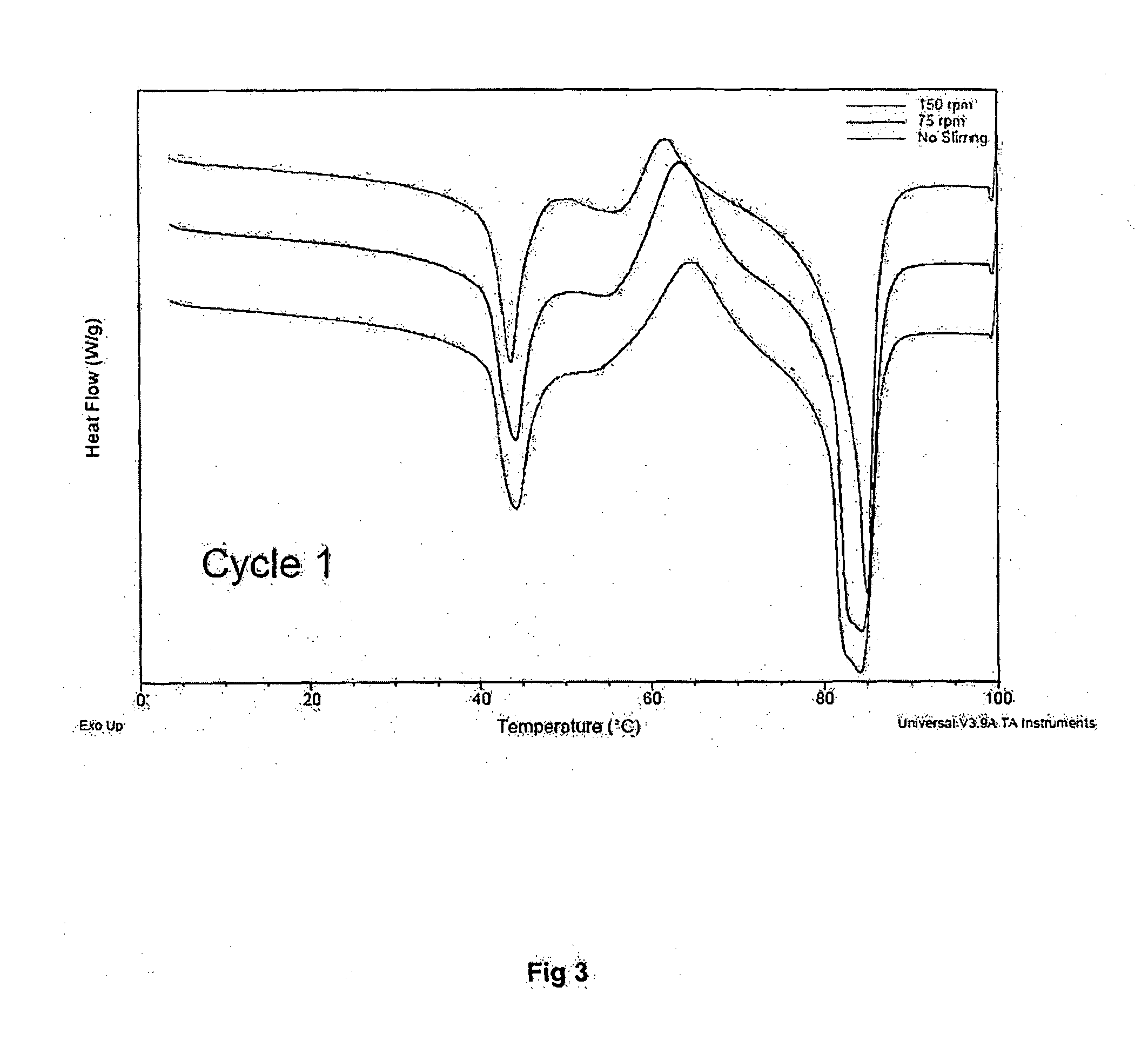
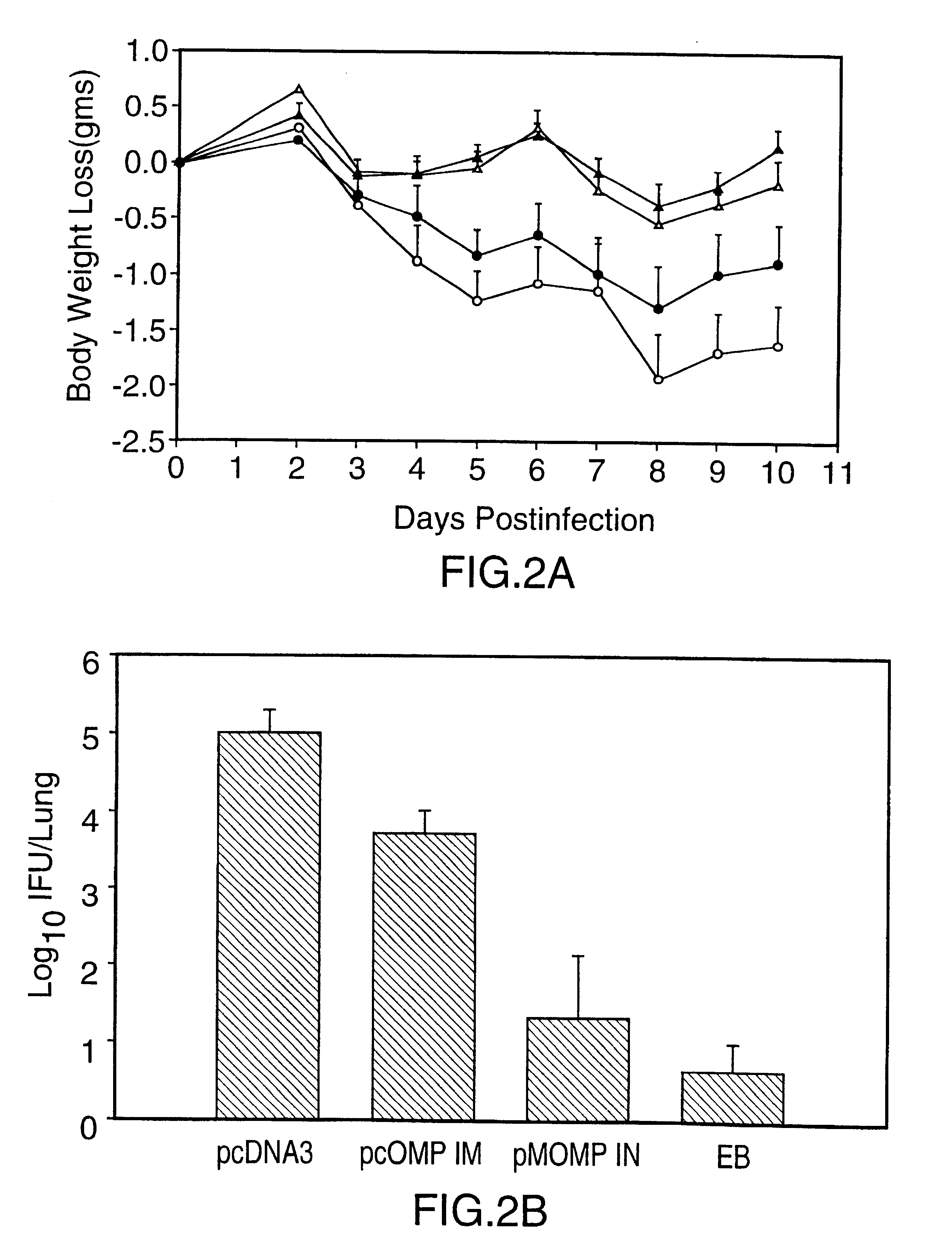
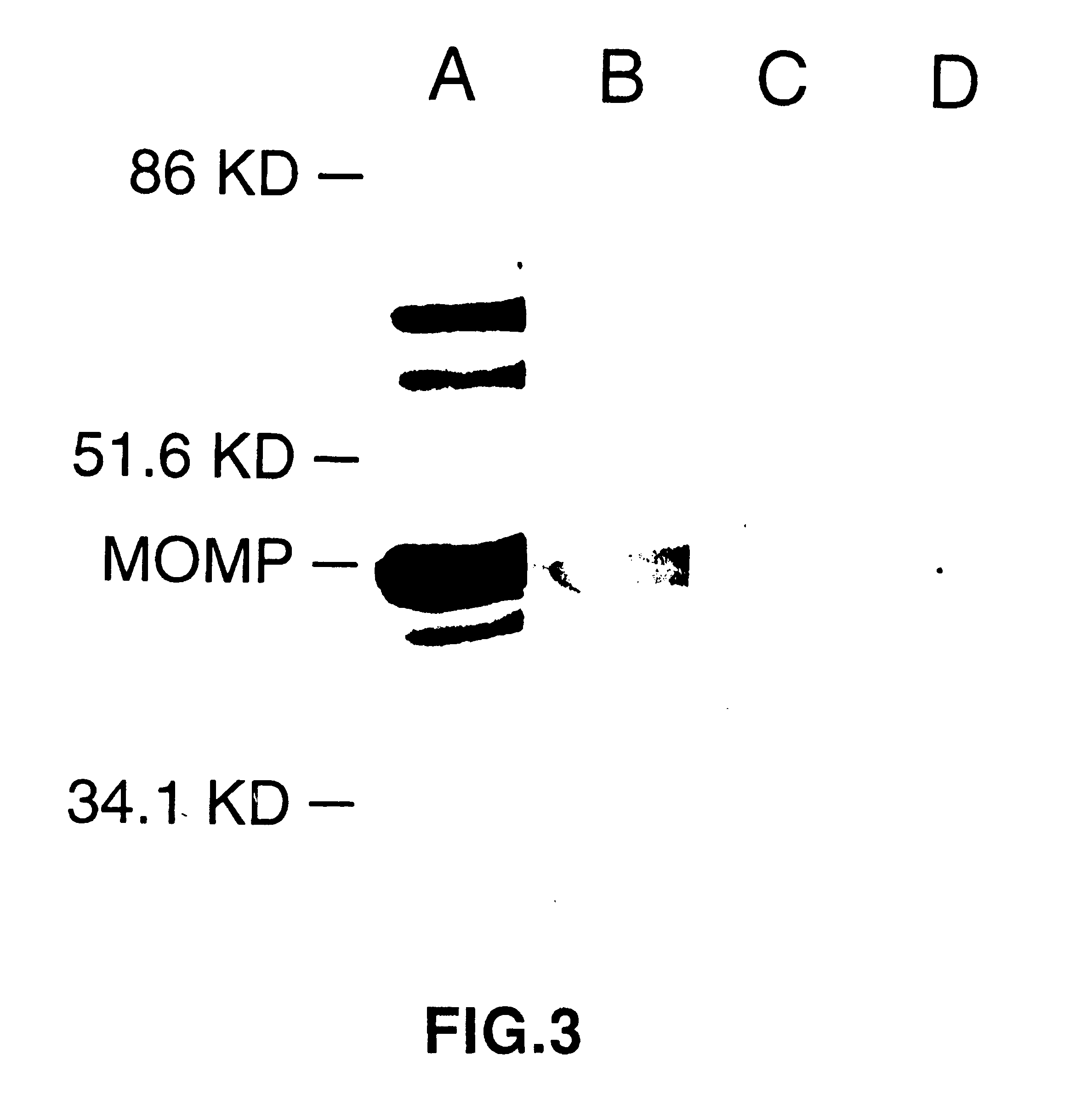
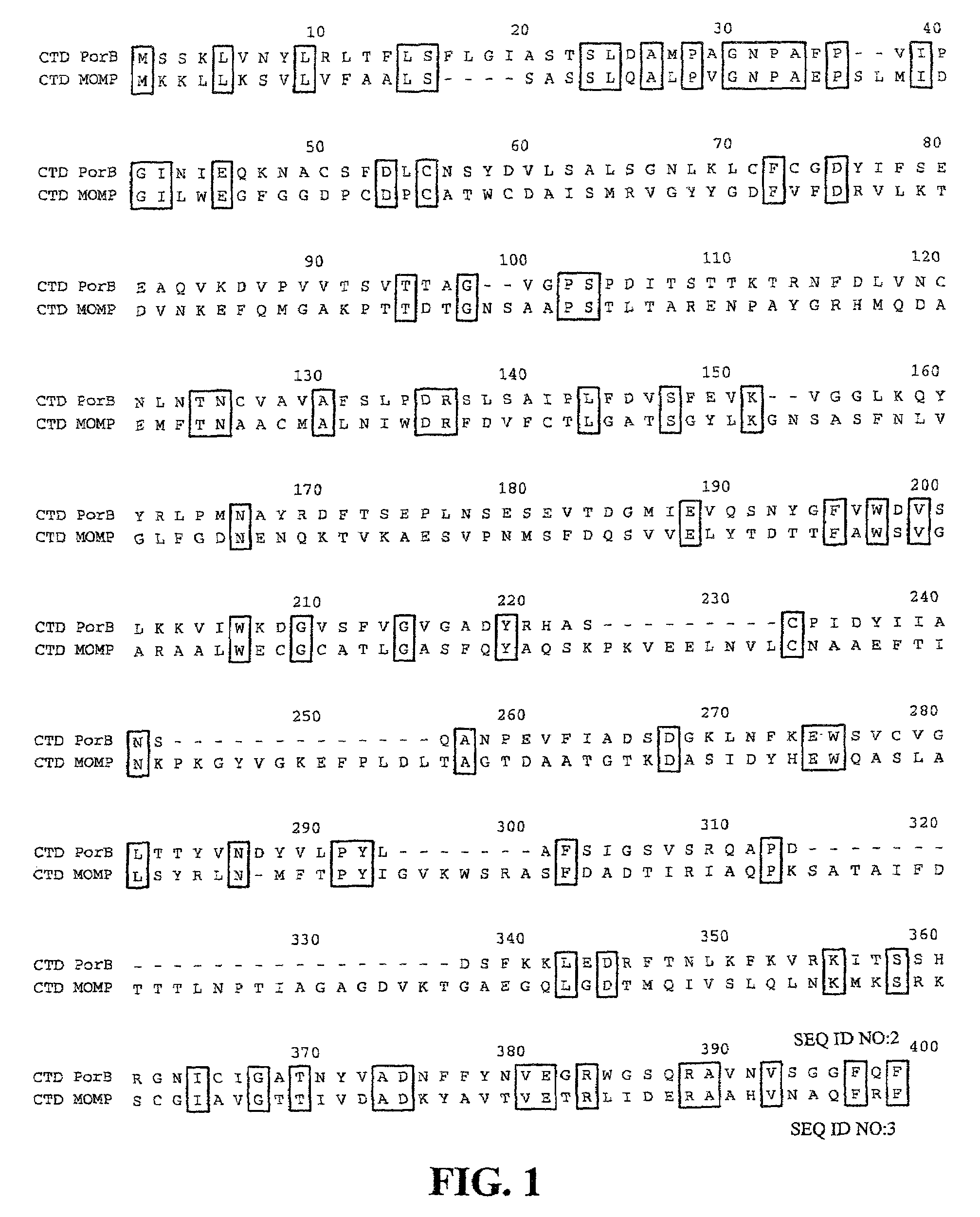Patents
Literature
232results about "Chlamydiaceae ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7332174B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAntigenAdjuvant
Mutant cholera holotoxins having single or double amino acid substitutions or insertions have reduced toxicity compared to the wild-type cholera holotoxin. The mutant cholera holotoxins are useful as adjuvants in antigenic compositions to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus, or parasite, a cancer cell, a tumor cell, an allergen, or a self-molecule.
