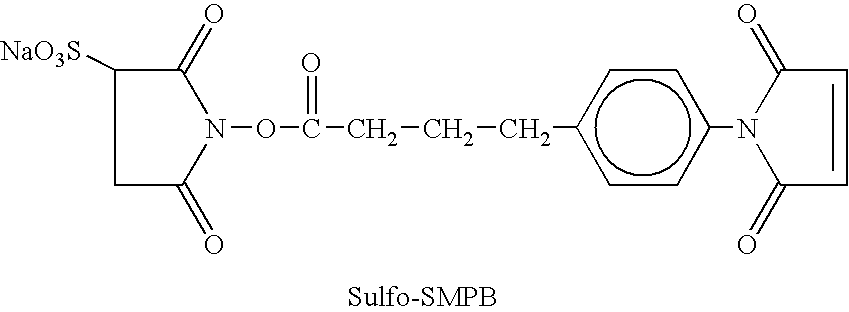
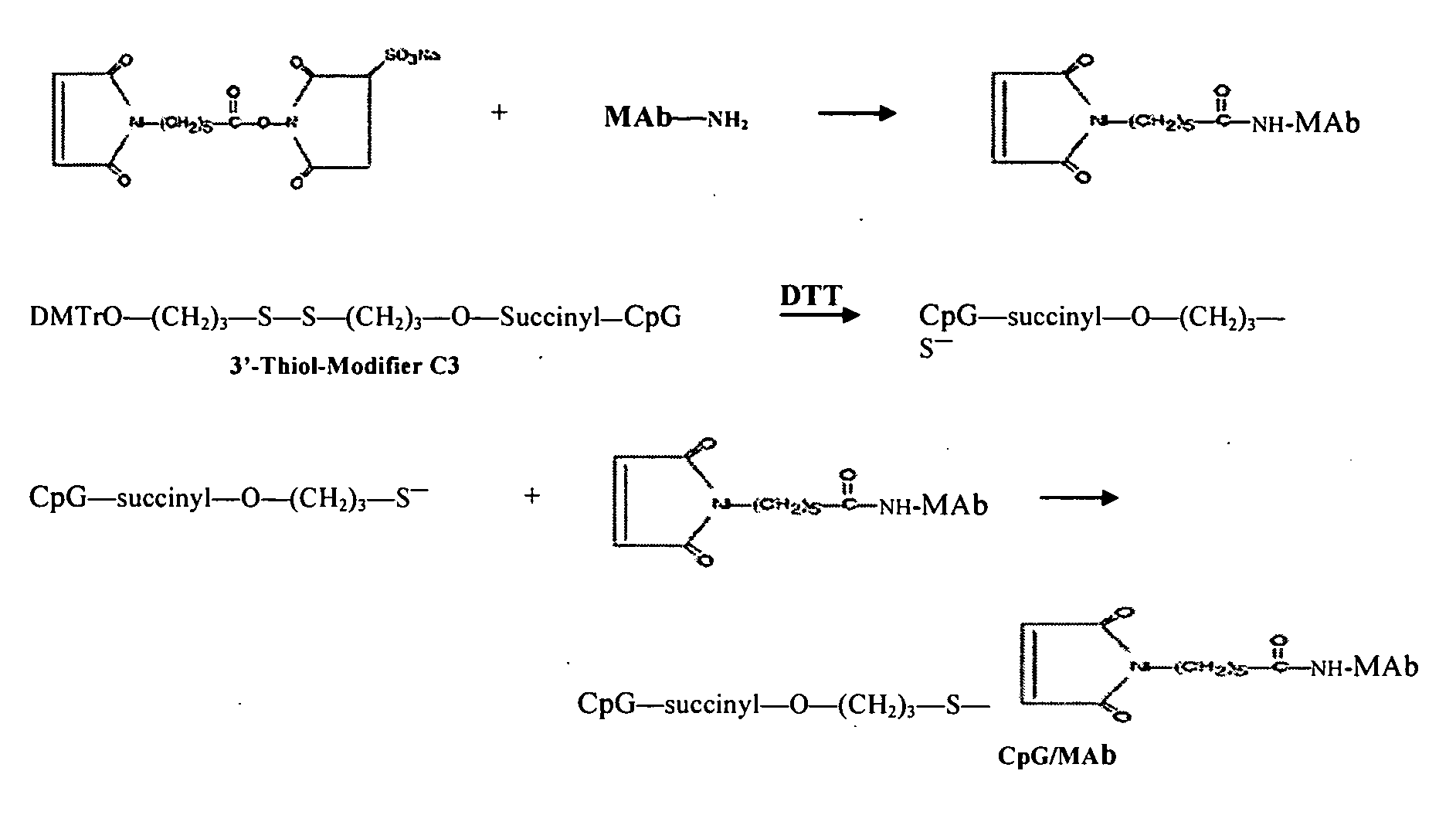
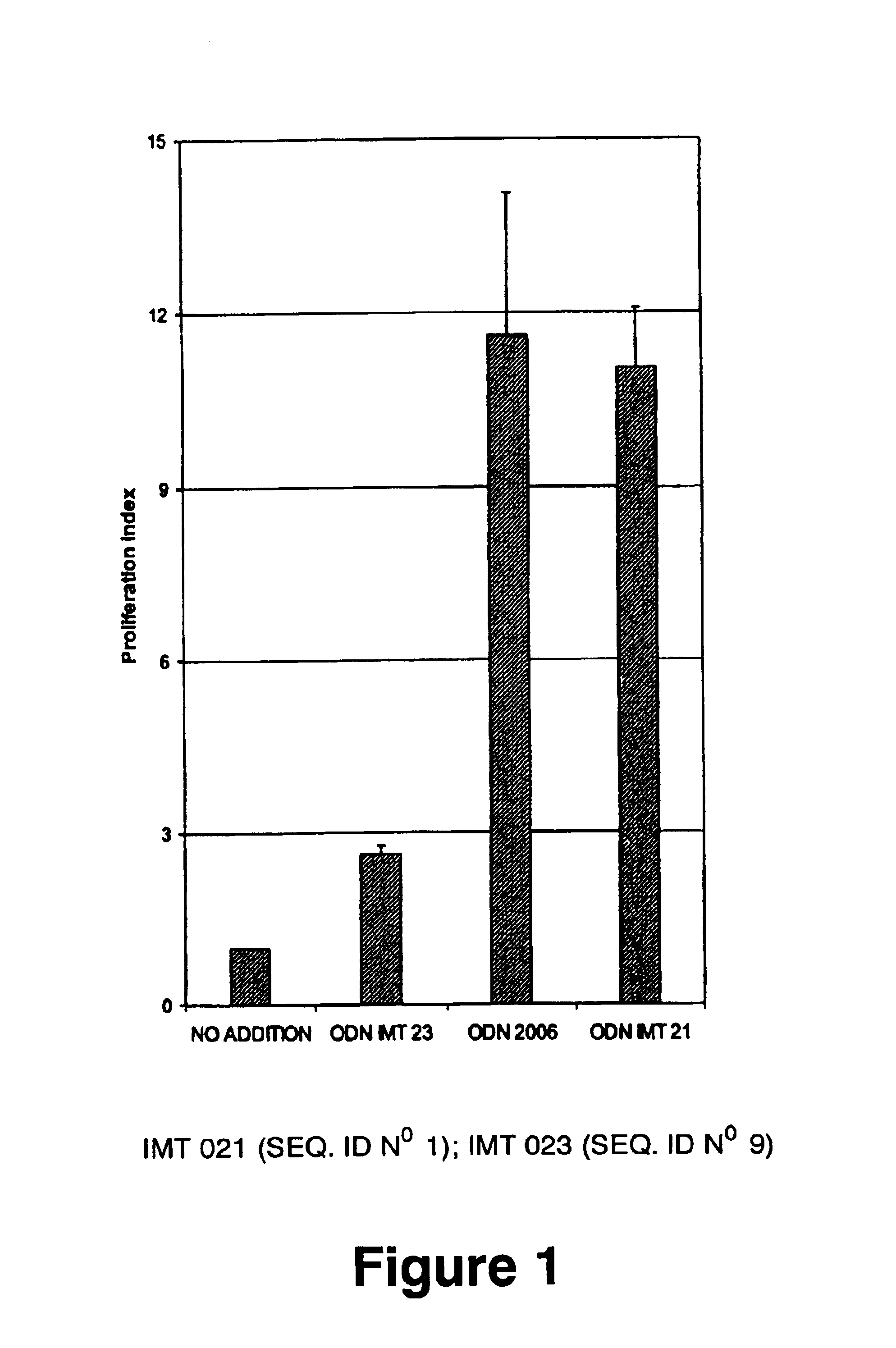
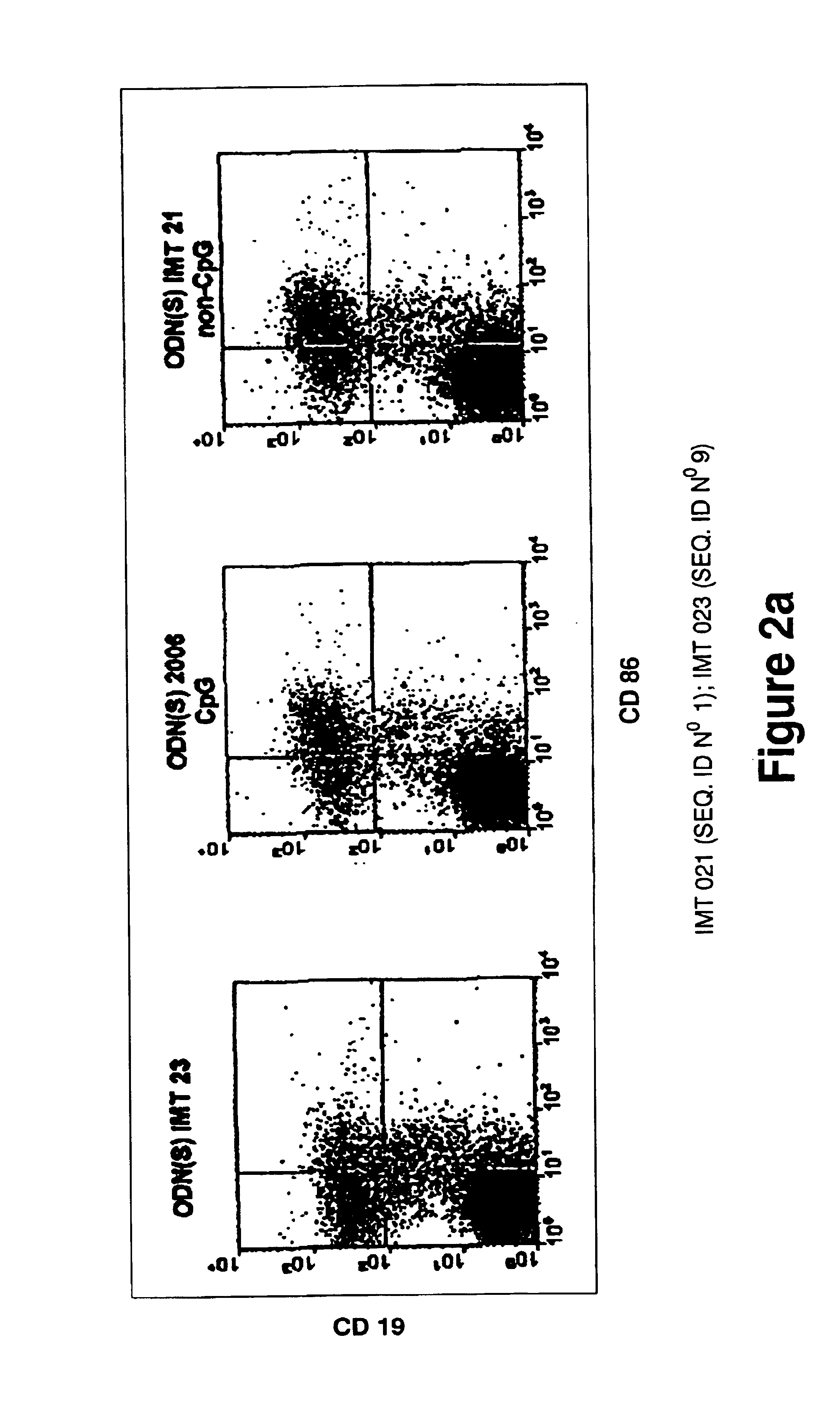
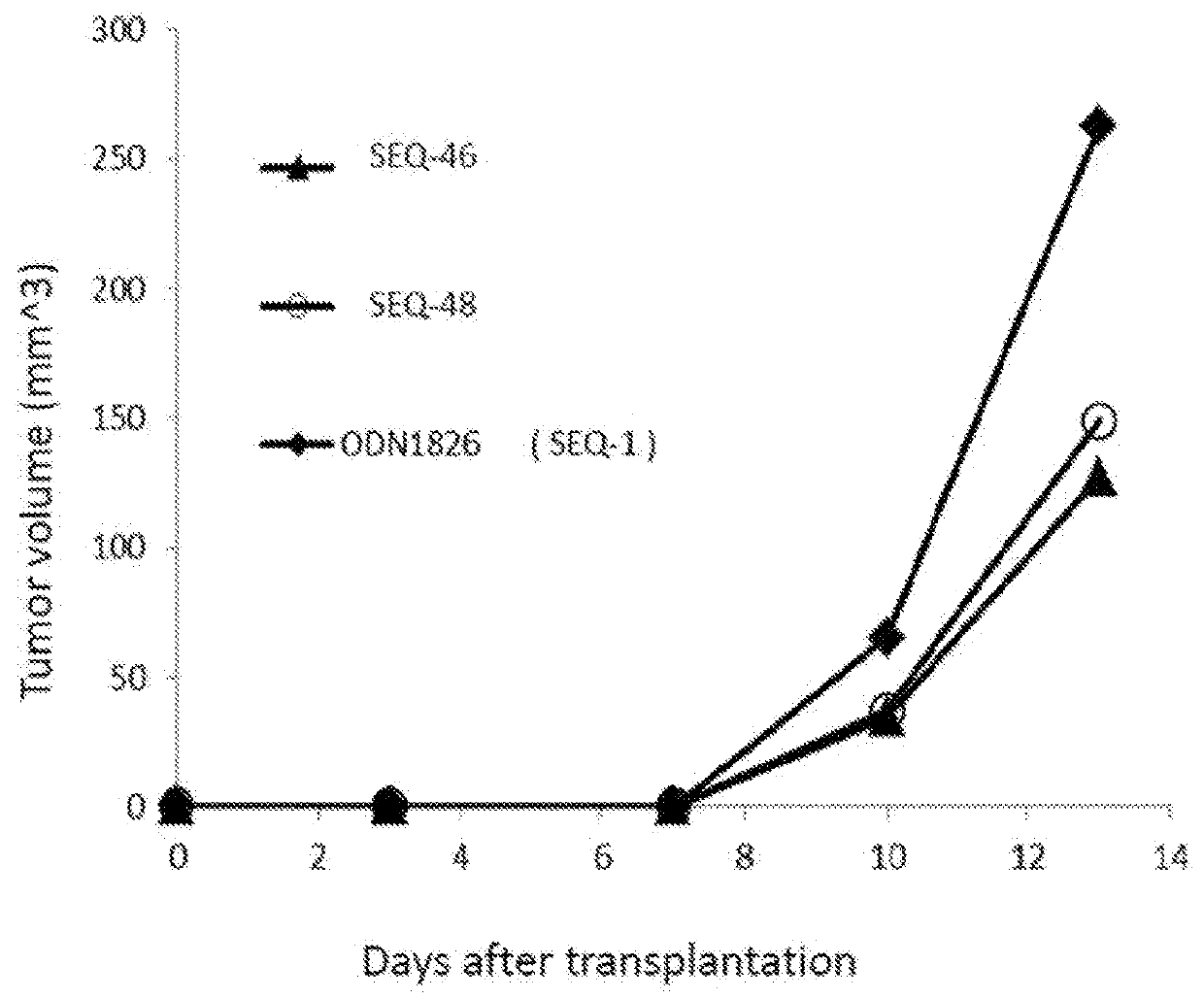
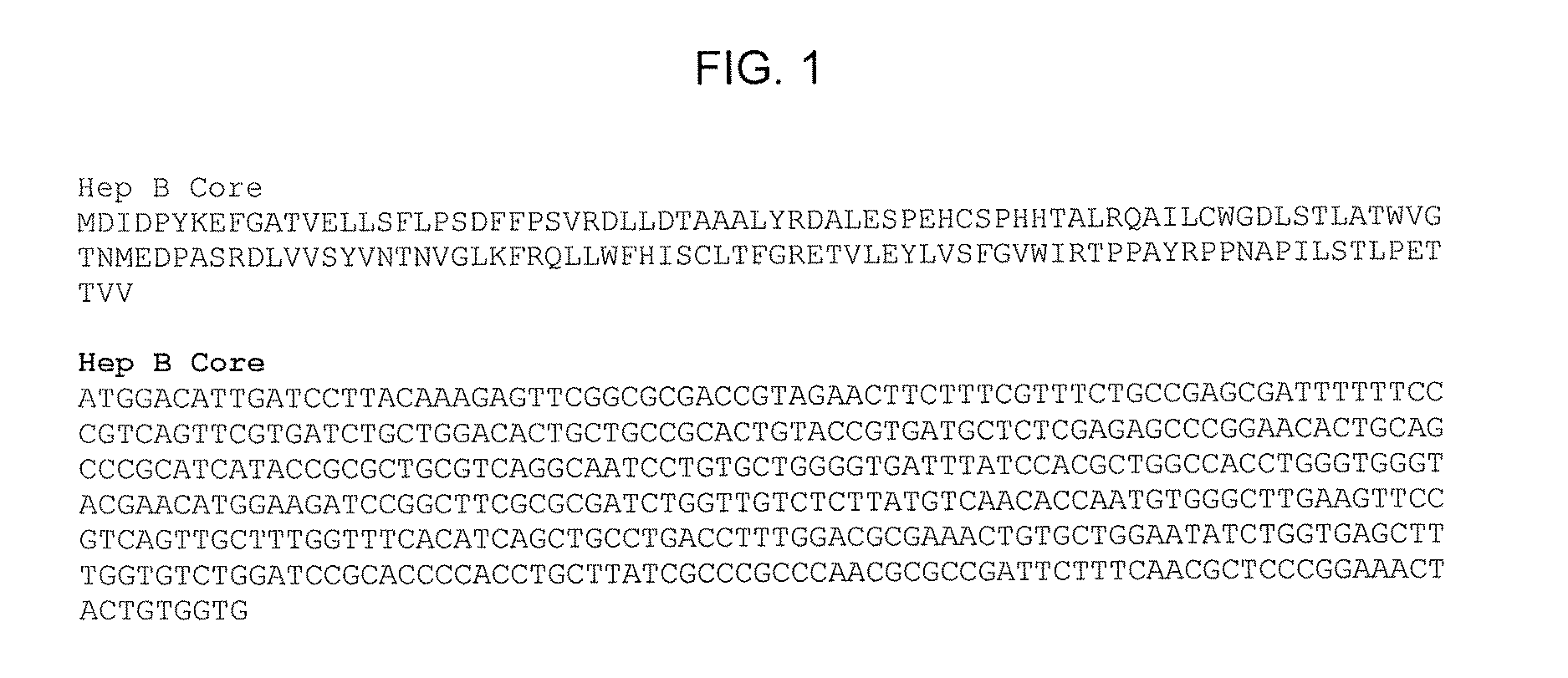
