein as the immunogen, for the first immunization, emulsify 50-100 μg / one of the recombinant antigen SARS-CoV-2-N protein with an equal volume of complete Freund’s adjuvant, and put it in BALB / c Mice were immunized by subcutaneous injection at multiple points on the back. For the second and third times, 50-100 μg / only recombinant antigen SARS-CoV-2-N protein was emulsified with an equal volume of incomplete Freund's adjuvant, and the immunization interval was 15 days. After three times of immunization, blood was collected from the tail vein of the mice, the serum was separated and the titer of the mouse serum anti-recombinant antigen SARS-CoV-2-N protein was detected by indirect ELISA.
[0034] 7 days before cell fusion, mice were intraperitoneally injected with 50-100 μg / only recombinant antigen SARS-CoV-2-N protein for booster immunization. The feeder...
Embodiment 3
[0036] Embodiment 3: Indirect method ELISA method detects monoclonal antibody titer
[0037] The SARS-CoV-2-N protein purified in Example 1 was coated with a microtiter plate at 1 μg / ml, 100 μl / well, and incubated overnight at 4°C. Wash the ELISA plate with PBST, add 200 μl / well of 5% skimmed milk powder, and block at 37°C for 2 hours. After the plate was washed, the monoclonal antibody in Example 2 was added at a doubling dilution concentration of 1:500, 1:1000, 1:2000, 1:4000, 1:8000, 1:16000, 1:32000, 1 :64000, 1:128000, 1:256000, 1:512000, 1:1024000, 100μl / well, and incubated at 37°C for 1h. After washing the plate, horseradish peroxidase-labeled goat anti-mouse antibody at appropriate dilution was added and incubated at 37°C for 1 hour. Add 100 μl / well of TMB chromogenic substrate to react for 10 min, add 50 μl 2mol / L sulfuric acid stop solution to terminate the reaction, and read the OD value of each well at 450 nm with a microplate reader. Test results such as Figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com