Owner:WYETH HOLDINGS CORP +1
Vaccines comprising aluminium adjuvants and histidine
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
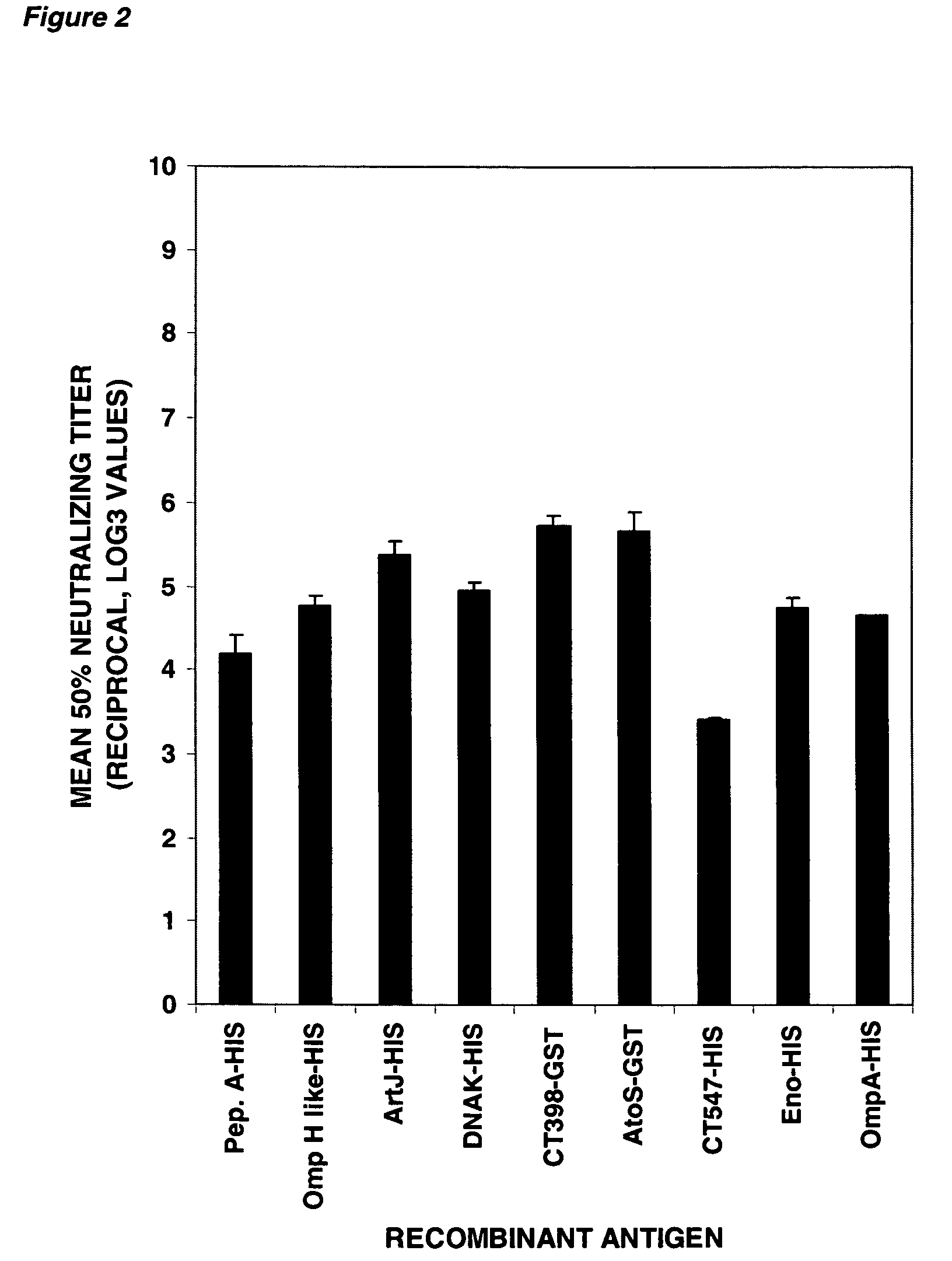
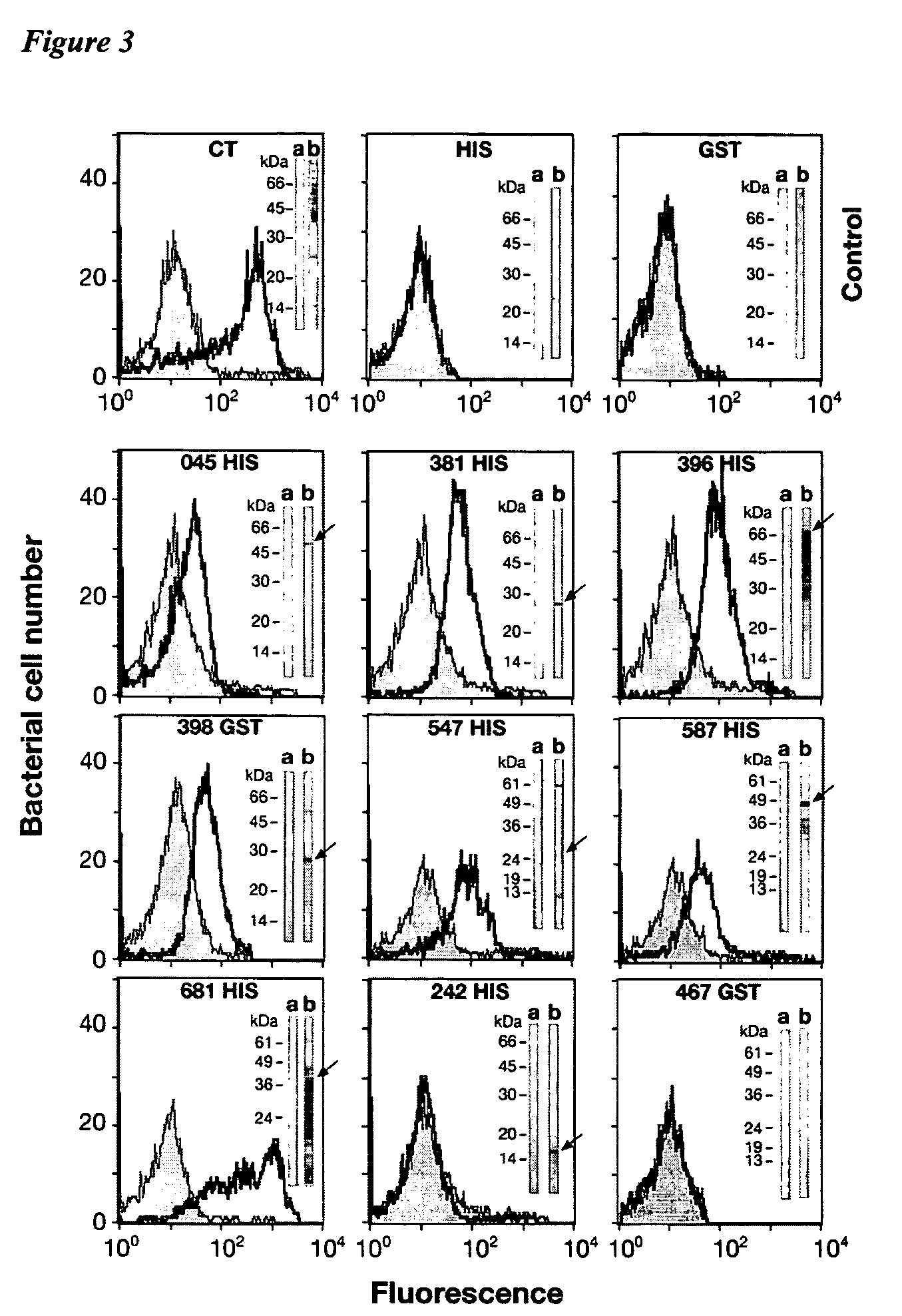
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Compounds and methods for treatment and diagnosis of chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
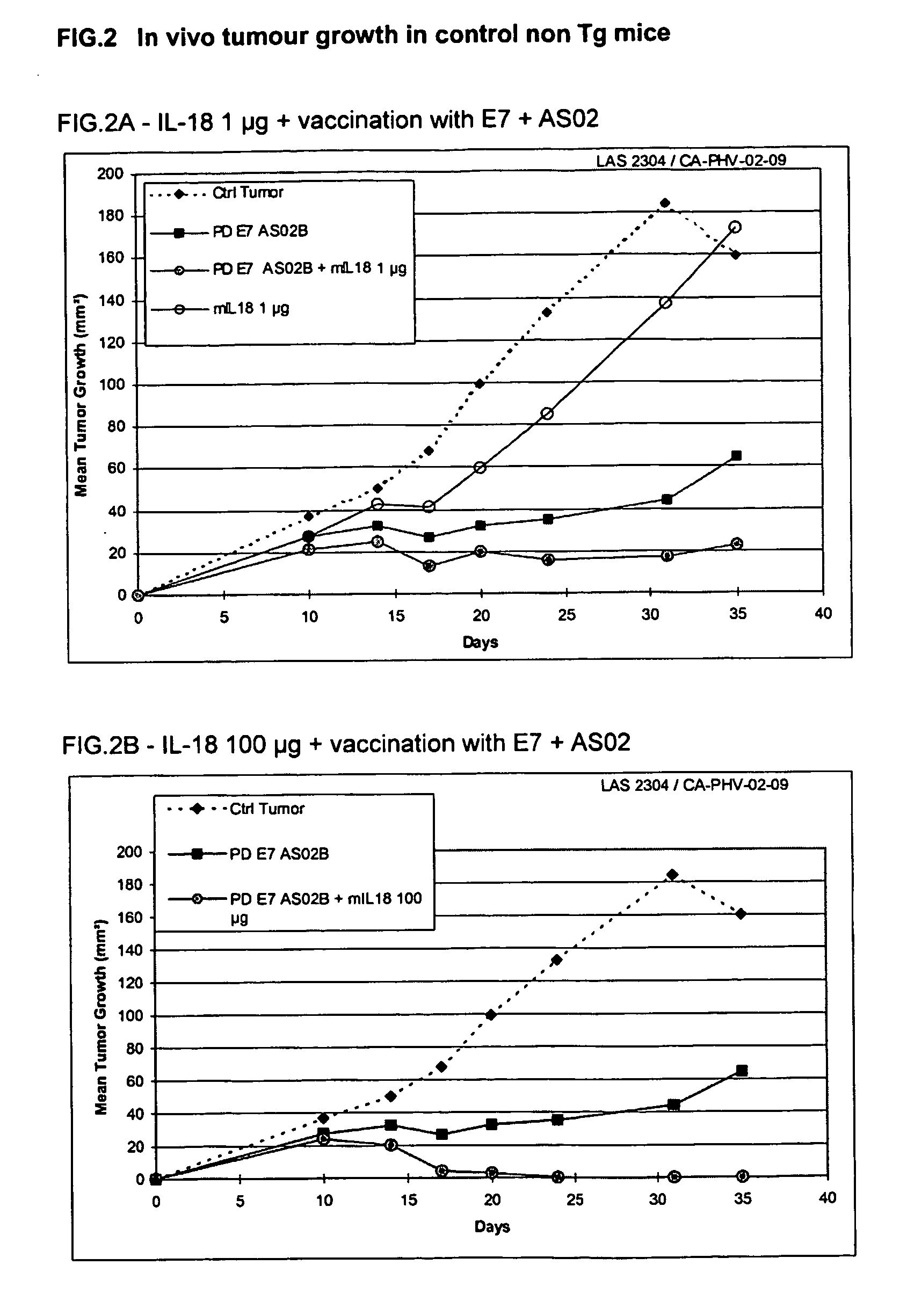
Vaccine Compositions Comprising An Interleukin 18 And Saponin Adjuvant System
InactiveUS20070212329A1Enhance immune responseReduce severityBiocidePeptide/protein ingredientsAdjuvantAutoimmune condition
This invention relates to a combination therapy that finds utility in the treatment or prophylaxis of infectious diseases, cancers, autoimmune diseases and related conditions. In particular, the combination therapy comprises the administration of a TH-1 cytokine, in particular, IL-18, and an immunogenic composition, in particular, a vaccine, comprising an antigen and a saponin adjuvant. In particular, the invention relates to the use of IL-18 or bioactive fragment or variant thereof and an immunogenic composition comprising a tumour-associated antigen and a saponin adjuvant, for the treatment of preneoplasic lesions or cancer.
Owner:SMITHKLINE BECKMAN CORP +1
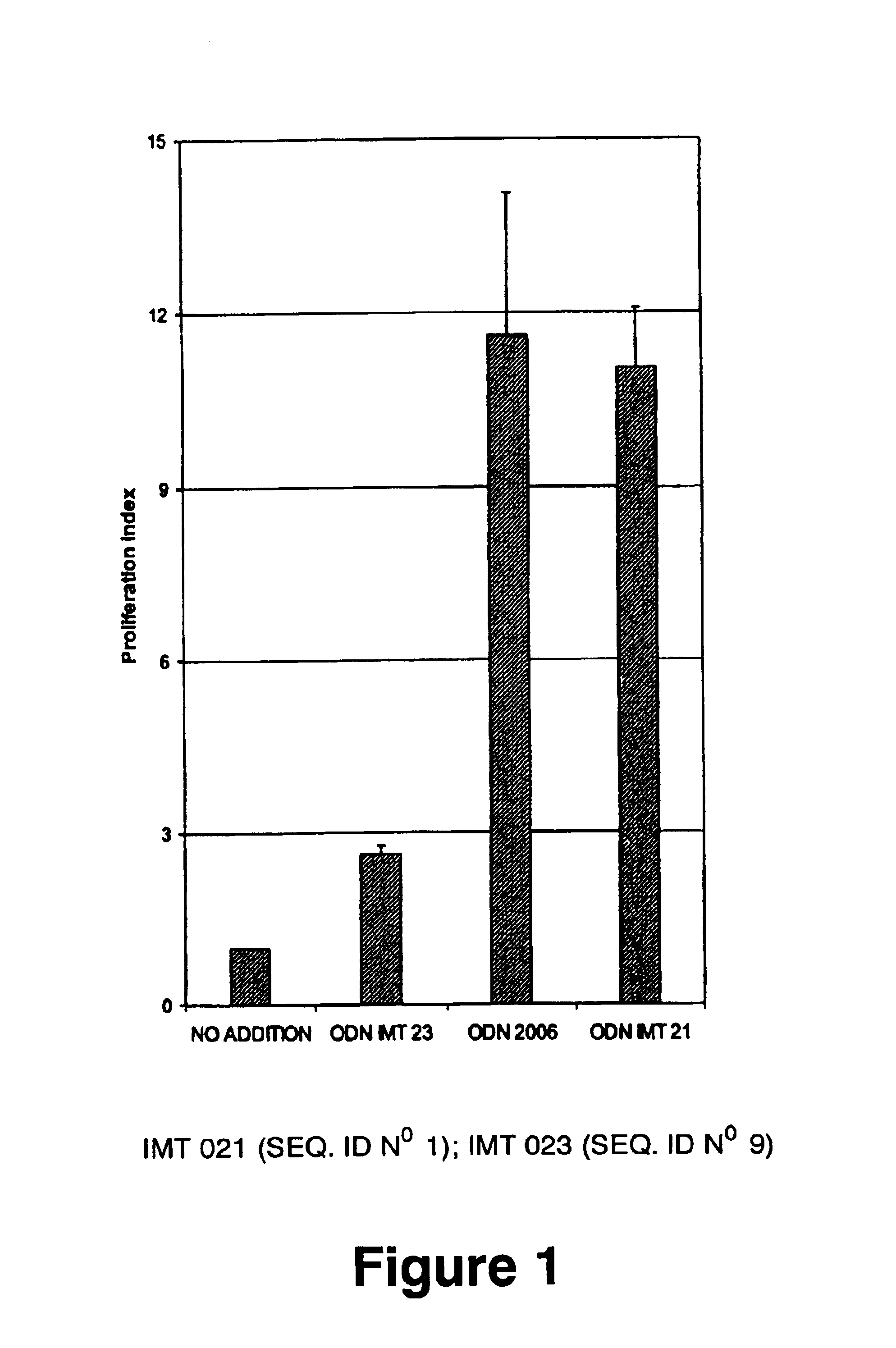
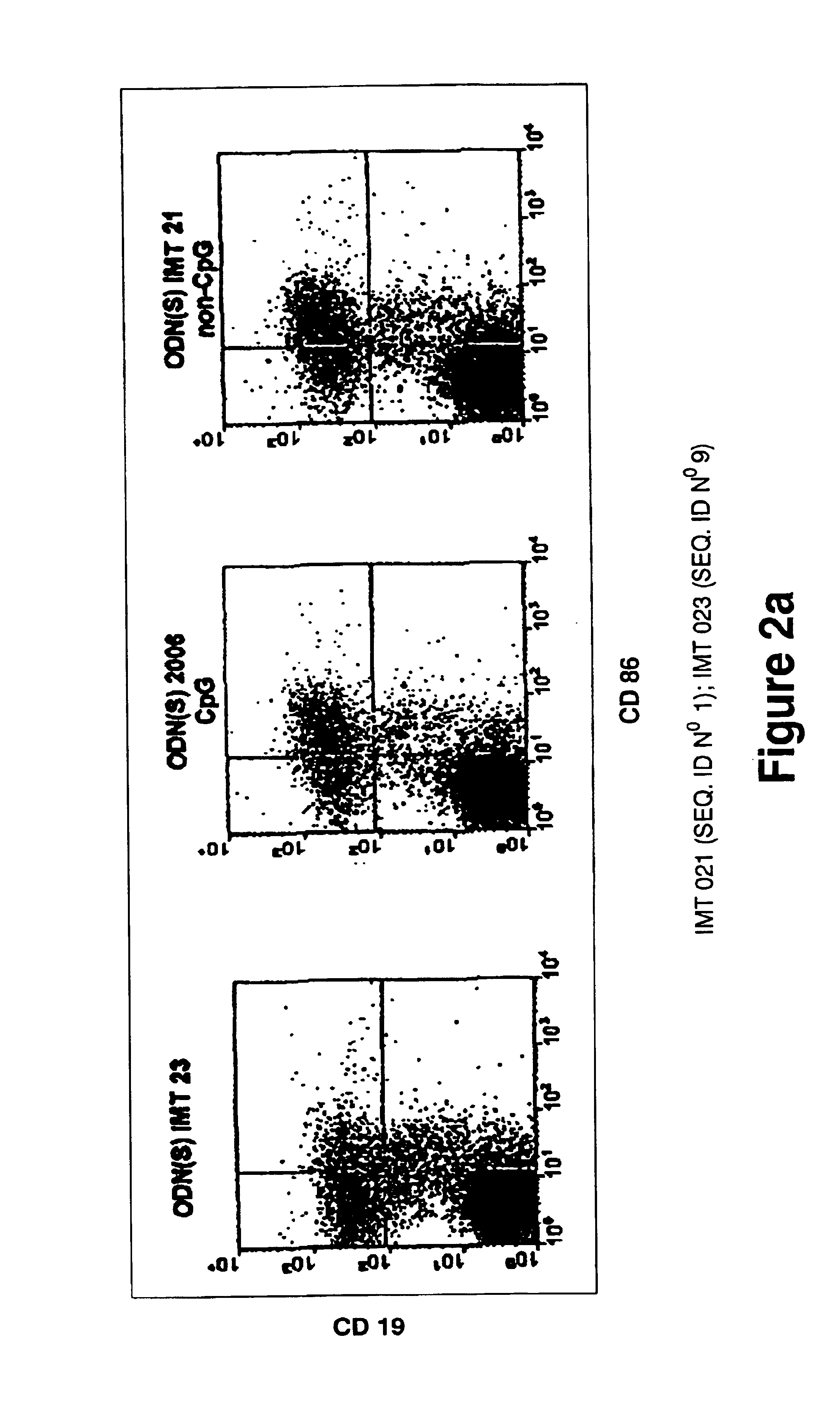
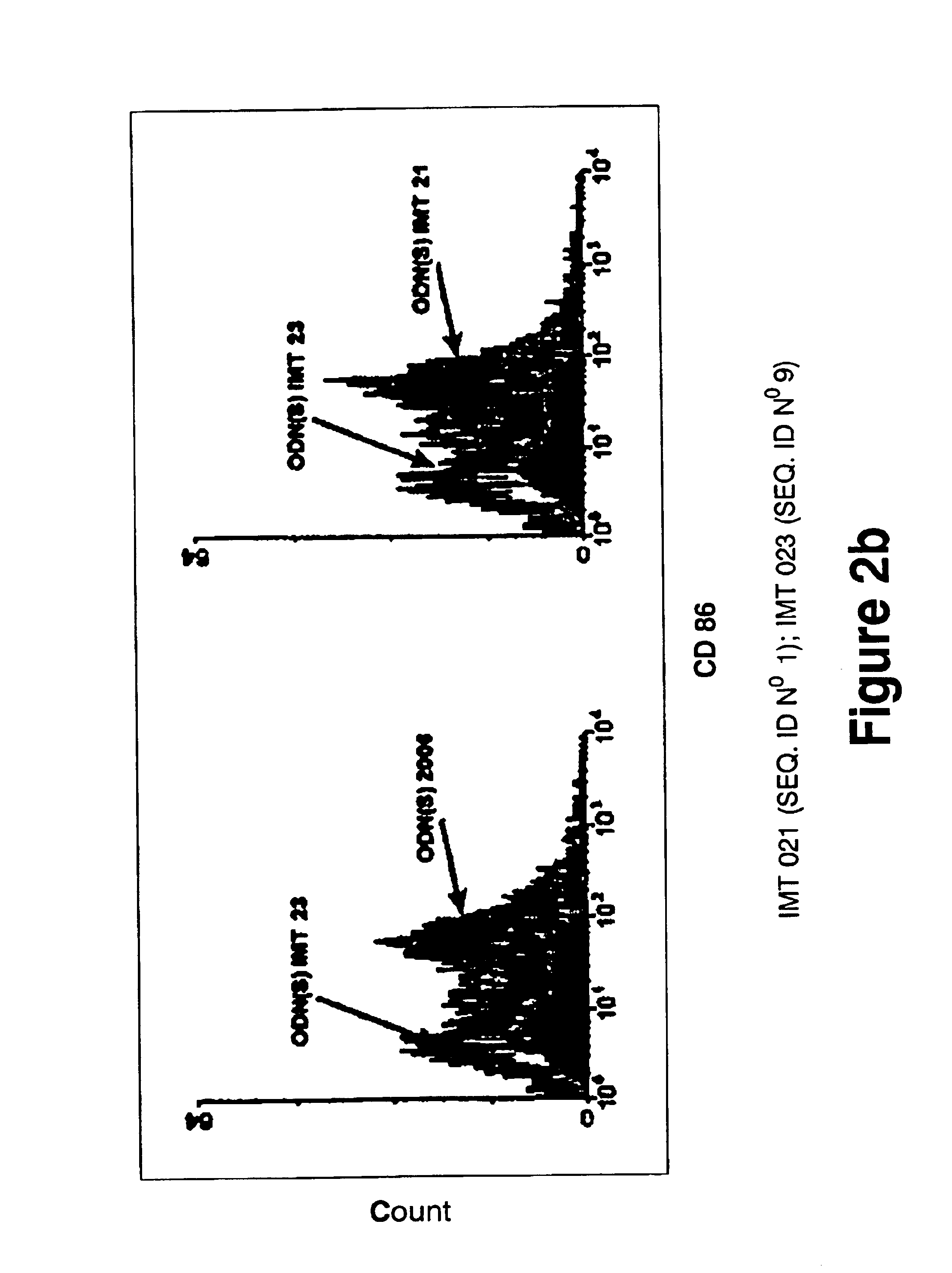
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Vaccines comprising aluminium adjuvants and histidine
InactiveUS20050158334A1Easy to controlMinimise adsorptionAntibacterial agentsOrganic active ingredientsAntigenAdjuvant
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Viable non-toxic gram-negative bacteria
ActiveUS20100272758A1Antibacterial agentsBacterial antigen ingredientsEscherichia coliLipopolysaccharide
The present invention provides non-toxic Gram-negative bacteria. In particular, the present invention provides viable Gram-negative bacteria (e.g., E. coli) substantially lacking lipopolysaccharide (LPS, endotoxin) within the outer membrane. The present invention further provides methods of generating viable non-toxic Gram-negative bacteria and uses thereof. The present invention also provides compositions and methods for inducing immune responses and for researching and developing therapeutic agents.