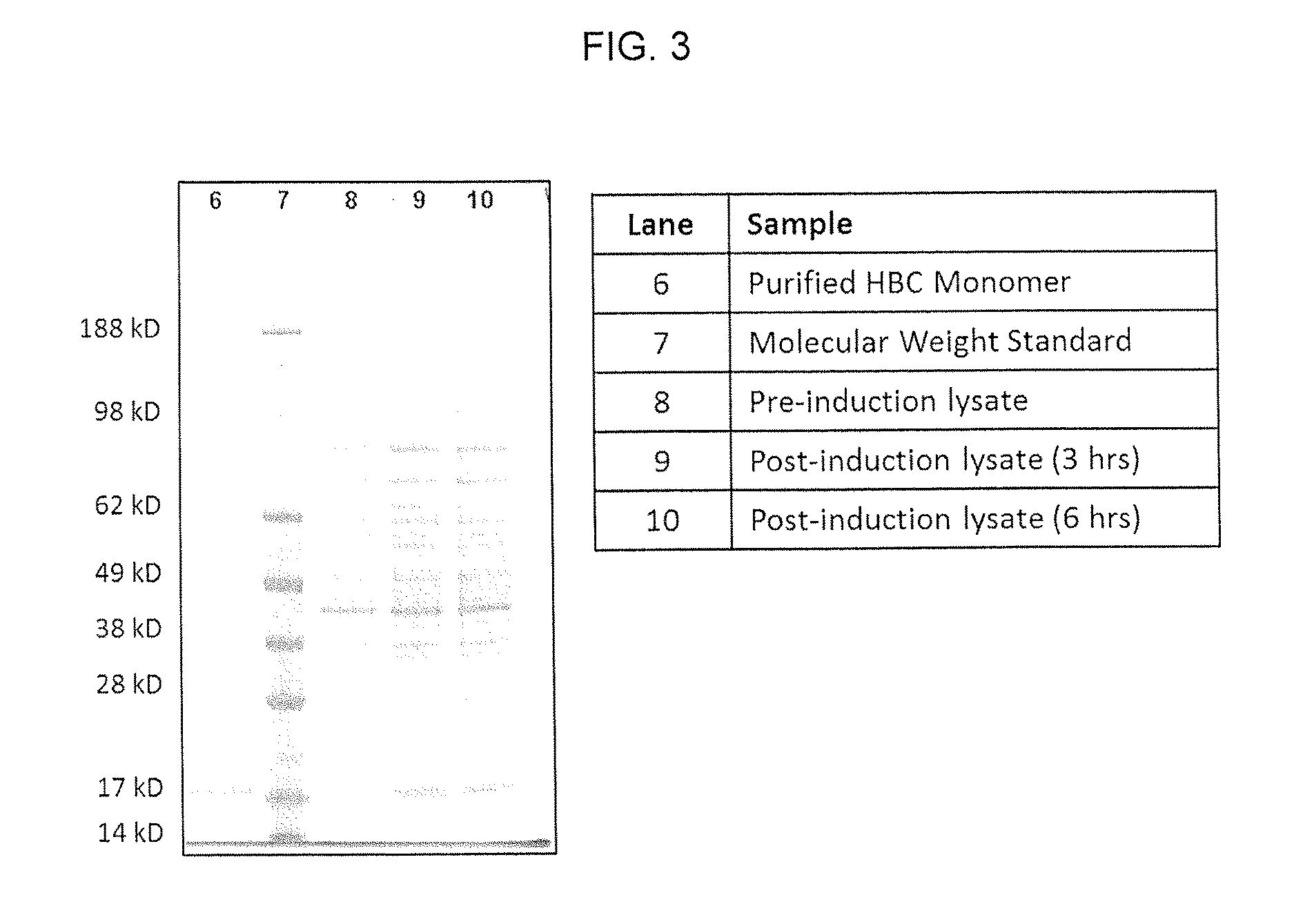
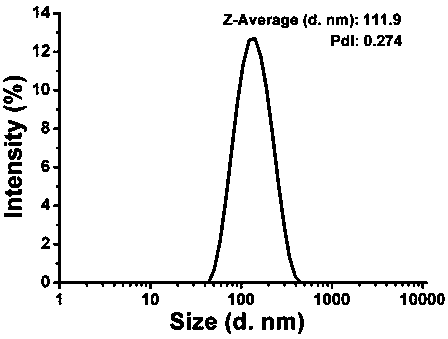
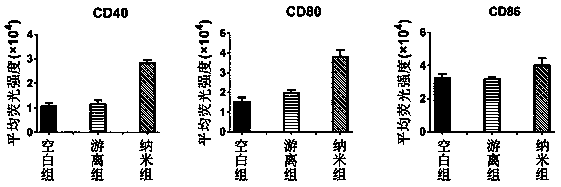
Patents
Literature
106 results about "CPG-oligonucleotide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CpG oligodeoxynucleotides (or CpG ODN) are short single-stranded synthetic DNA molecules that contain a cytosine triphosphate deoxynucleotide ("C") followed by a guanine triphosphate deoxynucleotide ("G").