Owner:RES CORP TECH INC
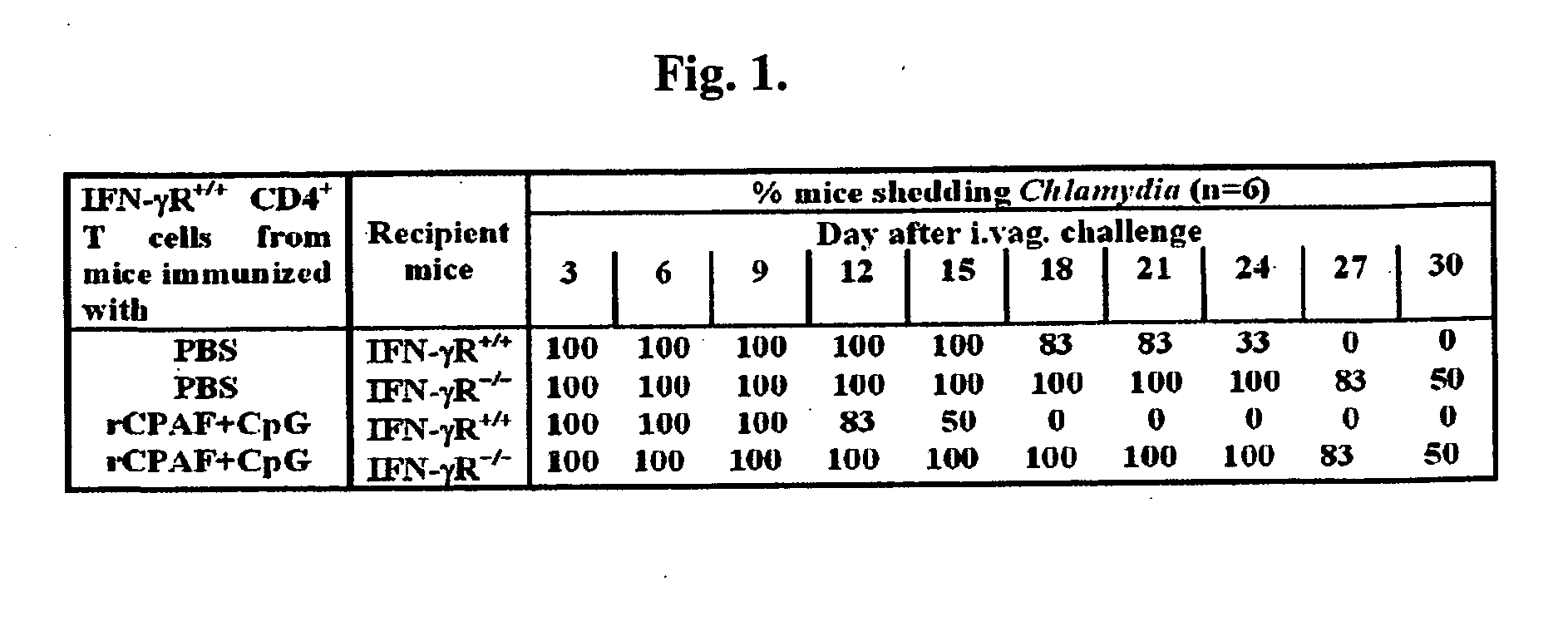
Methods and compositions for immunization against chlamydial infection and disease
ActiveUS20090098165A1Reduce morbidityReduce the possibilityAntibacterial agentsBacterial antigen ingredientsCHLAMYDIAL INFECTIONSDisease cause
The present invention provides Chlamydia proteins and compositions and methods of use in the treatment / prevention of chlamydial infection in a subject, for eliciting an immune response in a subject and for reducing the likelihood of infertility and reducing the incidence and / or degree of hydrosalpinx due to Chlamydia infection in a subject.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
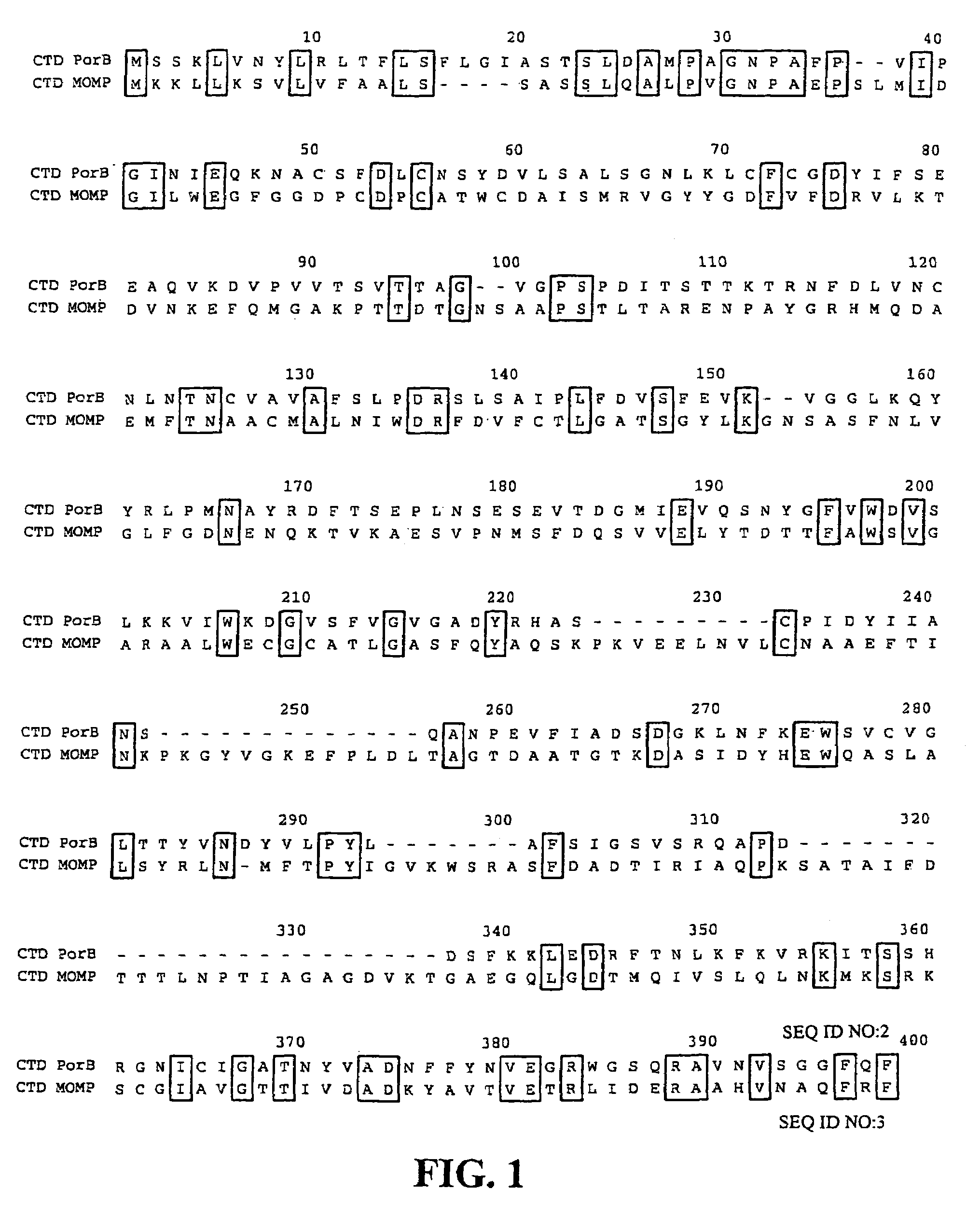
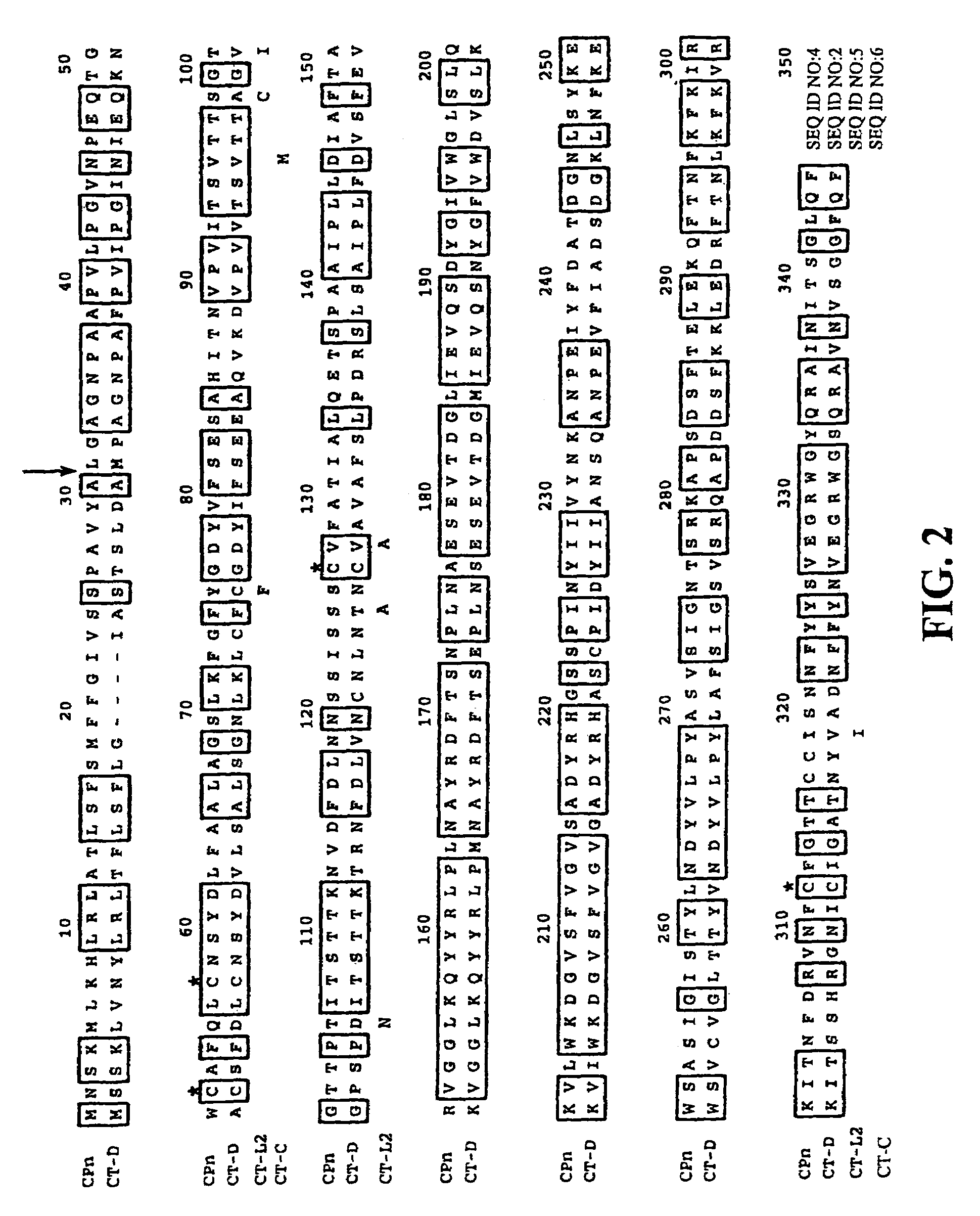
Porin B (PorB) as a therapeutic target for prevention and treatment of infection by Chlamydia
InactiveUS7105171B2Reduce the binding forceNeutralize infectivityAntibacterial agentsBiocideEpitopeNeutralizing antibody
The present invention features peptides of a PorB polypeptide, which PorB peptides are useful in production of antibodies that bind the full-length PorB polypeptide and as a therapeutic agent. In specific embodiments the invention features a composition comprising one or more PorB peptides (other than a full-length PorB polypeptide), which peptides contain at least one epitope that can elicit Chlamydia-neutralizing antibodies. The invention also features methods for induction of a protective immune response against infection by Chlamydia and Chlamydiophila.