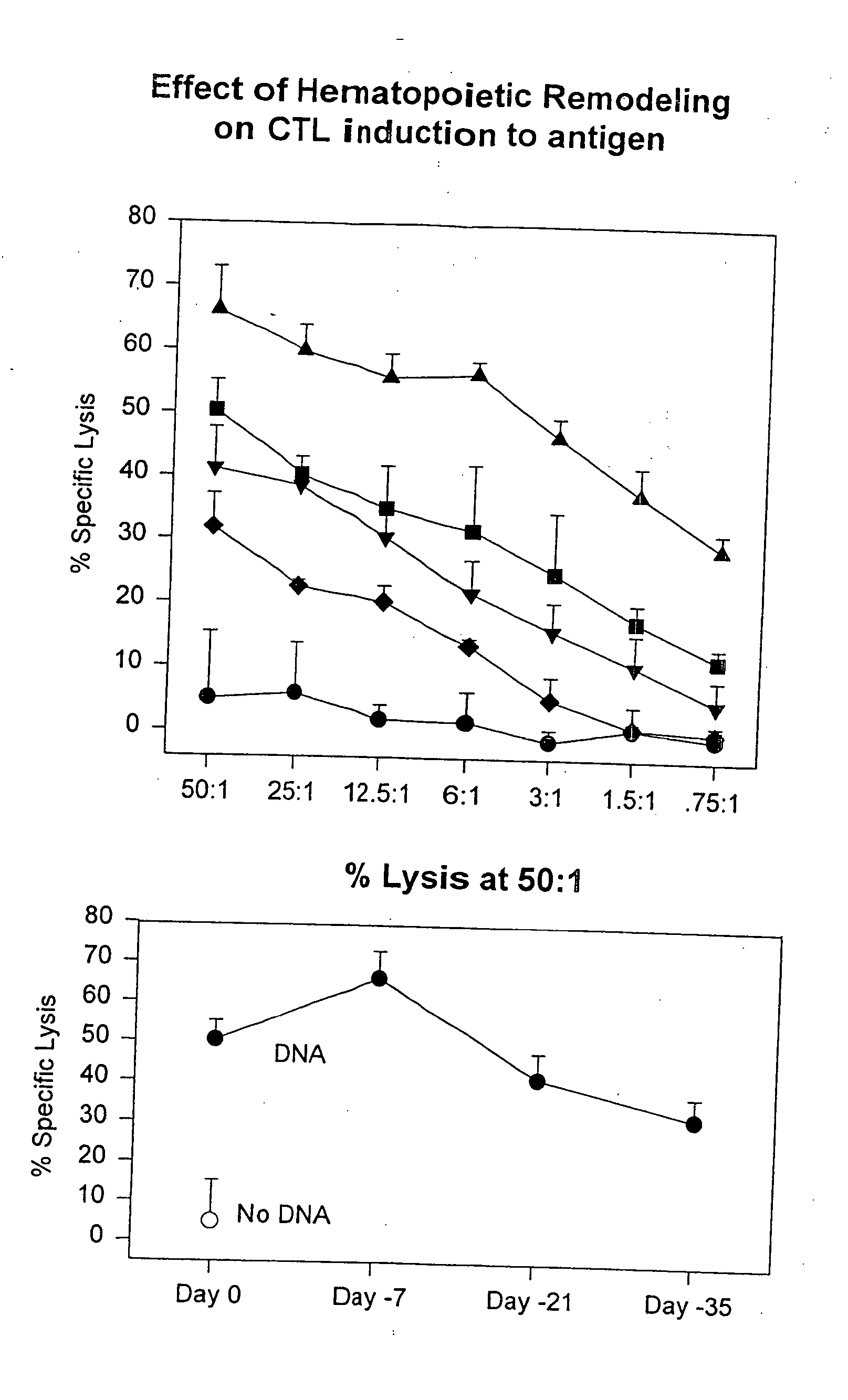
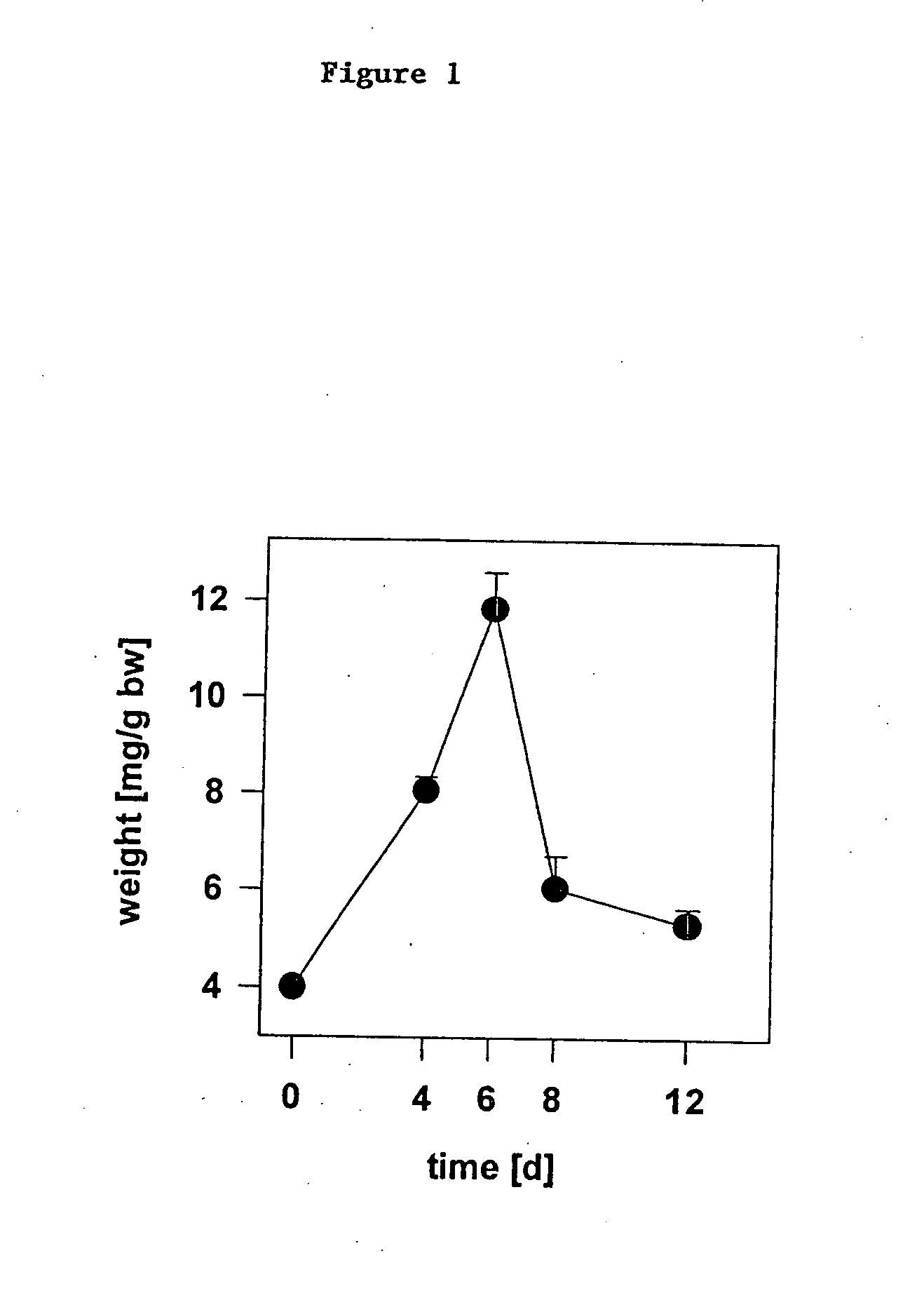
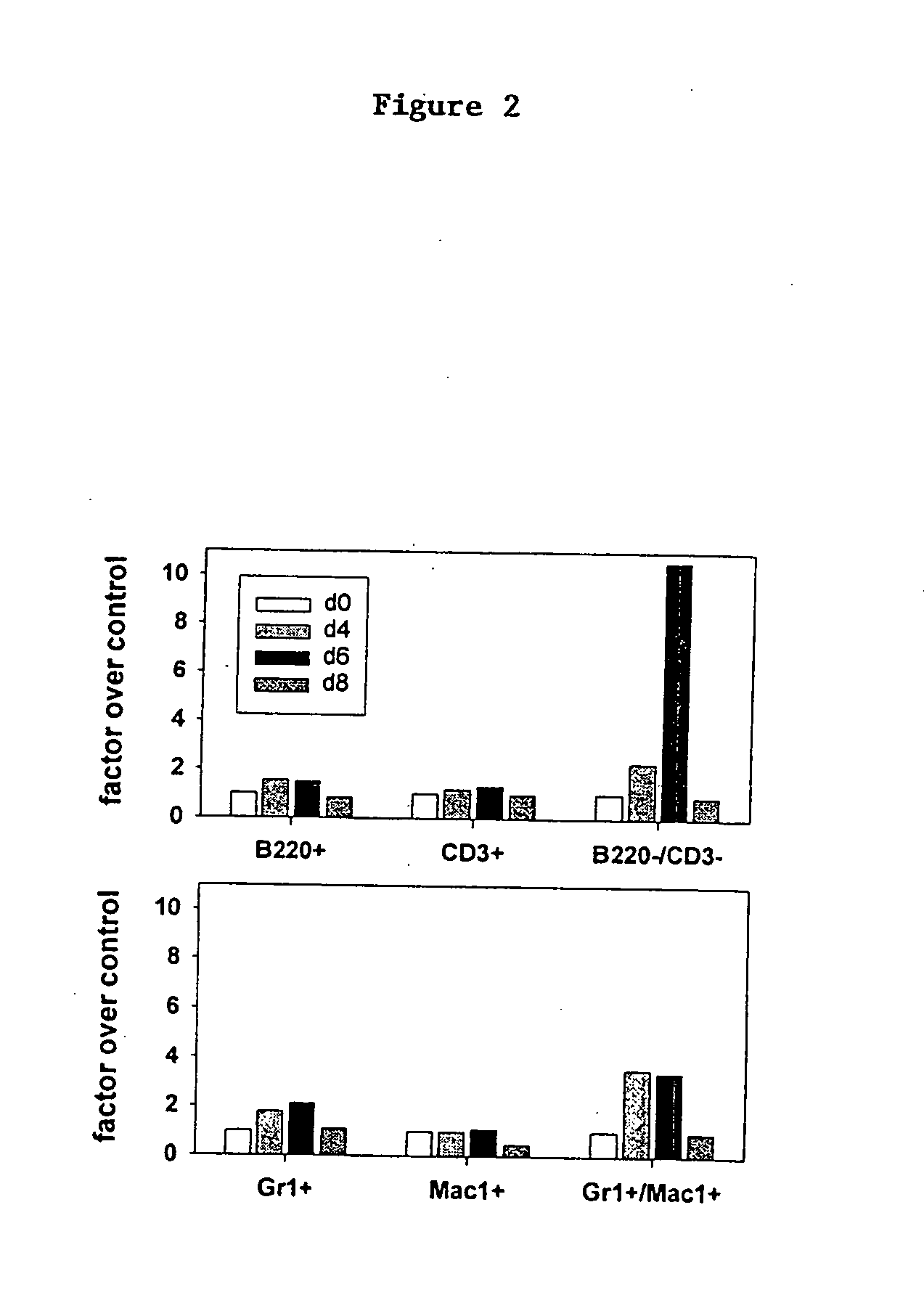
Methods for regulating hematopoiesis using CpG-oligonucleotides
InactiveUS20070184465A1Induce hematopoiesisInduces thrombocytopeniaSugar derivativesMicrobiological testing/measurementCpg oligonucleotidesPlatelet
The invention relates to methods for regulating hematopoiesis using CpG containing oligonucleotides. In particular, the invention relates to methods of treating thrombopoiesis and anemia by regulating hematopoiesis. The invention also relates to methods of regulating immune system remodeling by administering CpG oligonucleotides to control hematopoiesis.
Owner:COLEY PHARMA GMBH
Targeted innate immunity
InactiveUS20060135459A1Hybrid immunoglobulinsSugar derivativesAbnormal tissue growthCancer targeting