Owner:RGT UNIV OF CALIFORNIA
Vaccines against chlamydial infection
InactiveUS20090022755A1Stimulate immune responseProvide protectionBacterial antigen ingredientsChlamydiaceae ingredientsPolynucleotideChlamydia sp
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:CORIXA CORP +1
Chlamydia antigens and corresponding DNA fragments and uses thereof
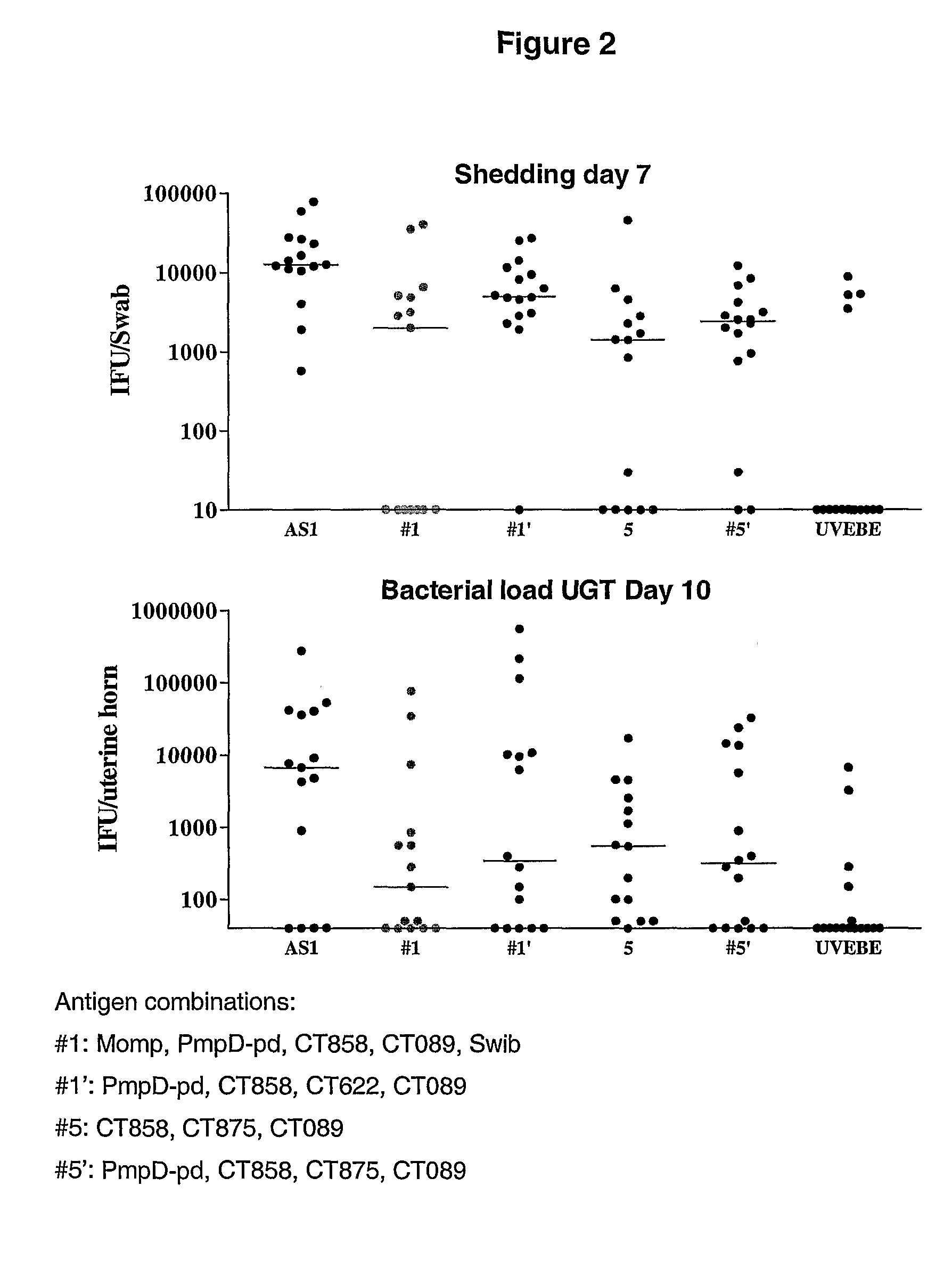
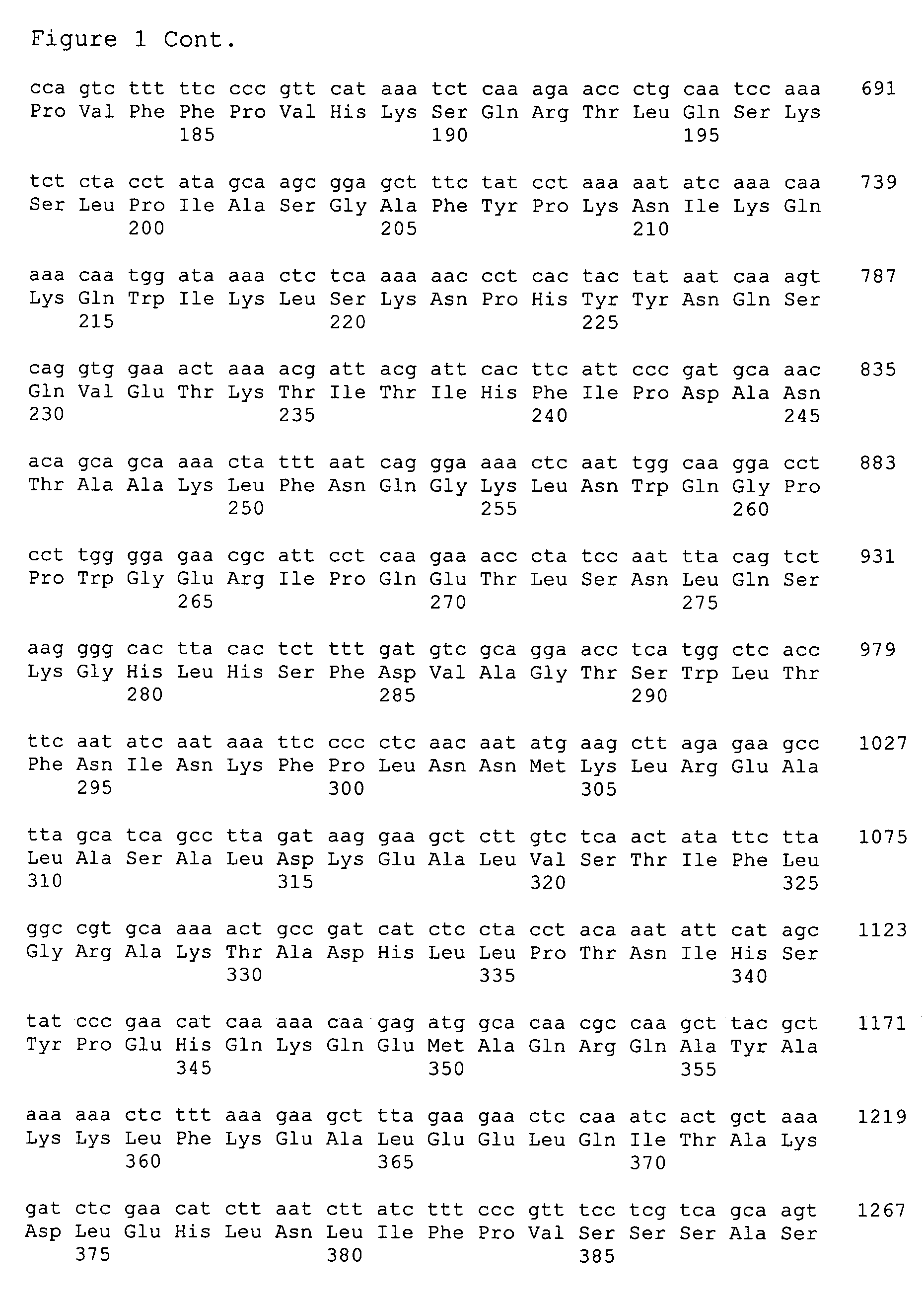
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae. The method employs a vector containing a nucleotide sequence encoding an ATP-binding cassette of a strain of Chlamydia pneumoniae and a promoter to effect expression of the ATP-binding cassette gene product in the host. Modifications are possible within the scope of this invention.
Owner:SANOFI PASTEUR LTD
Vaccine composition
The present invention relates to the field of Gram-negative bacterial vaccine compositions, their manufacture, and the use of such compositions in medicine. More particularly it relates to the field of useful Gram-negative bacterial outer membrane vesicle (or bleb) compositions comprising heterologously expressed Chlamydia antigens, and advantageous methods of rendering these compositions more effective and safer as a vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
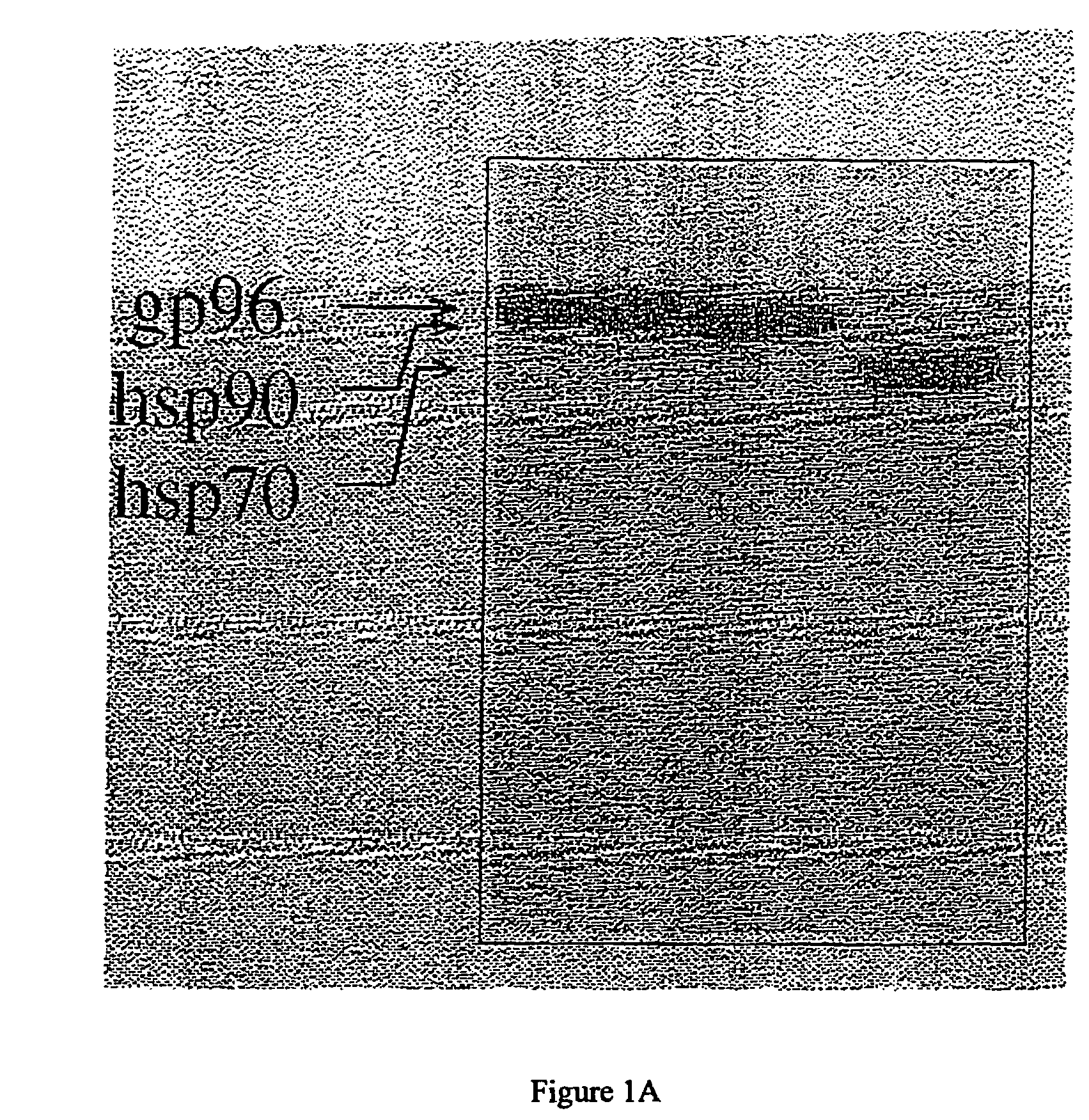
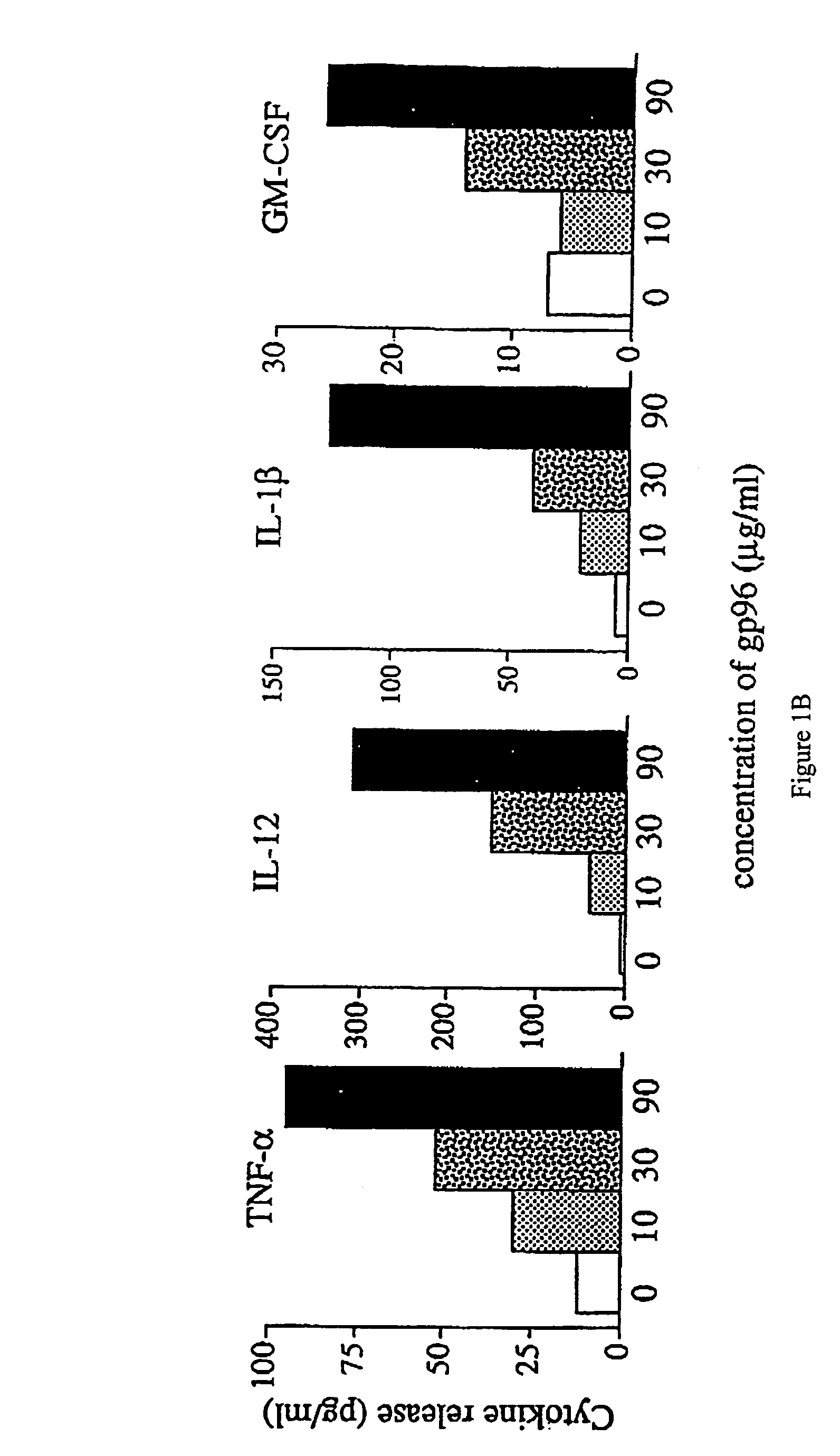
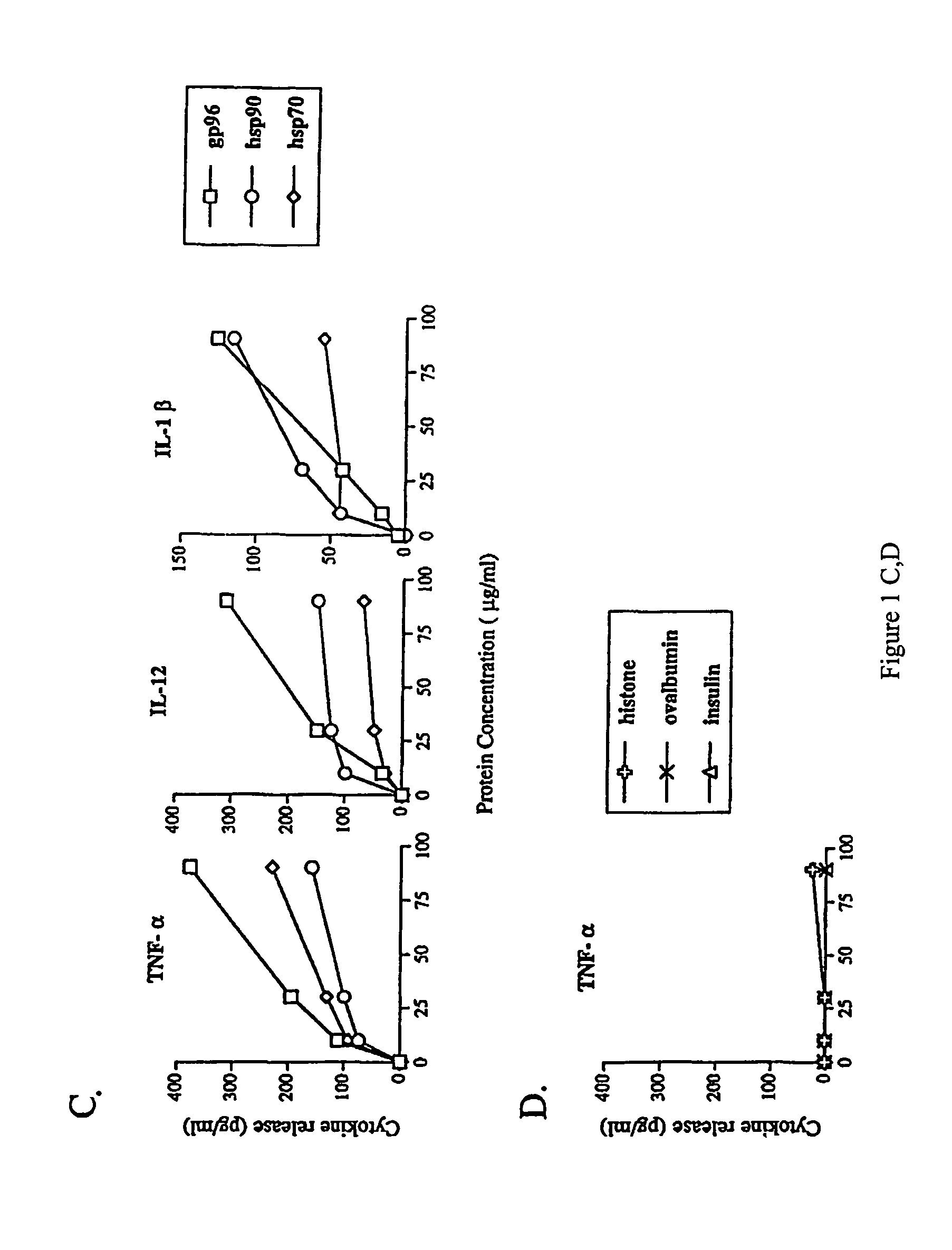
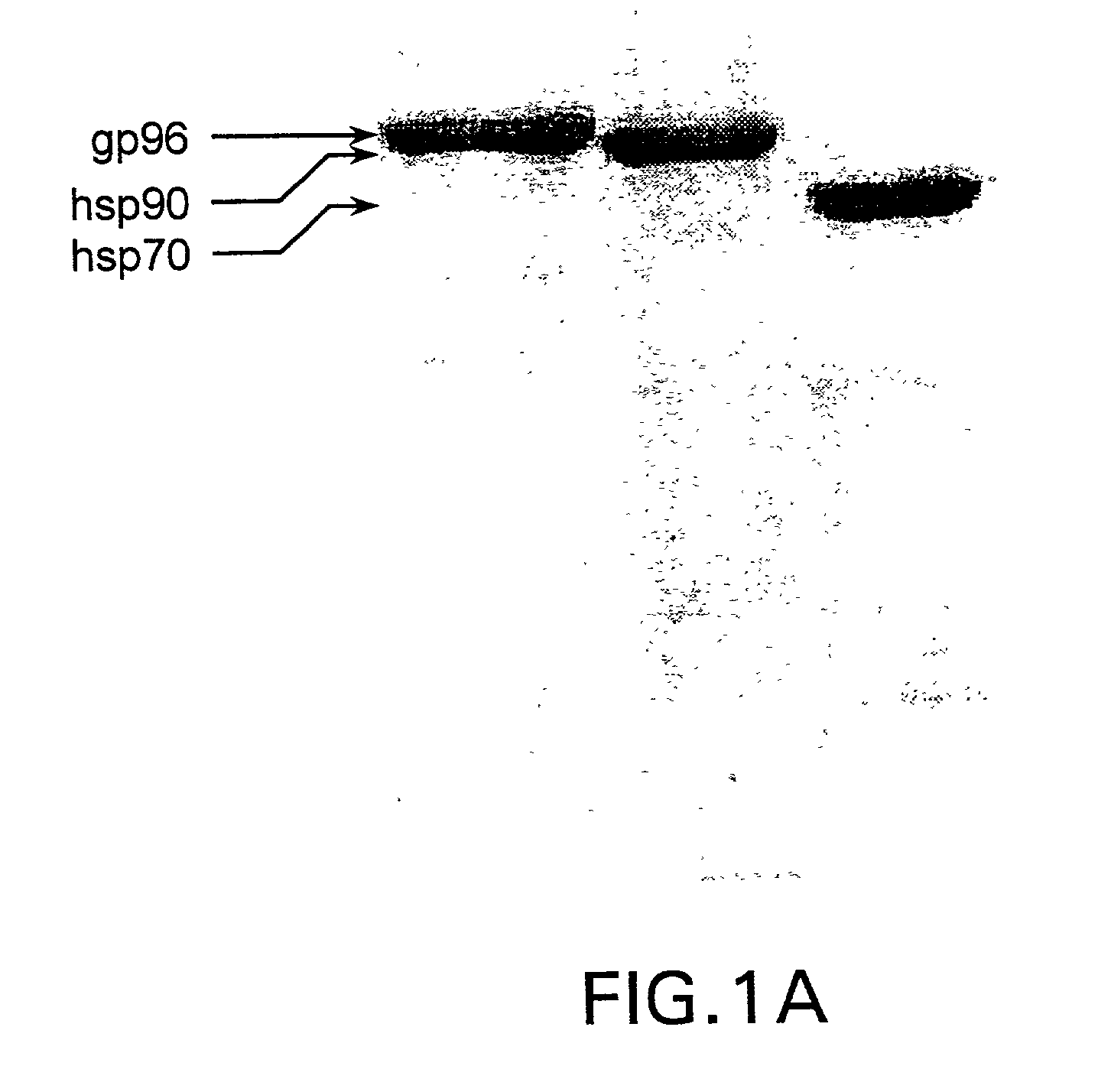
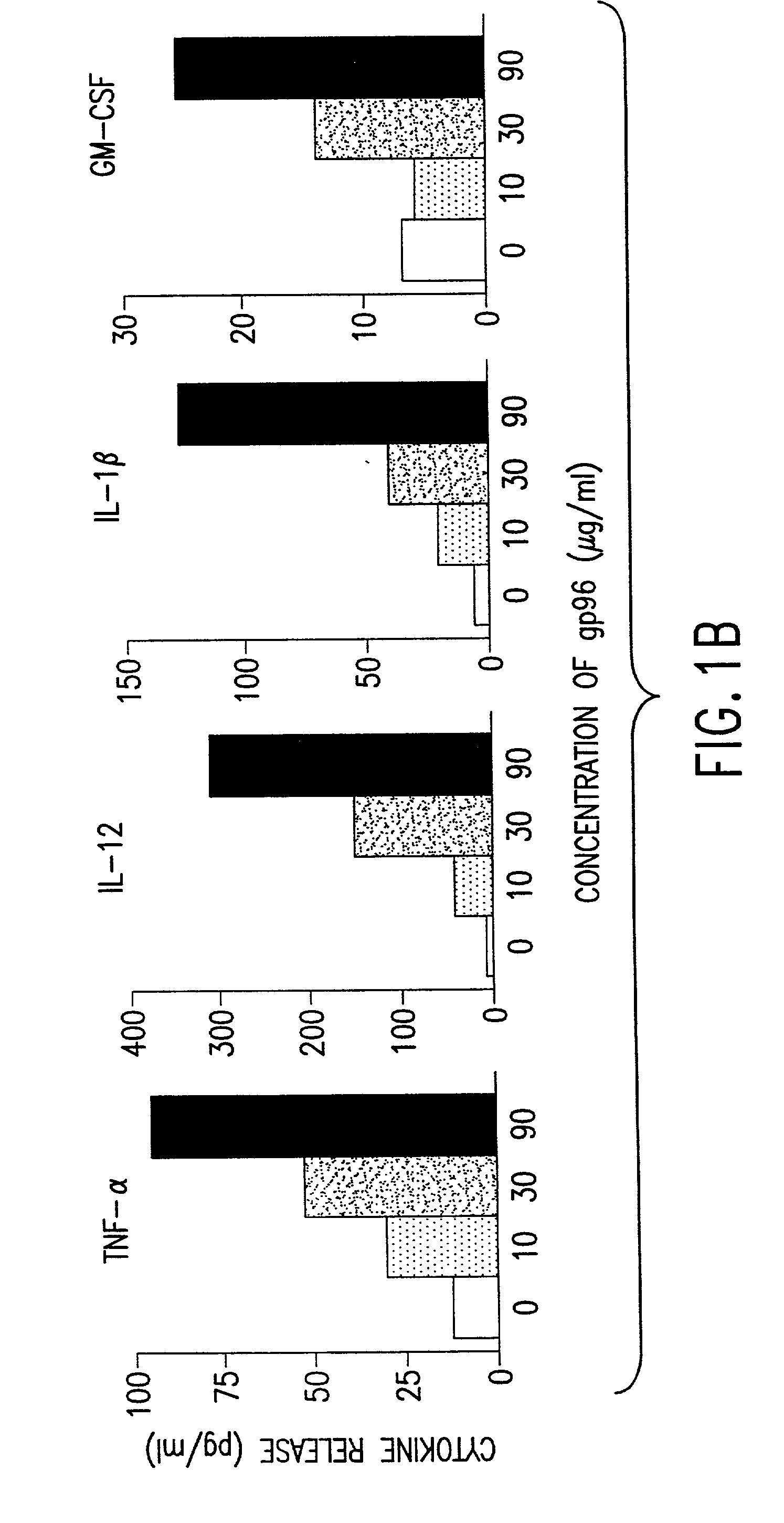
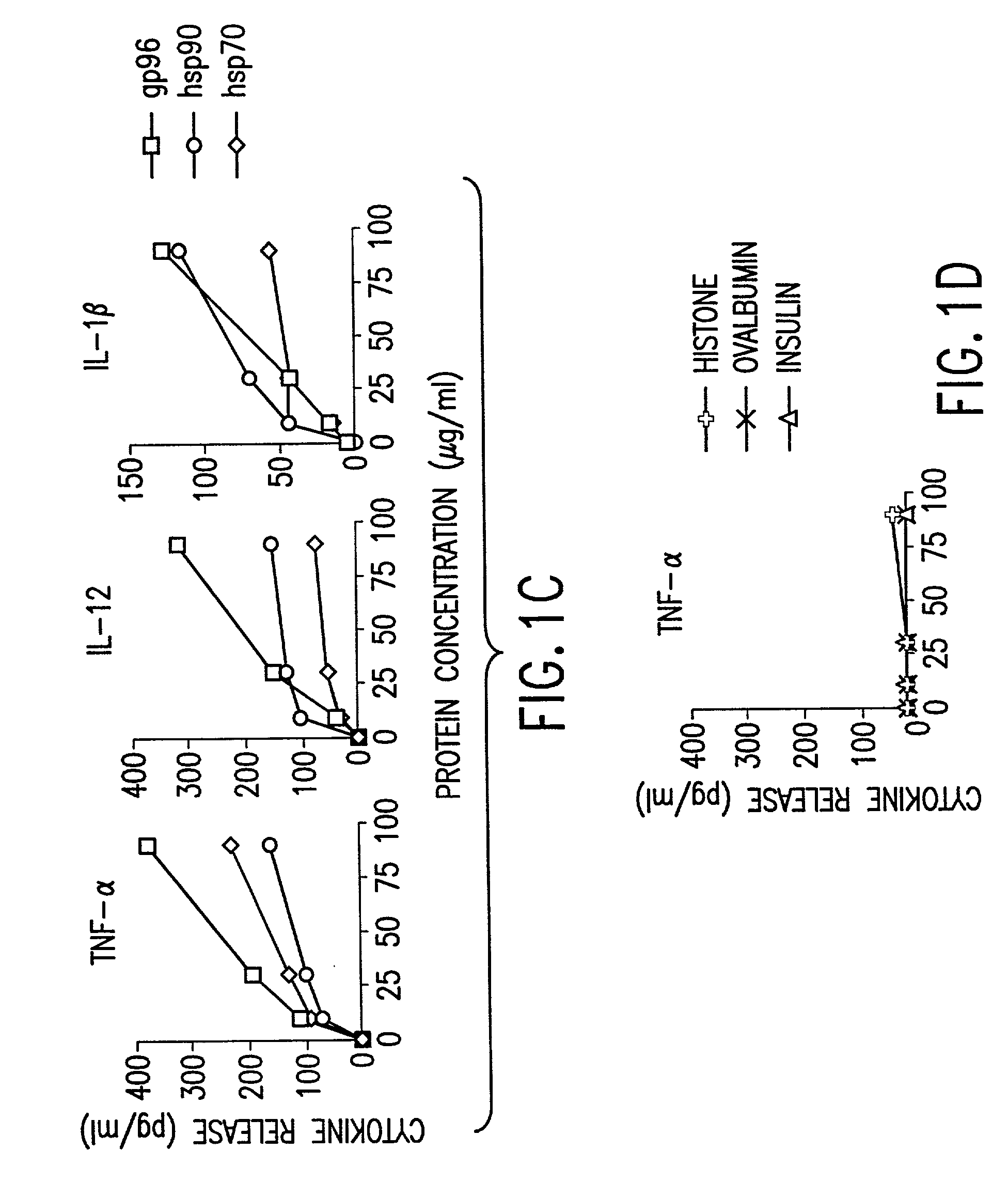
Using heat shock proteins to increase immune response
The present invention provides for a method of using heat shock proteins (HSPs) to amplify the immune response initiated by a vaccine. HSPs can be introduced into a subject before, concurrently, or after the administration of a vaccine. The HSPs can also be used to activate antigen presenting cells which are then introduced into a subject in conjunction with a vaccine. The HSPs used in the methods of the invention can be unbound or can be covalently or noncovalently bound to a peptide that is unrelated to the vaccine. The subject is preferably mammalian, and most preferably human. It is shown by way of example herein that HSPs induces secretion of cytokines and surface expression of antigen-presenting and co-stimulatory molecules. The invention also encompasses methods of treatment and prevention of cancer and infectious diseases in a subject.
Owner:UNIV OF CONNECTICUT HEALTH CENT
Using heat shock proteins to increase immune response
InactiveUS20020172682A1Improve treatment outcomesImprove efficiencyAntibacterial agentsBacterial antigen ingredientsAntigenVaccination
The present invention provides for a method of using heat shock proteins (HSPs) to amplify the immune response initiated by a vaccine. HSPs can be introduced into a subject before, concurrently, or after the administration of a vaccine. The HSPs can also be used to activate antigen presenting cells which are then introduced into a subject in conjunction with a vaccine. The HSPs used in the methods of the invention can be unbound or can be covalently or noncovalently bound to a peptide that is unrelated to the vaccine. The subject is preferably mammalian, and most preferably human. It is shown by way of example herein that HSPs induces secretion of cytokines and surface expression of antigen-presenting and co-stimulatory molecules. The invention also encompasses methods of treatment and prevention of cancer and infectious diseases in a subject.
Owner:UNIV OF CONNECTICUT HEALTH CENT
Compounds and methods for treatment and diagnosis of Chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
Methods and composition for oral vaccination
InactiveUS20060171960A1Provide protectionBacterial antigen ingredientsDispersion deliveryFlavorVaccination
The present invention encompasses methods and compositions both for providing protection against disease in an animal and for inducing increased intake of an orally administered vaccine by an animal. The methods of the invention are directed to admixing a bacterial or viral antigen with a water soluble palatable flavorant, further admixing the antigen and flavorant mixture with a water soluble vehicle for oral administration of the vaccine to an animal in order to provide protection against disease associated with infection by the admixed antigen and to induce the increased intake of the vaccine with the flavorant. The present invention thus encompasses methods and compositions for the oral vaccination of healthy animals through drinking water or syrups as an aid in the prevention of disease. The admixing of the palatable flavorant provides for a vaccine formulation with a desirable taste in order to promote self-administration of the vaccine formulation and / or to prevent rejection of the formulation when administered by an animal handler.
Owner:WYETH LLC
Methods for producing liposomes
InactiveUS20140112979A1Easy to handleEnhance immune responseSsRNA viruses negative-senseBiocideHigh concentrationLiposome
The invention discloses a method for the formation of liposomes by using high shear mixing of aqueous solution of lipid powder; the lipid powder can be produced by any known technique. Components of the liposomes include but are not limited to cationic lipids, immunostimulatory / immunopotentiators and macromolecules as components for the liposome formation. The disclosed method describes the formulation of stable liposomes solitary or complexing high concentrations of macromolecules such as proteins, DNA and RNA having opposite charge of the liposomes by high shear mixing where aggregation is avoided due to the formulation method.
Owner:STATENS SERUM INST
Compounds and methods for treatment and diagnosis of chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
Porin B (PorB) as a therapeutic target for prevention and treatment of infection by Chlamydia
The present invention features peptides of a PorB polypeptide, which PorB peptides are useful in production of antibodies that bind the full-length PorB polypeptide and as a therapeutic agent. In specific embodiments the invention features a composition comprising one or more PorB peptides (other than a full-length PorB polypeptide), which peptides contain at least one epitope that can elicit Chlamydia-neutralizing antibodies. The invention also features methods for induction of a protective immune response against infection by Chlamydia and Chlamydiophila.
Owner:RGT UNIV OF CALIFORNIA
Porin B (PorB) as a therapeutic target for prevention and treatment of infection by chlamydia
InactiveUS6899880B2Reduce the binding forceNeutralize infectivityBiocideCompound screeningTreatment chlamydiaChlamydia
The present invention features the use of PorB polypeptide as a therapeutic agent. In specific embodiment the invention features a chlamydial vaccine based on a PorB polypeptide, as well as methods for induction of a protective immune response against infection by Chlamydia and Chlamydiophila. The invention further features methods for identifying agents that affect PorB function such as in transport of α-ketoglutarate and which are effective as anti-chlamydial chemotherapeutic agents.
Owner:RGT UNIV OF CALIFORNIA
Salmonella vectored vaccines against Chlamydia and methods of use
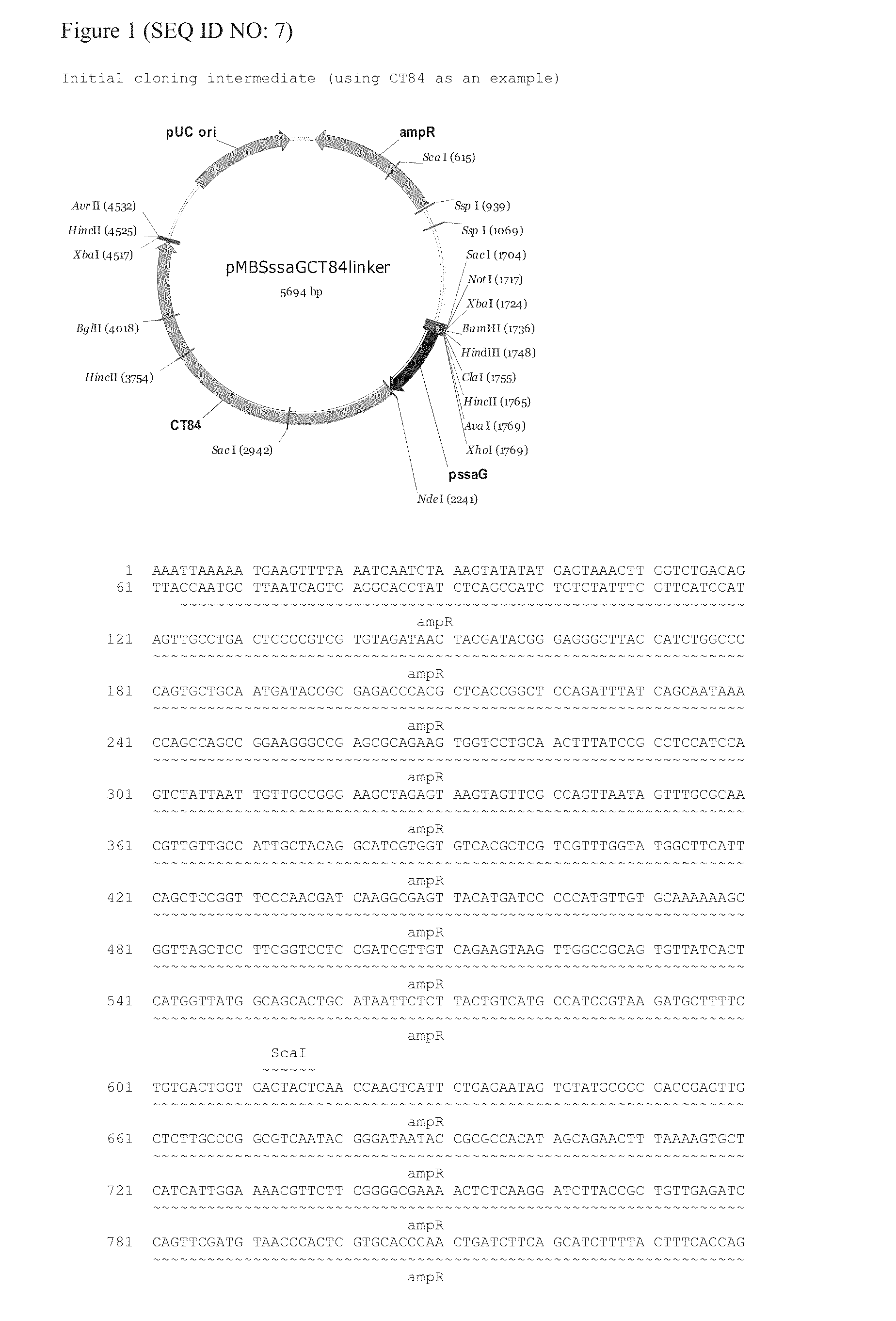
The invention provides an attenuated Salmonella vaccine vector comprising one or more heterologous polynucleotides that encode immunogenic Chlamydial peptides. In one embodiment, the attenuated Salmonella vaccine vector comprises aroC and ssaV attenuating mutations. The heterologous polynucleotides encoding the immunogenic Chlamydial peptides can be under the control of an inducible promoter such as a Salmonella ssaG promoter. In one embodiment of the invention, the immunogenic Chlamydial peptide is a PmpG peptide, for instance, a CT110, CT84 or CT40 peptide.