Provided is a cancer therapeutic agent comprising a cancer targeting molecule linked to a CpG oligodeoxynucleotide. Also provided are methods of reducing the size of a tumor or inhibiting the growth of cancer cells in an individual or inhibiting the development of metastatic cancer, comprising administering an effective amount of the cancer therapeutic agent. The methods may also include reducing immunoregulatory T cell activity in the individual.
Owner:UNIV OF SOUTHERN CALIFORNIA
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Therapeutic use of cpg oligodeoxynucleotide for skin disease
InactiveUS20090062224A1Good conditionReduce expressionOrganic active ingredientsSugar derivativesDiseaseCpg oligonucleotides
Disclosed is the therapeutic use of CpG oligodeoxynucleotides for skin diseases. The CpG oligodeoxynucleotides (CpG ODNs) of the present invention show excellent immunoactive effects against skin diseases in both cases of CpG ODNs with a phosphorothioate backbone and CpG ODNs with a phosphodiester backbone.
Owner:BIO CLUE & SOLUTION +2
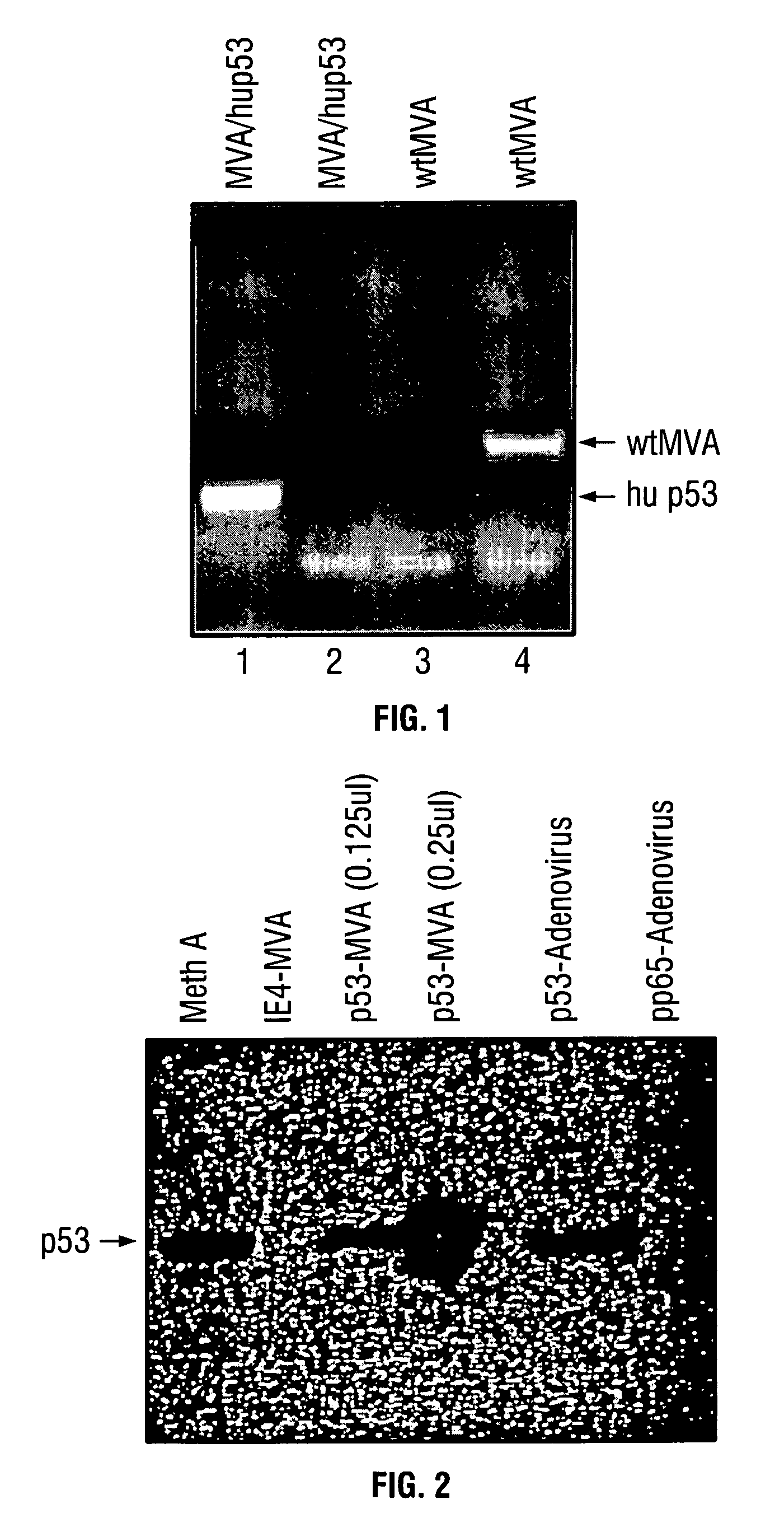
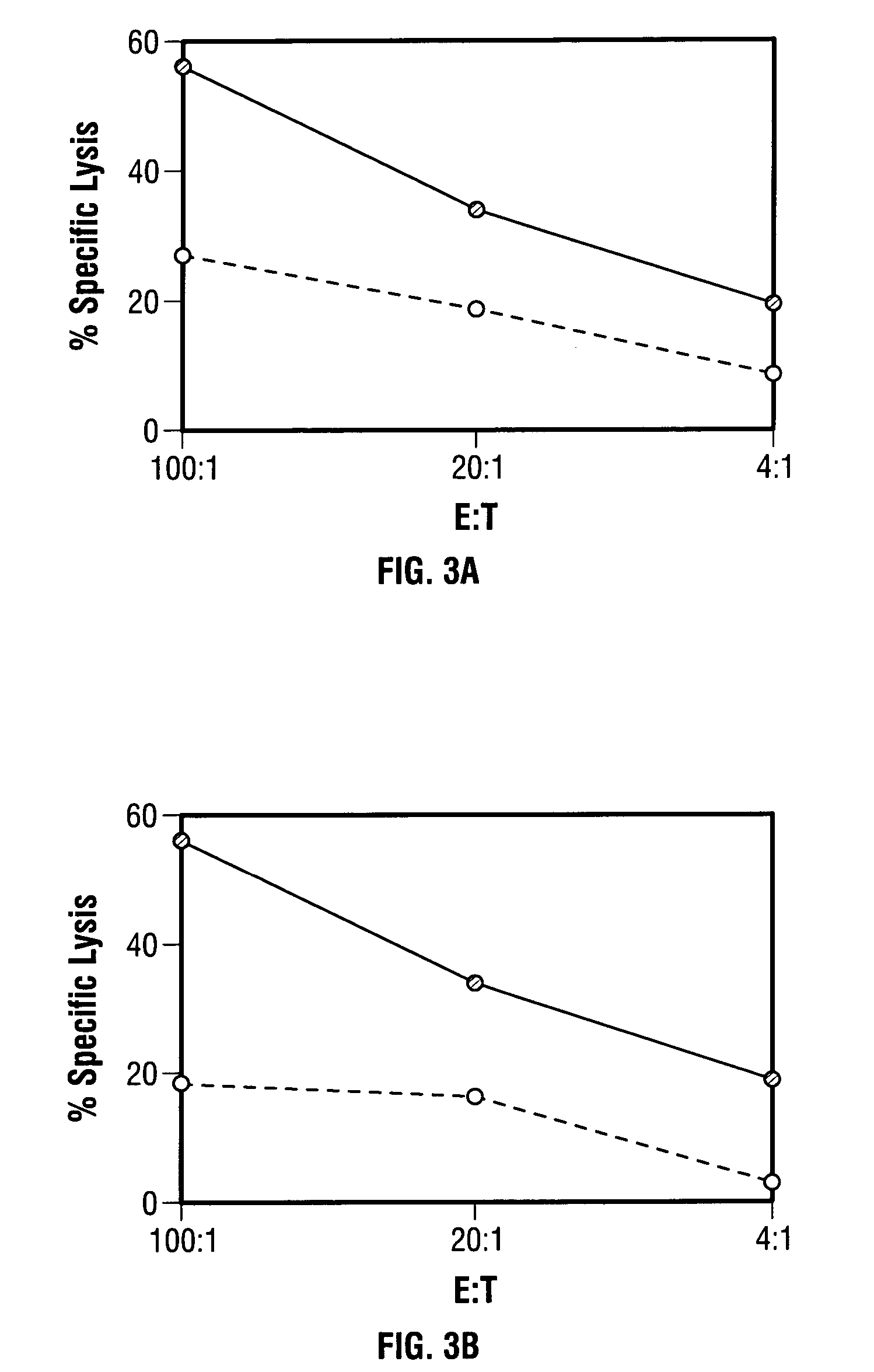
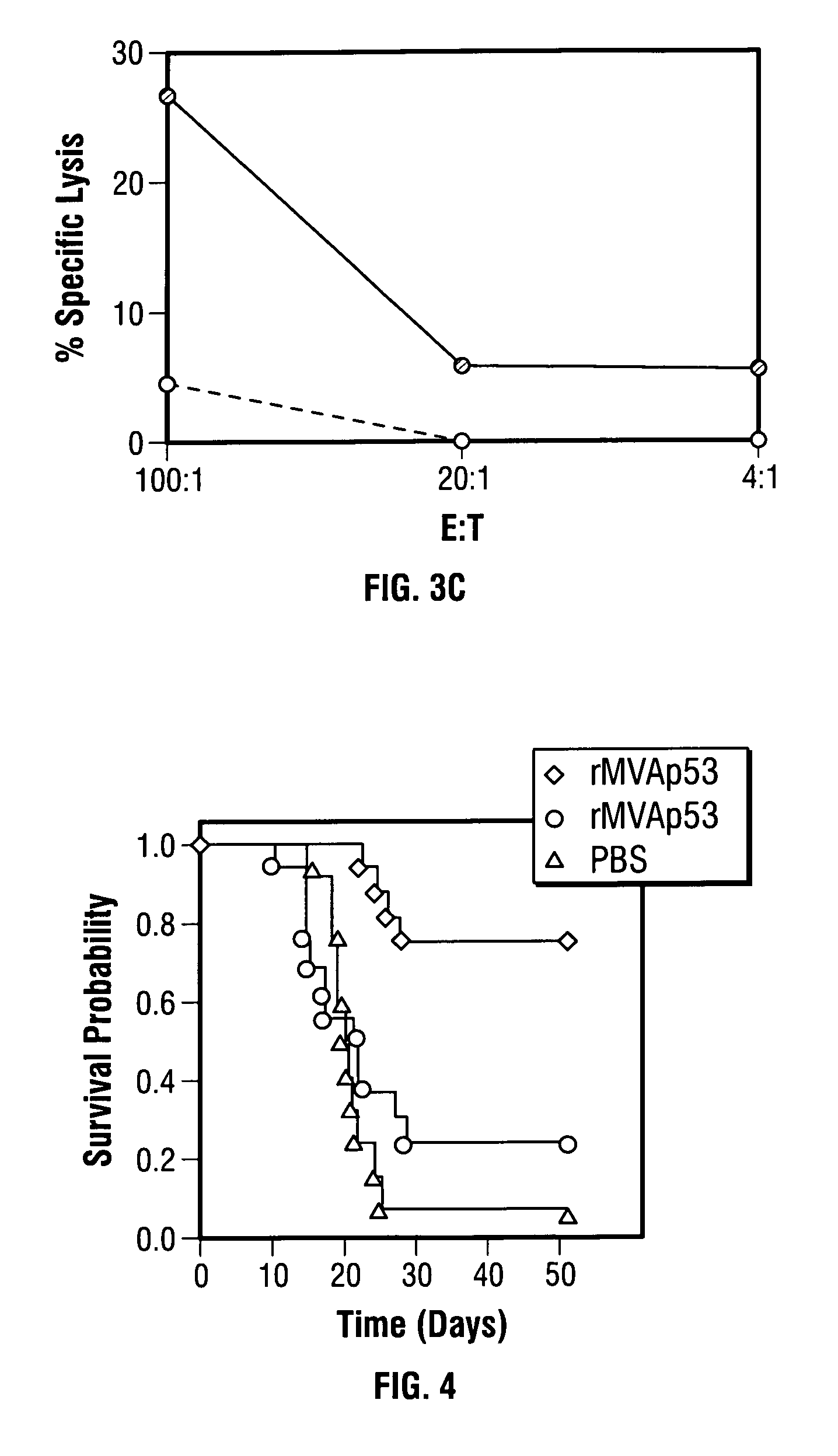
Modified vaccinia Ankara expressing p53 in cancer immunotherapy
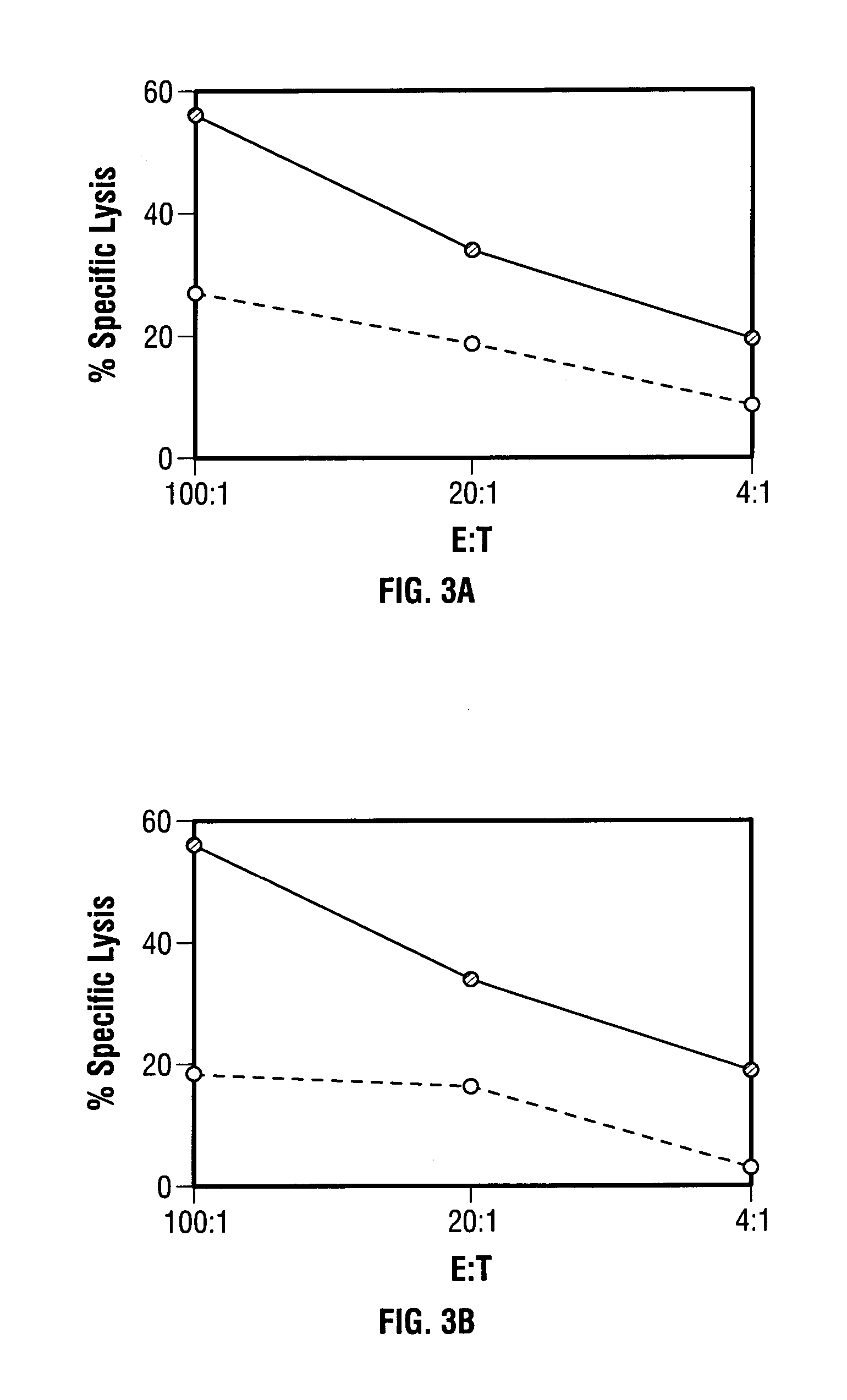
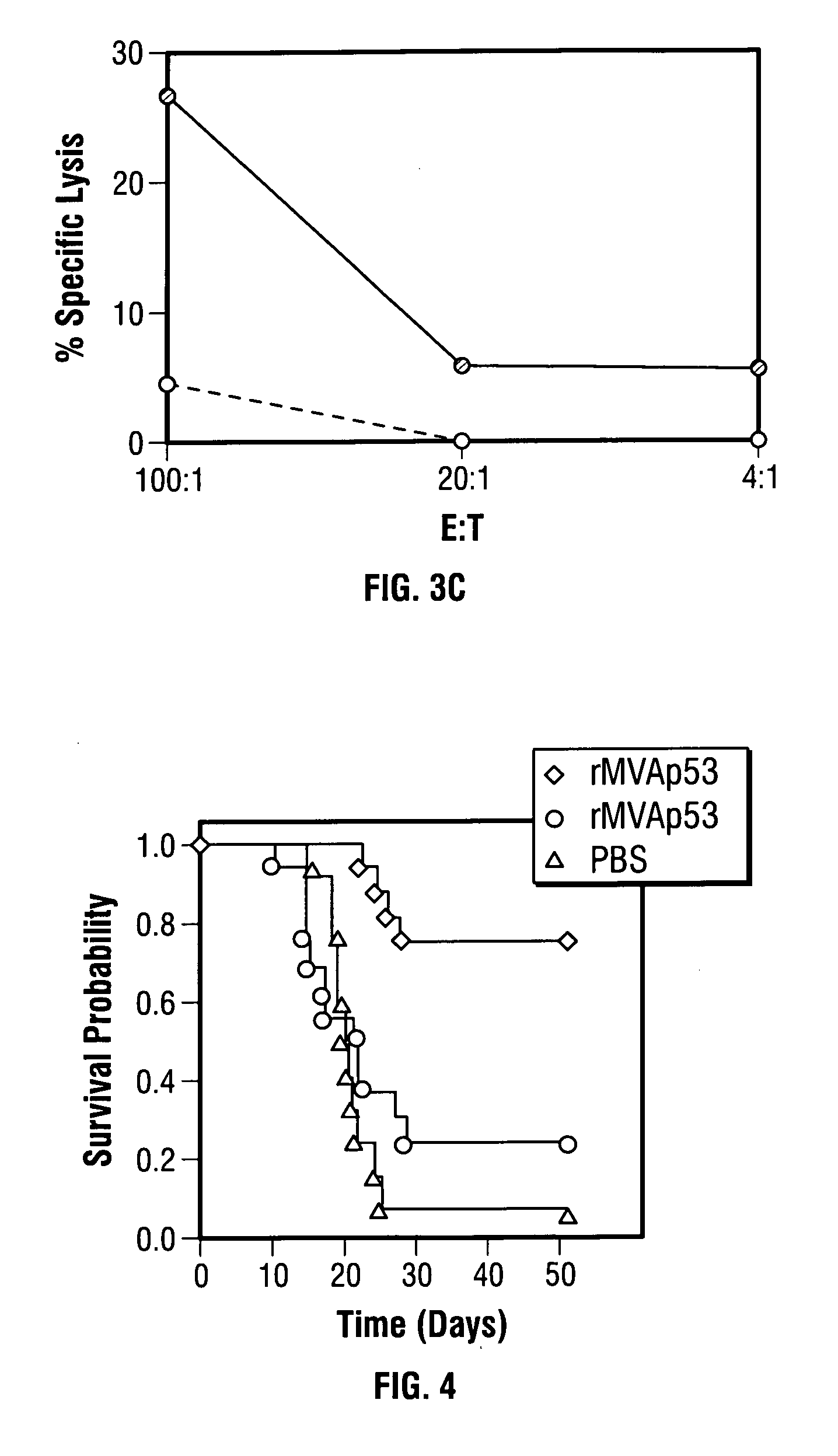
ActiveUS7256037B2Limit therapeutic efficacyReduce developmentBiocideGenetic material ingredientsModified vaccinia AnkaraADAMTS Proteins
Mutations to the tumor suppressor protein p53 have been observed in 40-60% of all human cancers. These mutations are often associated with high nuclear and cytoplasmic concentrations of p53. Since many tumors exhibit highly elevated p53 levels, the protein is an attractive target for cancer immunotherapy. Unfortunately, p53 is an autoantigen that is likely to be tolerated as a self-protein by the immune system. The present invention is based on the discovery that this self-tolerance can be overcome by administration of recombinant modified vaccinia Ankara (MVA) containing a nucleic acid that encodes p53 (rMVAp53). The invention discloses a method of generating a p53-specific CTL-response to tumor cells expressing mutated p53 by administering a composition comprising rMVAp53. Administration of rMVAp53 decreases tumor development, tumor growth, and mortality in a variety of malignant cell types. These effects are enhanced by administration of CTLA-4 blocker and / or CpG oligodeoxynucleotide immunomodulators.