Owner:PROKARIUM
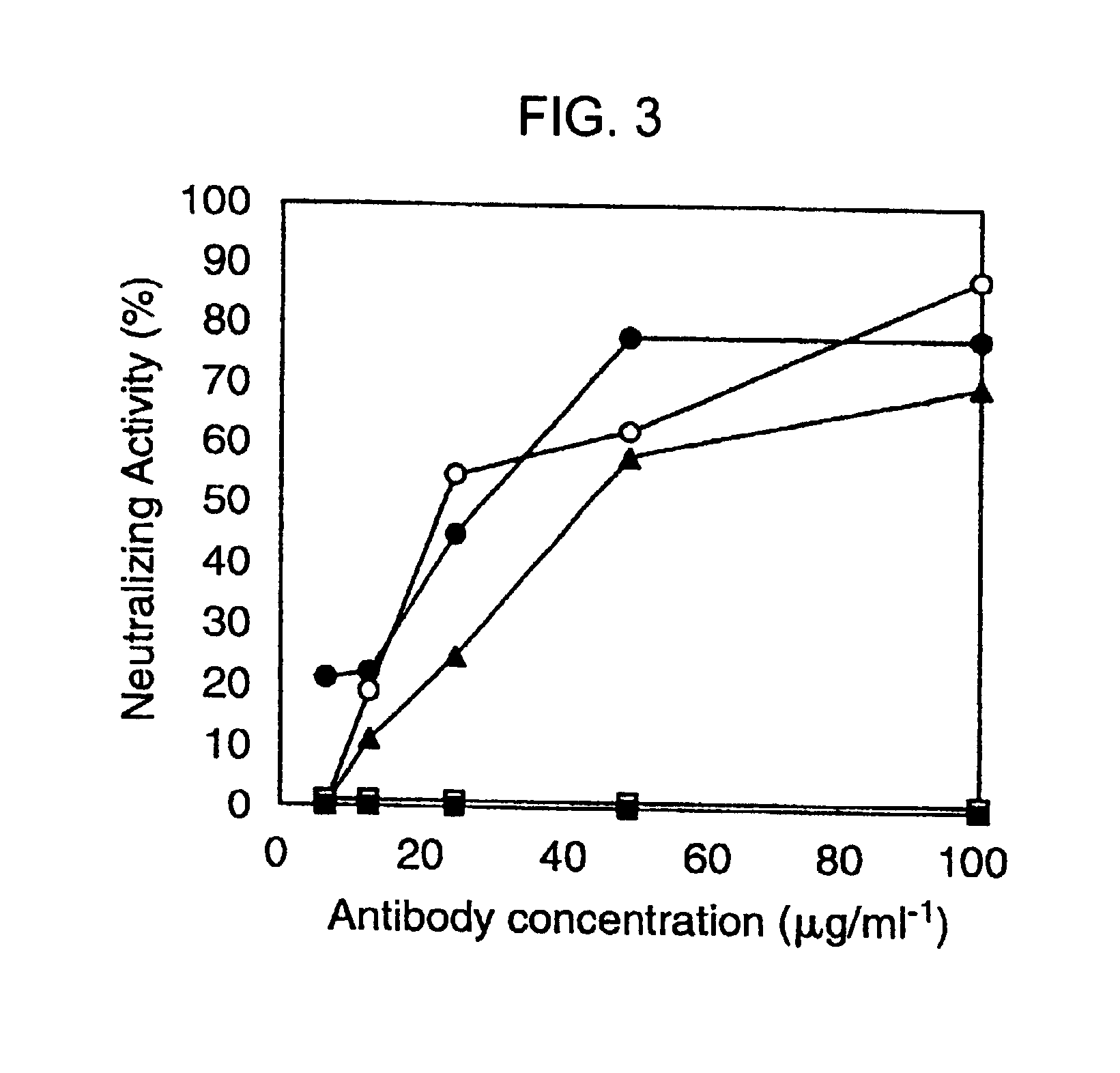
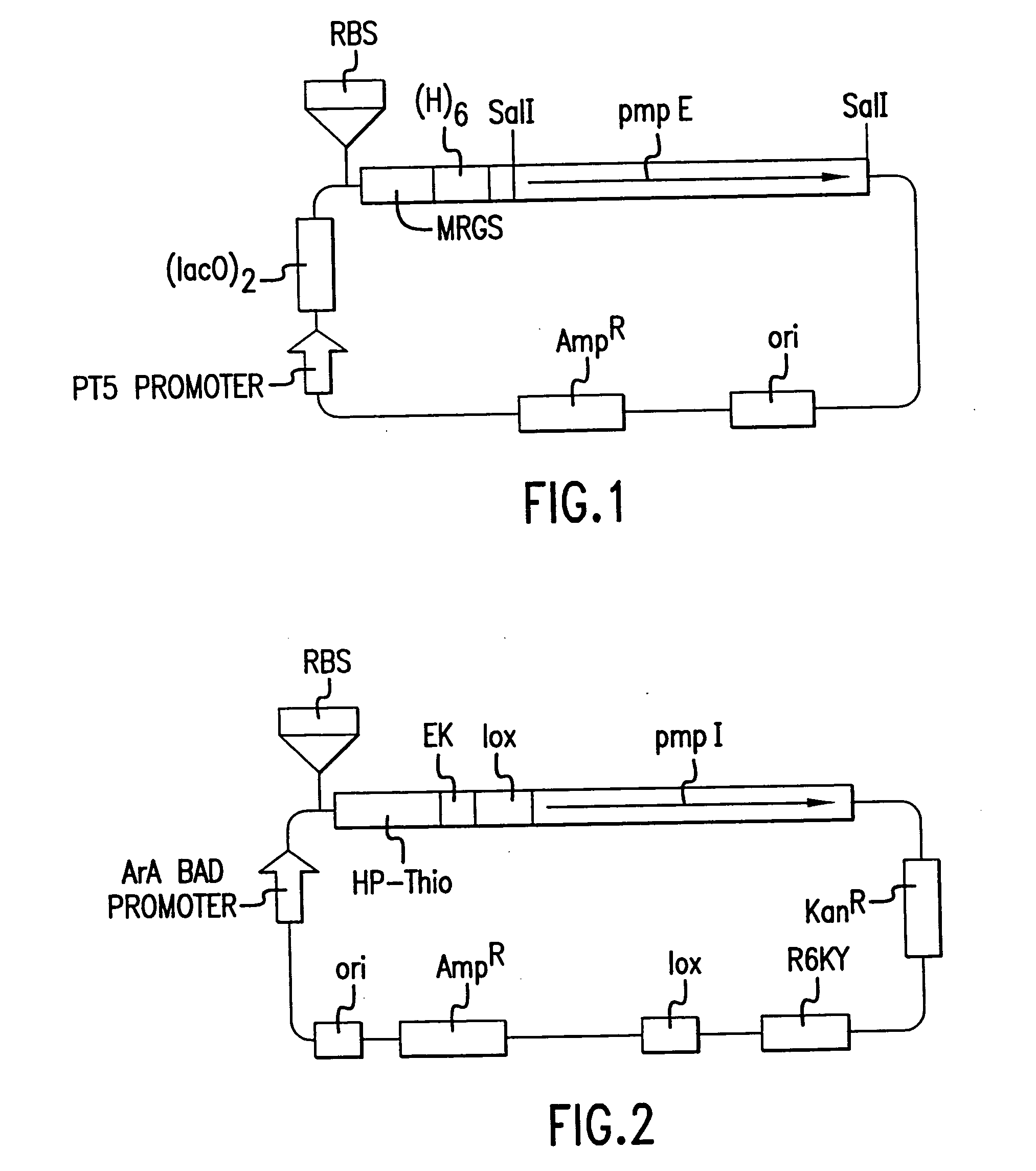
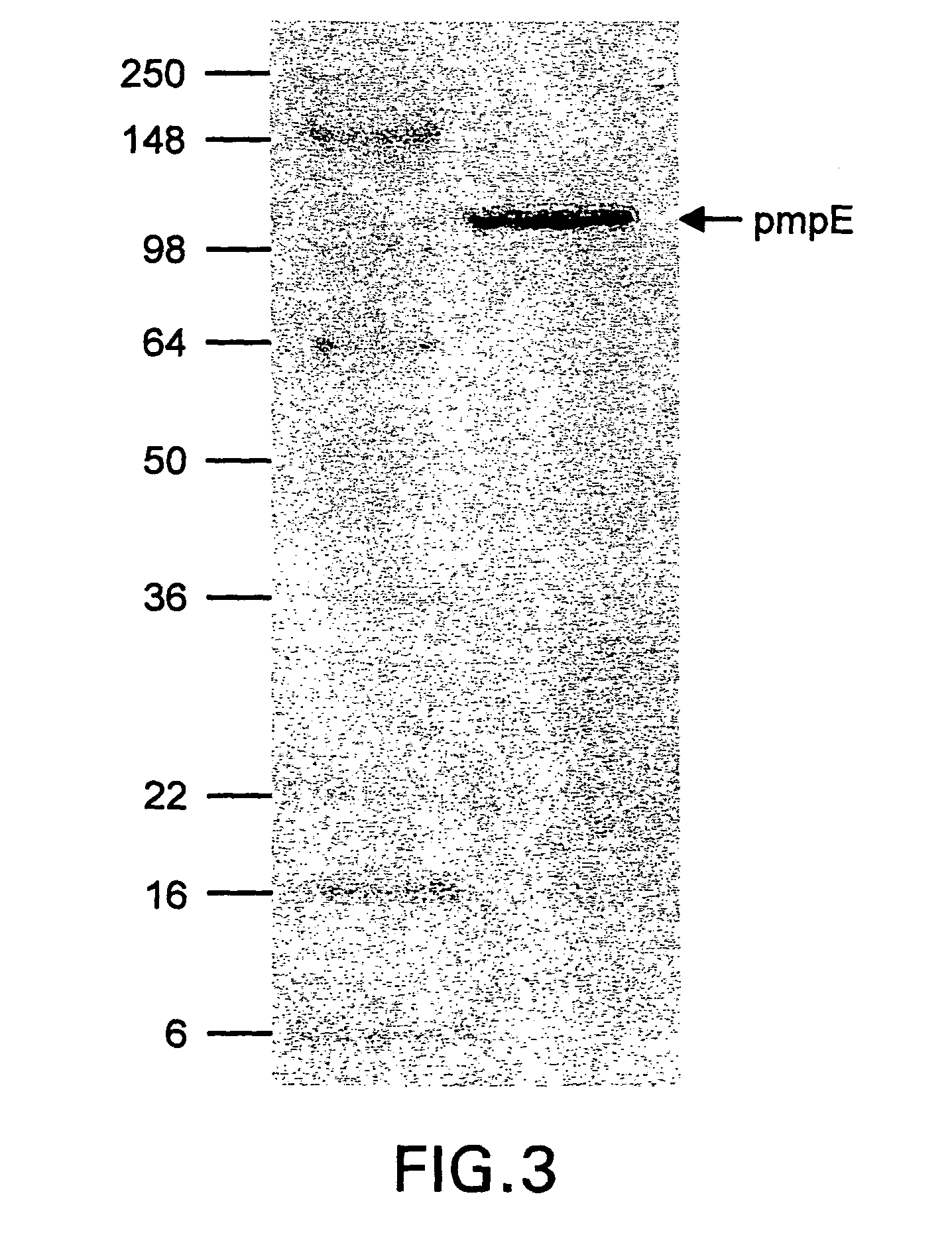
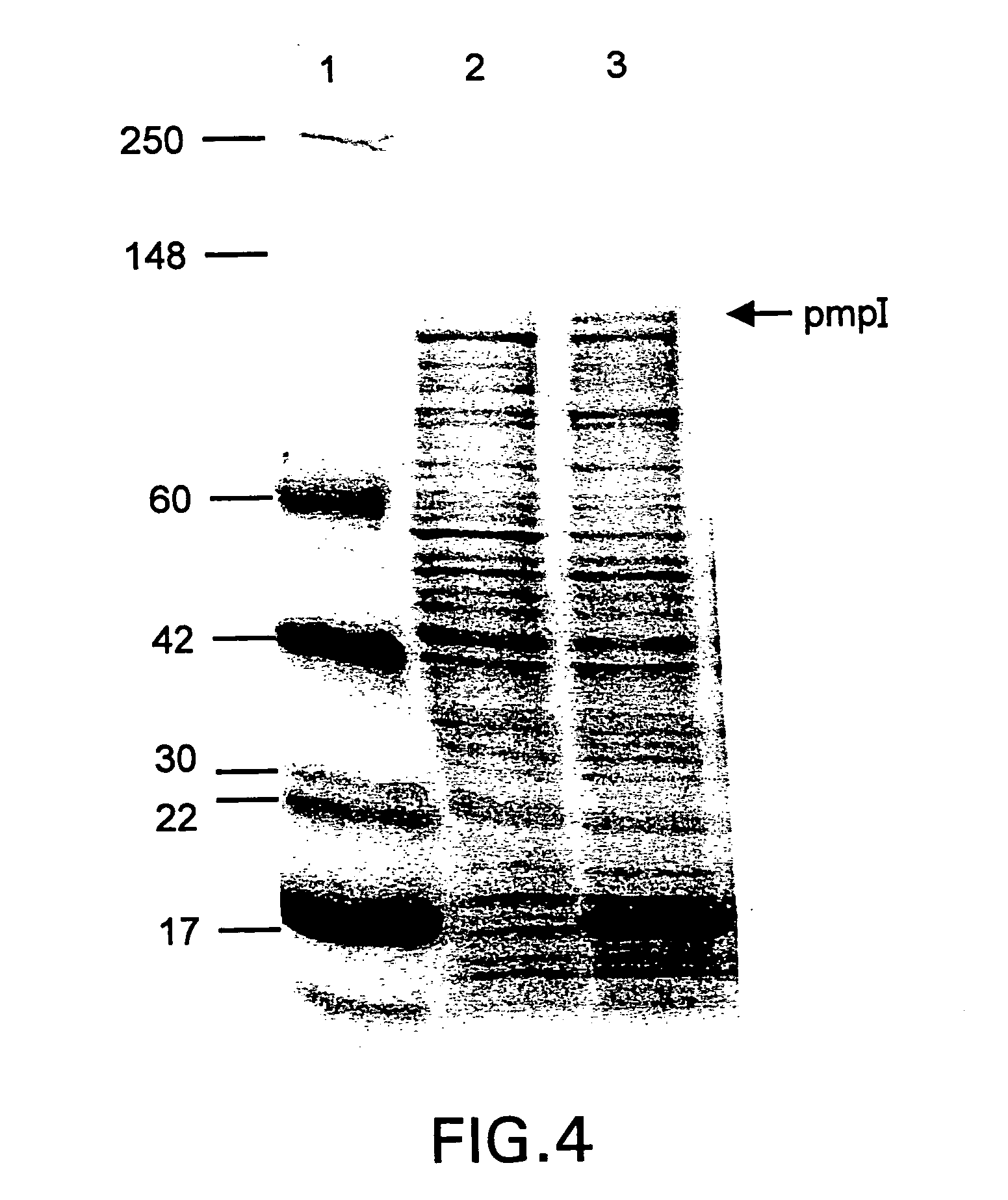
Chlamydia pmp proteins, gene sequences and uses thereof
InactiveUS20040037846A1Compounds screening/testingAntibacterial agentsChlamydiaceae InfectionsNucleotide
The invention discloses the Chlamydia PMPE and PMPI polypeptide, polypeptides derived therefrp, (PMP-derived polypeptides), nucleotide sequences encoding said polypeptides, antibodies that specifically bind the PMP polypeptides and PMP-derived polypeptides and T-cells specific for PMP polypeptides and PMP-derived polypeptides. Also disclosed are prophylactic and therapeutic compositions, including immunogenic compositions, e.g., vaccines, comprising PMP polypeptides or PMP-derived polypeptides or antibodies thereto. The invention additionally discloses methods of inducing in animals an immune response to Chlamydia cells, Chlamydia elementary bodies, and / or cells expressing Chlamydial proteins, e.g., cell infected with Chlamydia.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
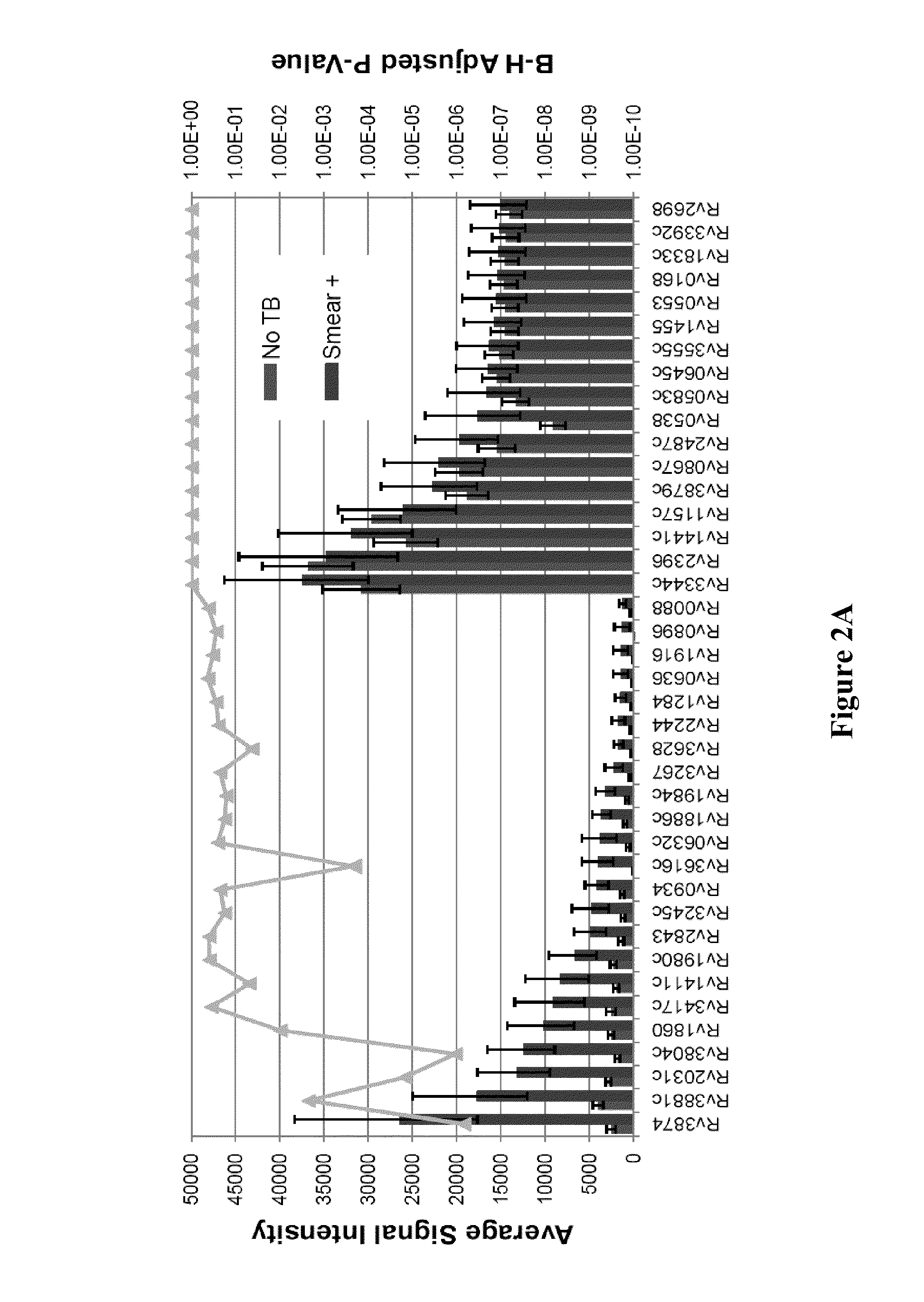
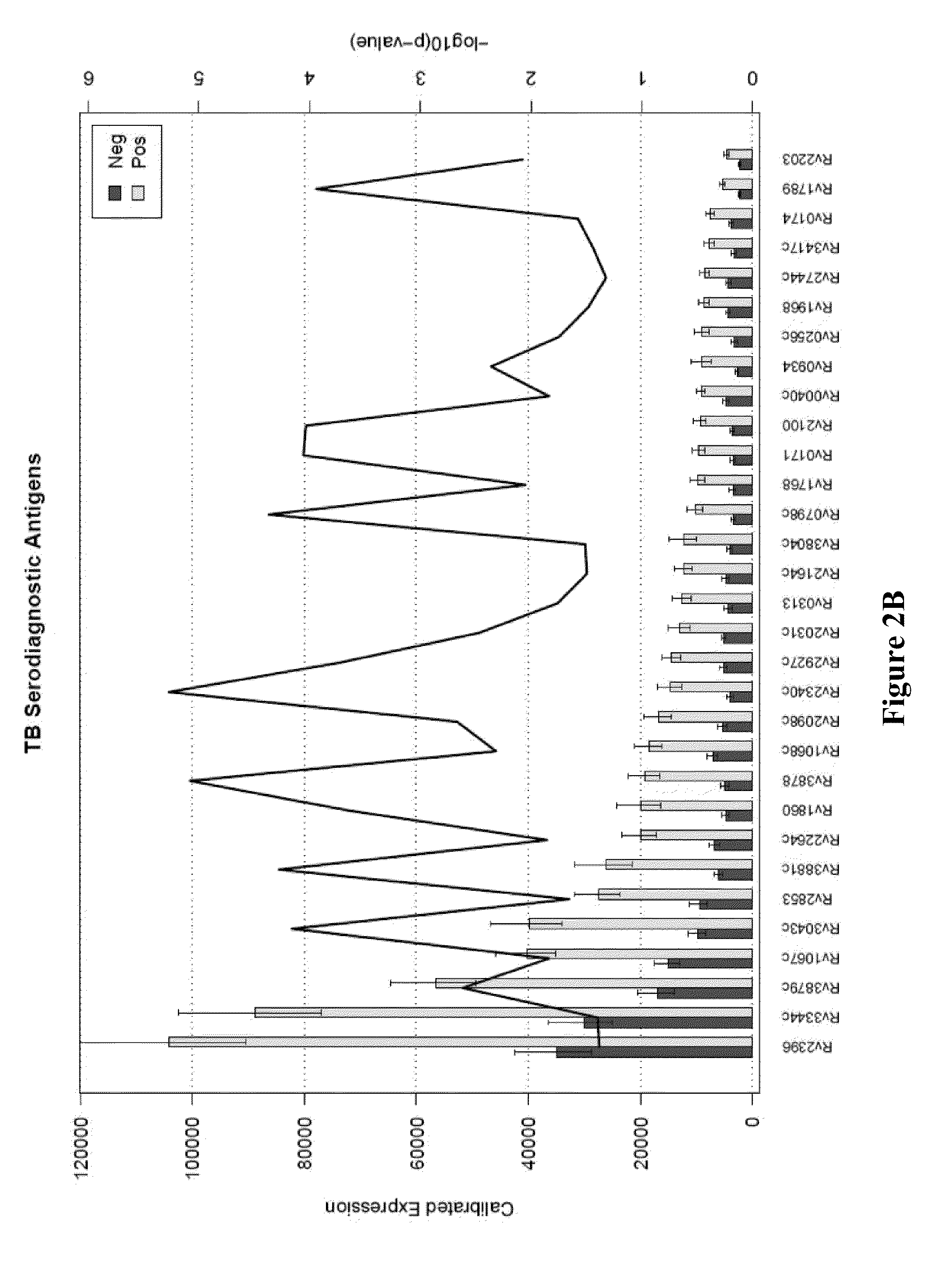
Compositions and methods for immunodominant antigens of Mycobacterium tuberculosis
Contemplated compositions, devices, and methods are drawn to various antigens from the pathogen M. tuberculosis and their use in vaccines, therapeutic agents, and various diagnostic tests. In particularly preferred aspects, the antigens are immunodominant and have quantified and known relative reactivities with respect to sera of a population infected with the pathogen, and / or have a known association with a disease parameter.
Owner:RUTGERS THE STATE UNIV +1
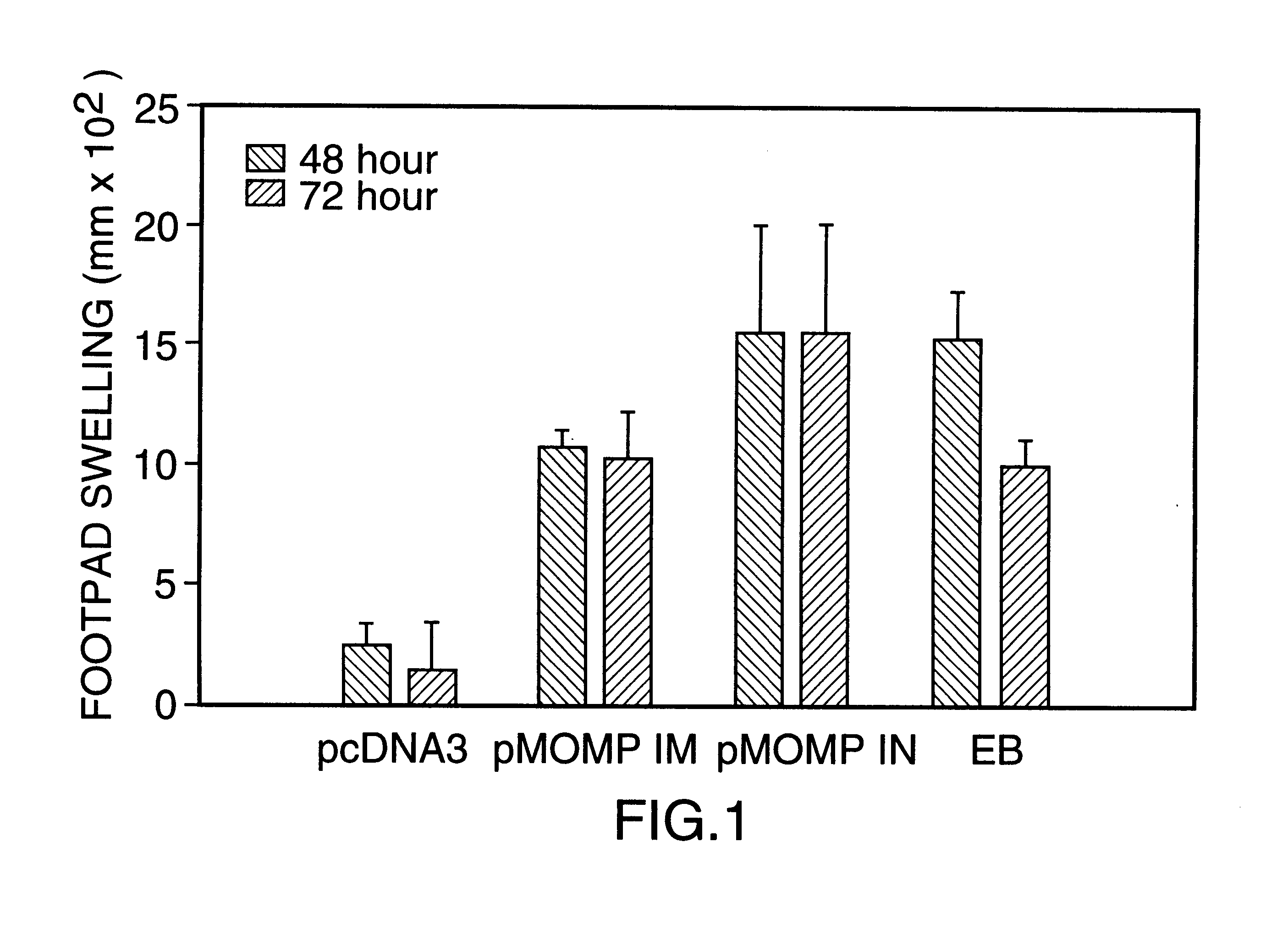
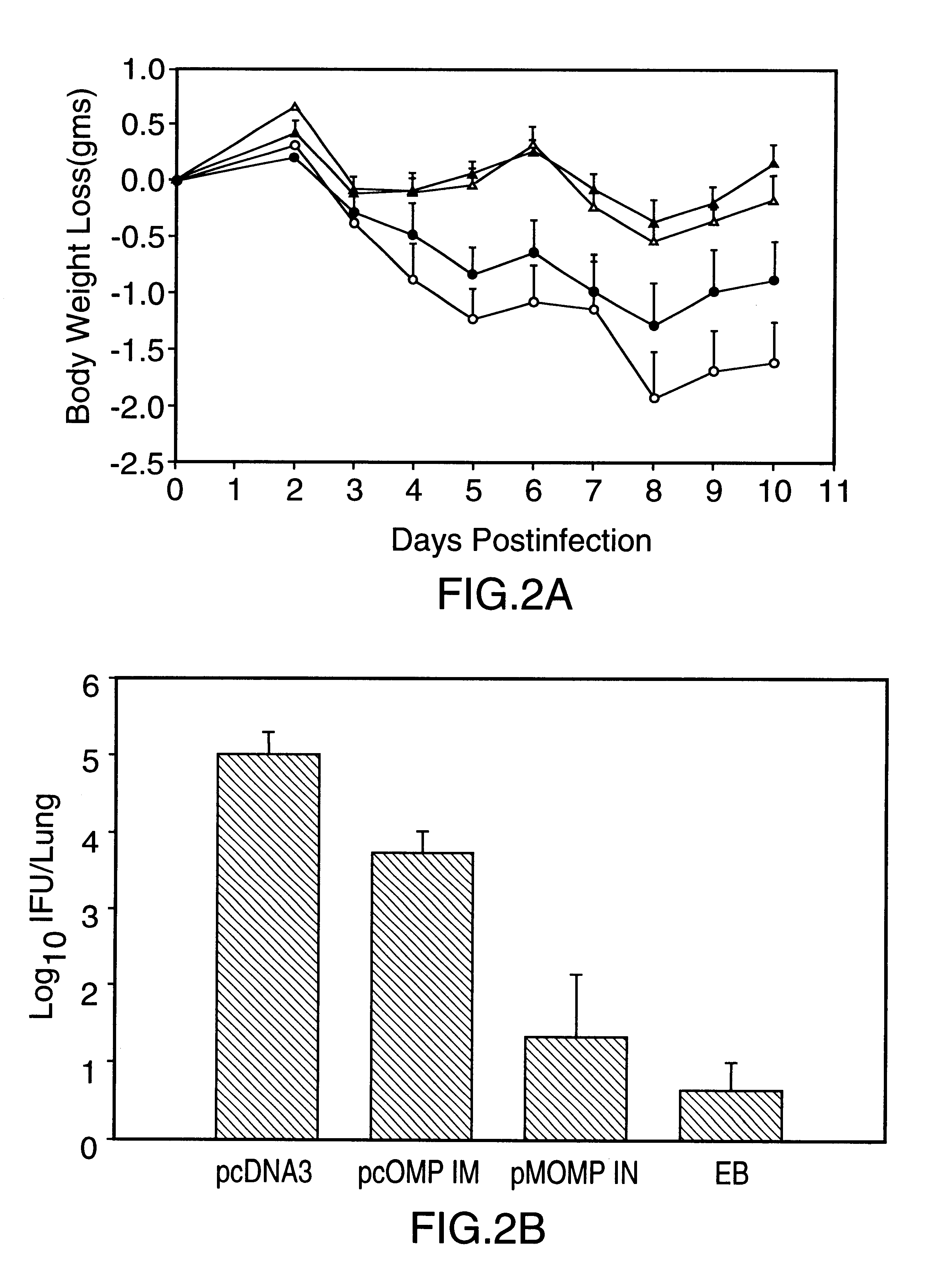
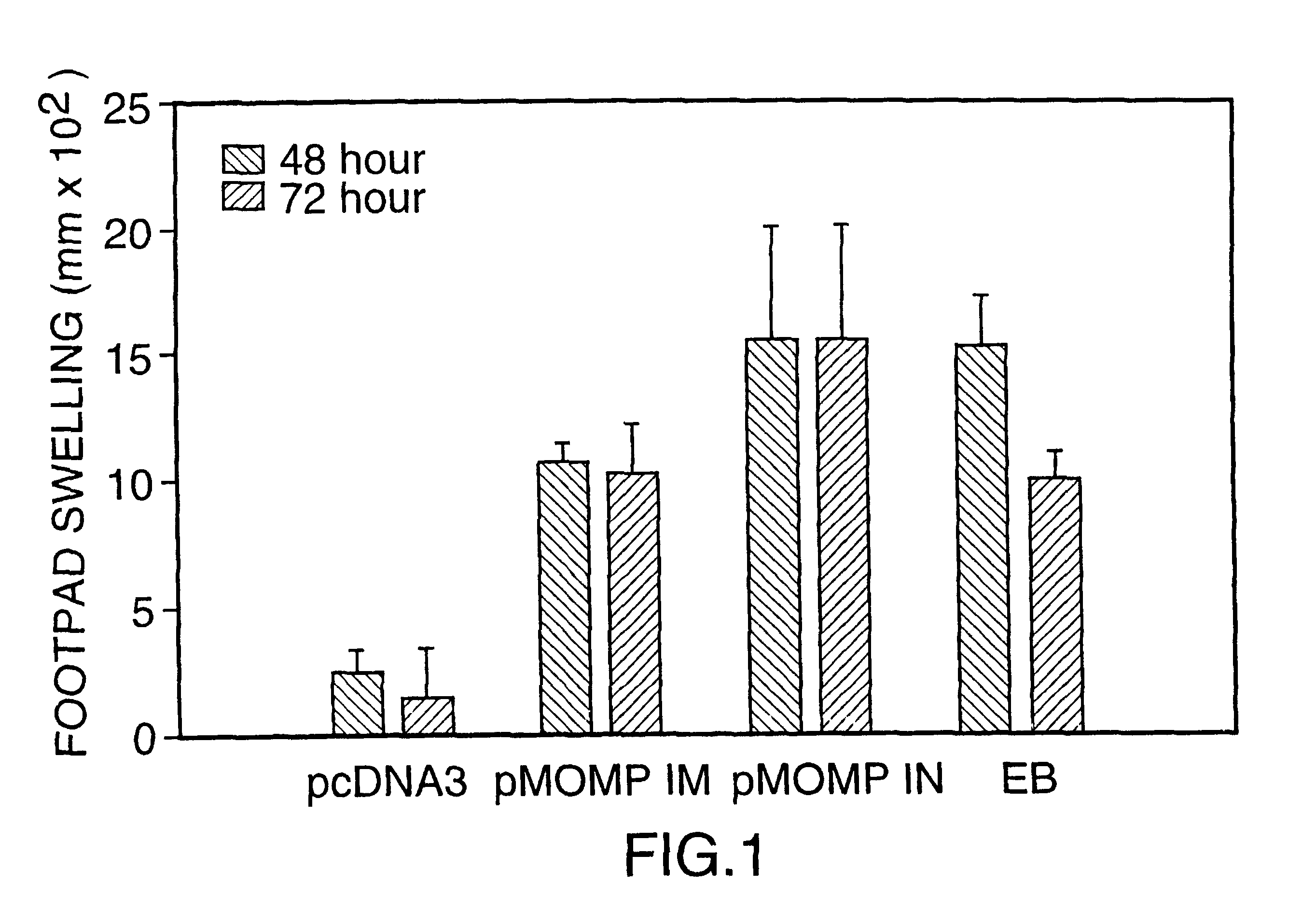
DNA immunization against chlaymdia infection
InactiveUS6235290B1Effectively induces protective immunityGood curative effectAntibacterial agentsVirusesDna immunizationIn vivo
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.
Owner:MANITOBA UNIV OF THE
Oil-based adjuvants
The instant invention provides various formulations comprising combinations of immunostimulating oligonucleotides, polycationic carriers, sterols, saponins, quaternary amines, TLR-3 agonists, glycolipids, and MPL-A or analogs thereof in oil emulsions, use thereof in preparations of immunogenic compositions and vaccines, and use thereof in the treatment of animals.
Owner:ZOETIS SERVICE LLC
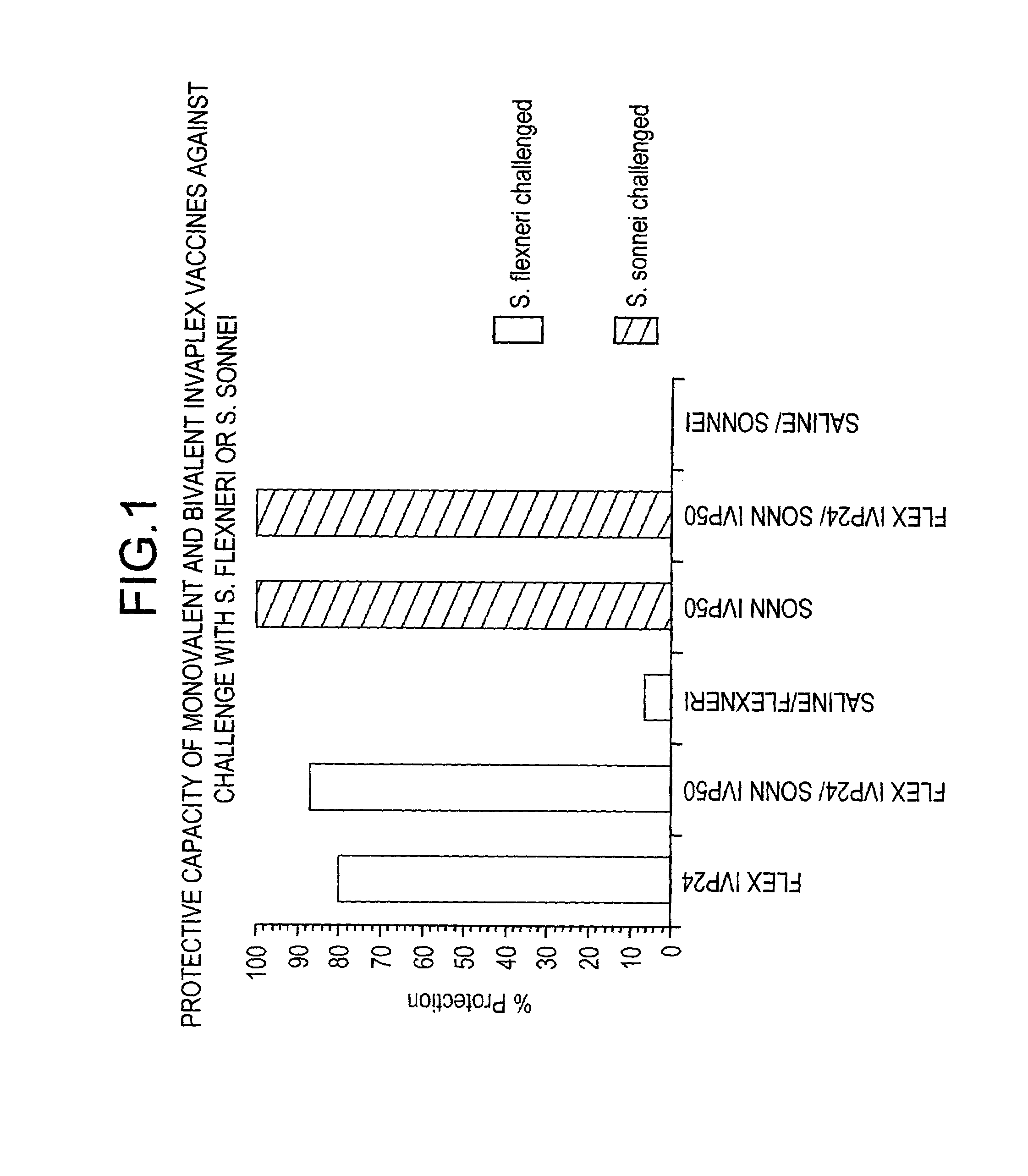
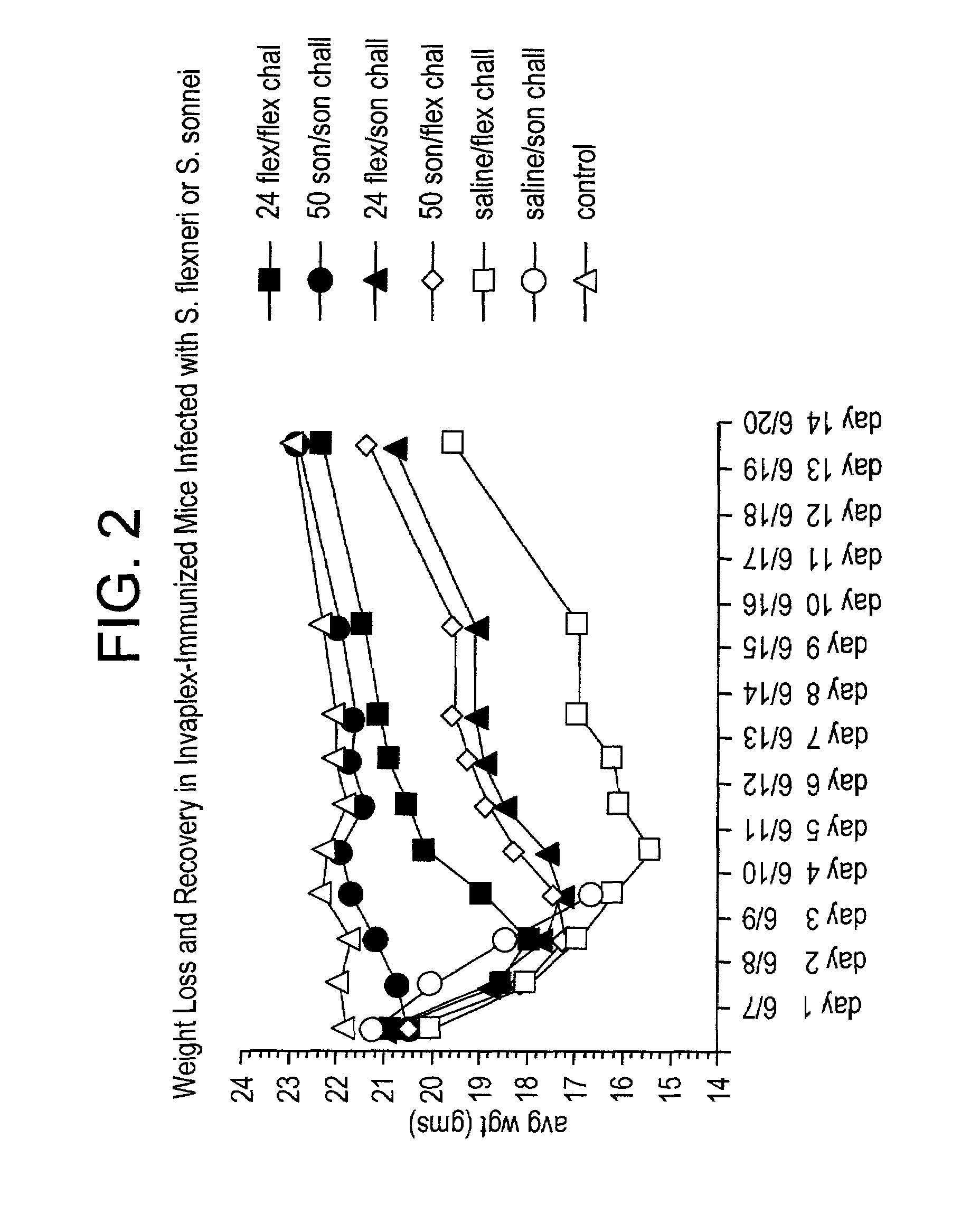
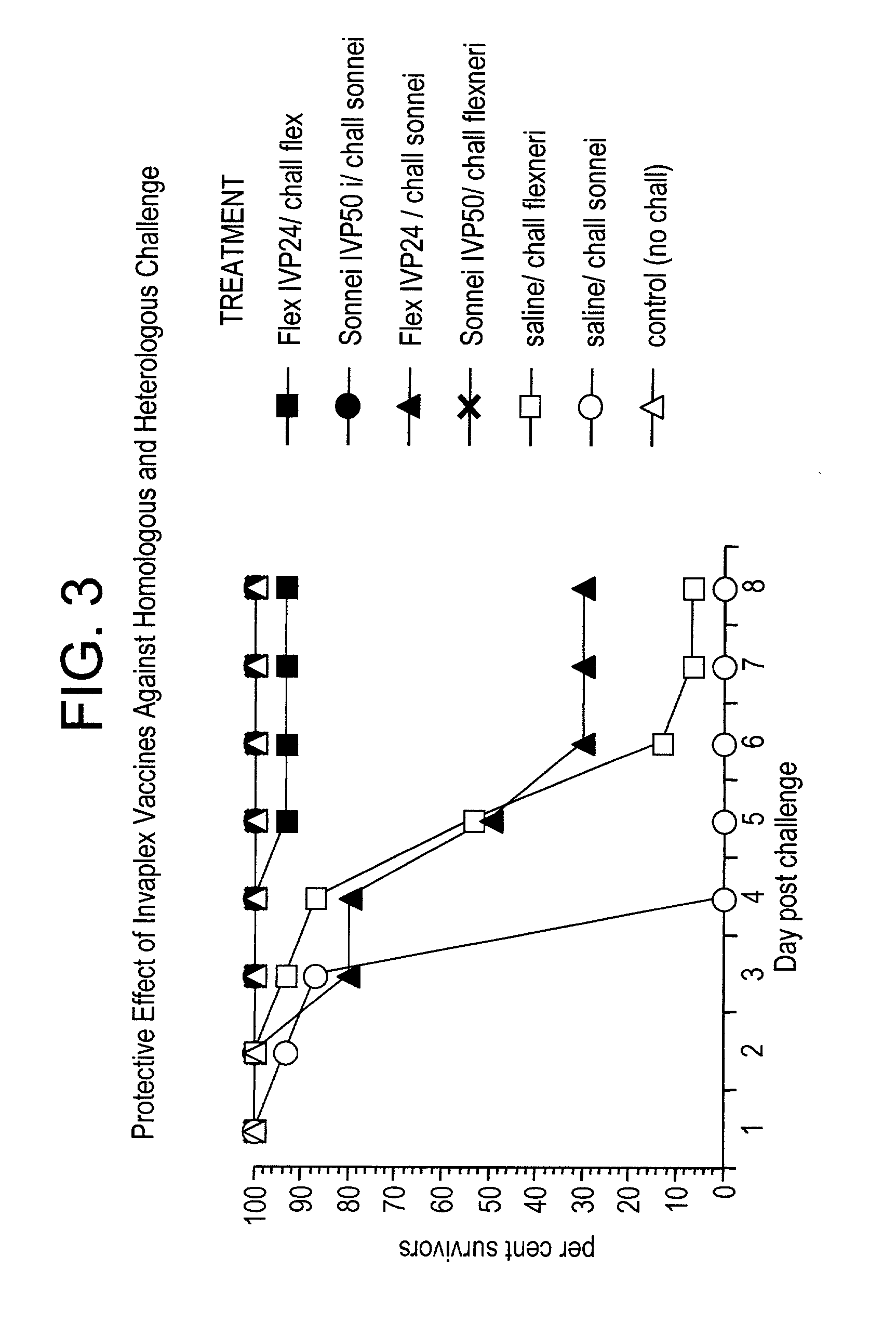
Heterologous protection induced by immunization with invaplex vaccine
InactiveUS7258863B2Enhance immune responseExtended half-lifeAntibacterial agentsBacterial antigen ingredientsHeterologousMicrobiology
In this application is described a composition, Invaplex, derived from a gram negative bacteria for use in generating an immune response in a subject against one or more heterologous species or strains of gram-negative bacteria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
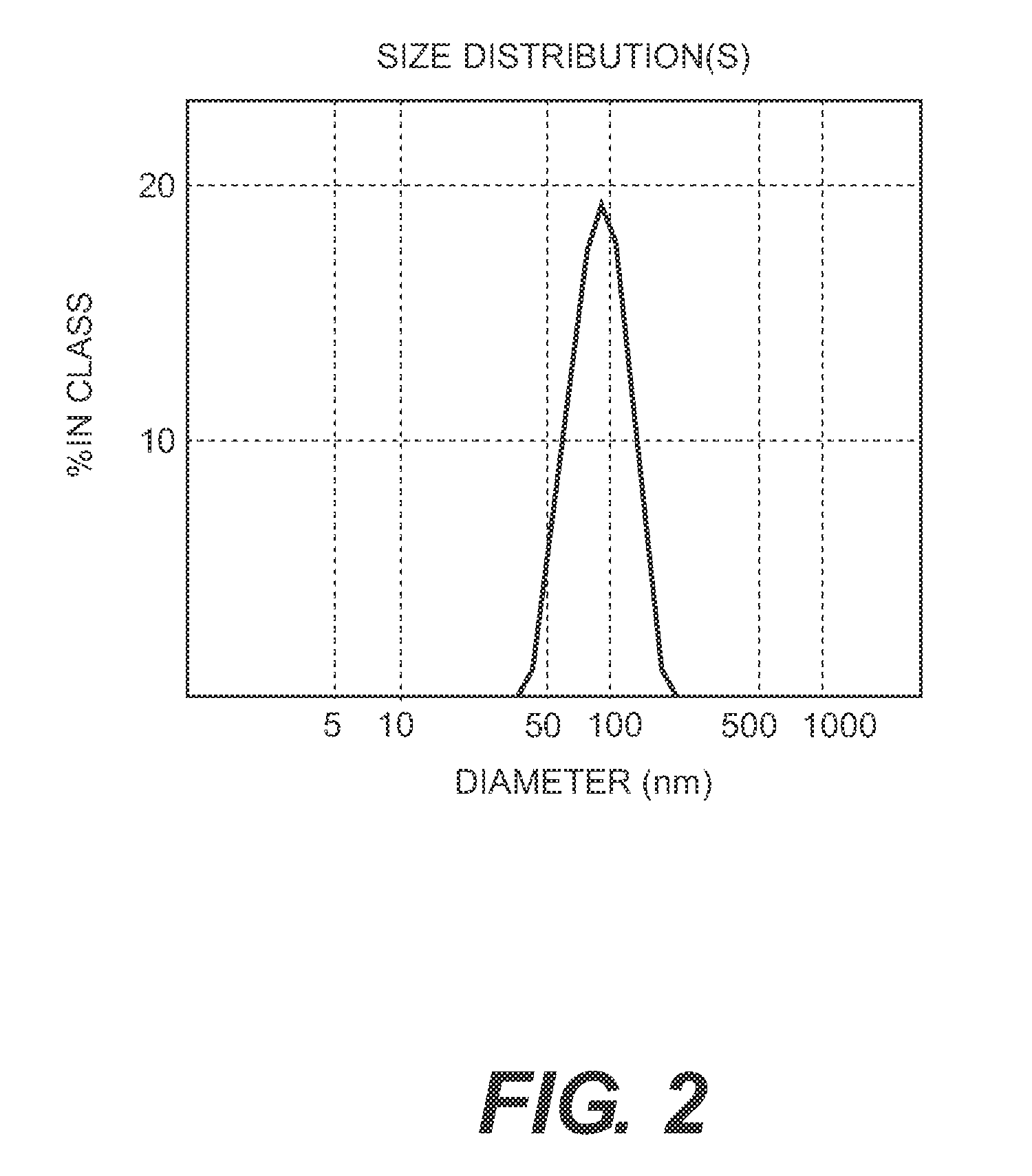
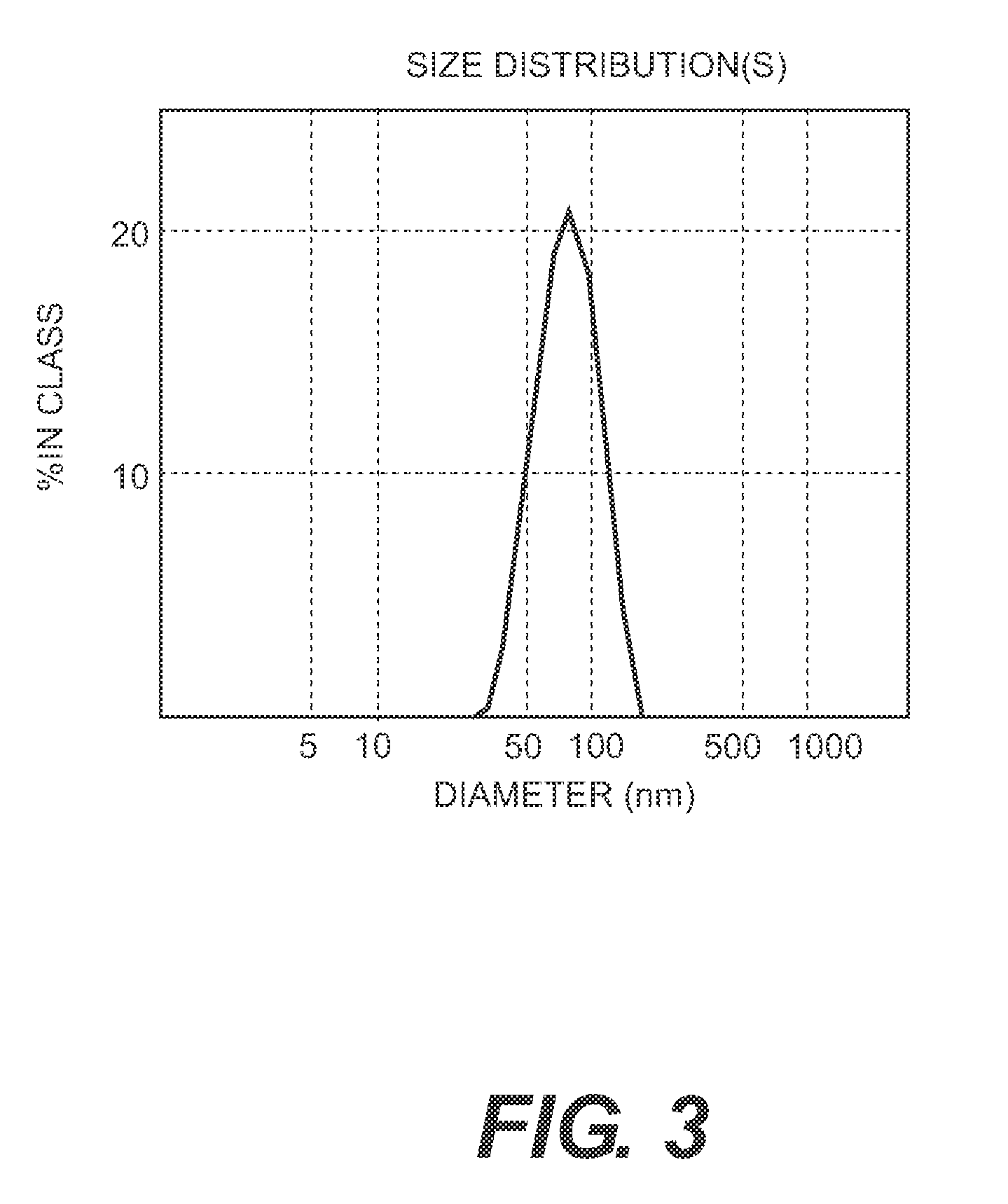
Vaccine composition comprising virus-like particles of human papillomavirus
The present invention relates to a vaccine composition comprising VLPs containing L1 proteins or functional L1 protein derivatives from HPV 16, HPV 18, HPV 31 and HPV 45 genotypes.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
DNA immunization against chlamydia infection
InactiveUS6696421B2Significantly more efficient in inducing protective immunityRapid recruitment of dendritic cellsBiocideGenetic material ingredientsNucleotidePhylum Chlamydiae
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Immunogenic compositions for protection against Chlamydial infection
InactiveUS20050065106A1Improve efficacySugar derivativesChlamydiaceae ingredientsNucleotideProtection sex
Owner:MURDIN ANDREW +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com