Owner:CITY OF HOPE
Modified vaccinia ankara expressing p53 in cancer immunotherapy
Mutations to the tumor suppressor protein p53 have been observed in 40-60% of all human cancers. These mutations are often associated with high nuclear and cytoplasmic concentrations of p53. Since many tumors exhibit highly elevated p53 levels, the protein is an attractive target for cancer immunotherapy. Unfortunately, p53 is an autoantigen that is likely to be tolerated as a self-protein by the immune system. The present invention is based on the discovery that this self-tolerance can be overcome by administration of recombinant modified vaccinia Ankara (MVA) containing a nucleic acid that encodes p53 (rMVAp53). The invention discloses a method of generating a p53-specific CTL-response to tumor cells expressing mutated p53 by administering a composition comprising rMVAp53. Administration of rMVAp53 decreases tumor development, tumor growth, and mortality in a variety of malignant cell types. These effects are enhanced by administration of CTLA-4 blocker and / or CpG oligodeoxynucleotide immunomodulators.
Owner:CITY OF HOPE
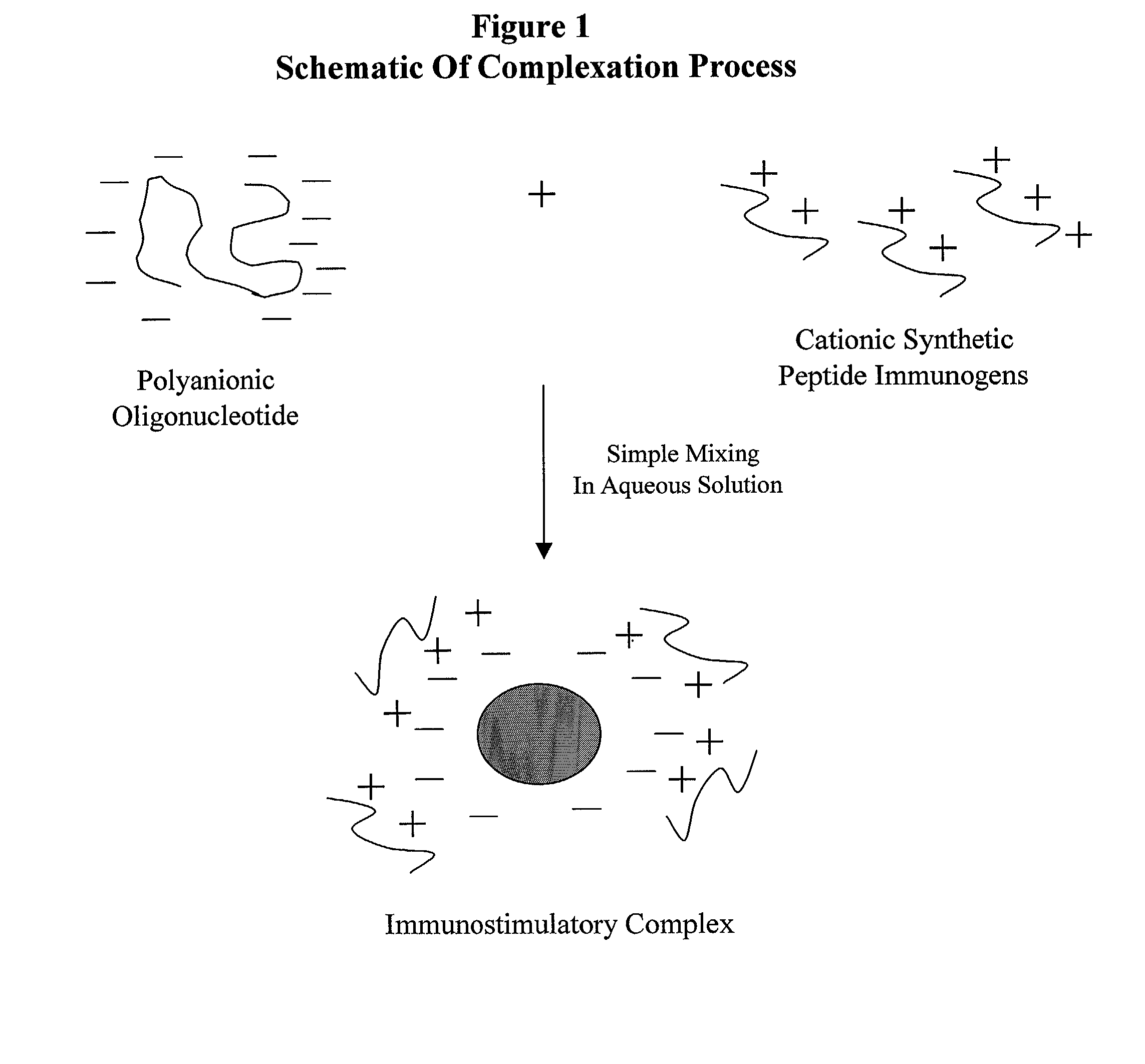
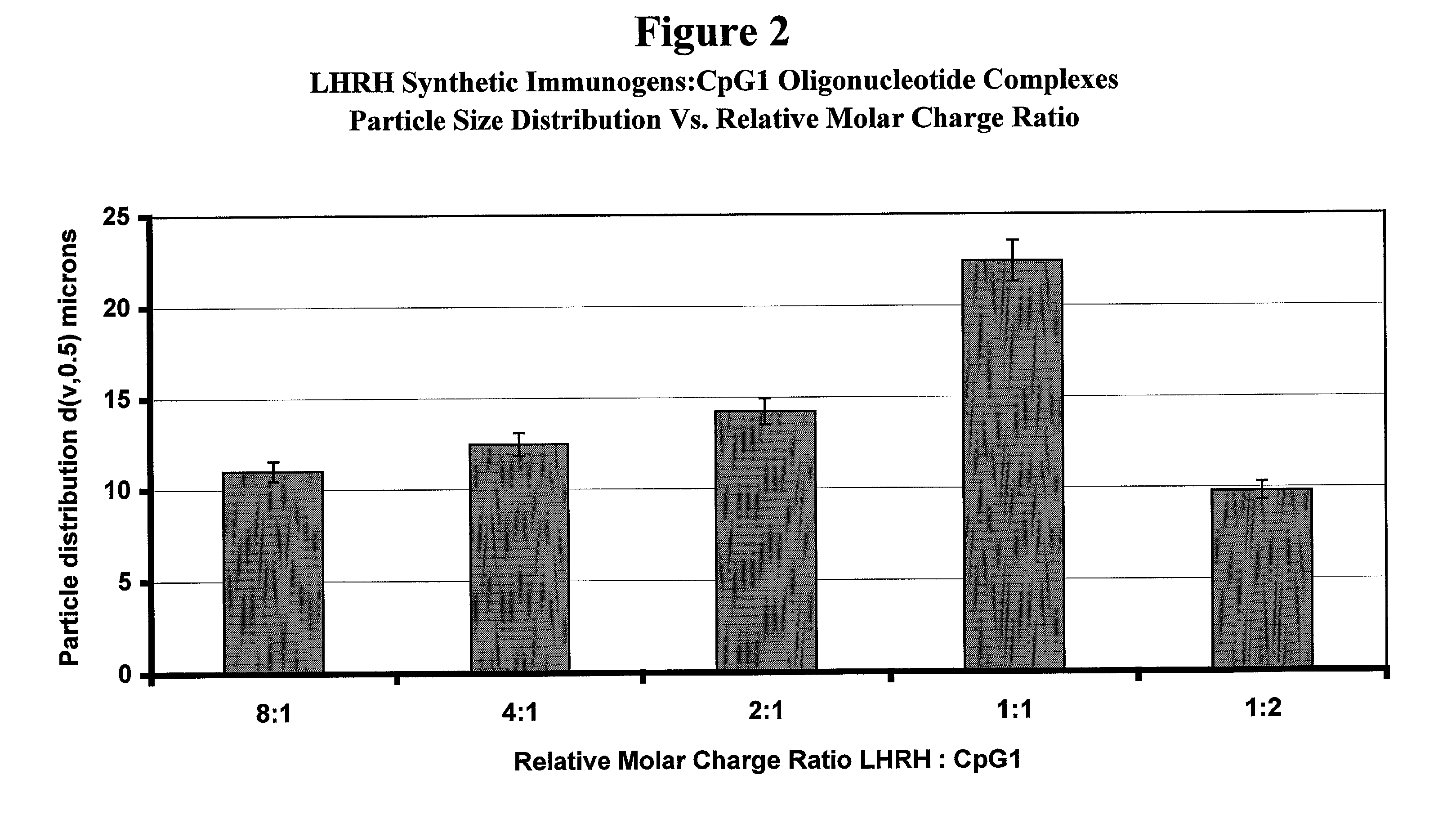
Stabilized synthetic immunogen delivery system
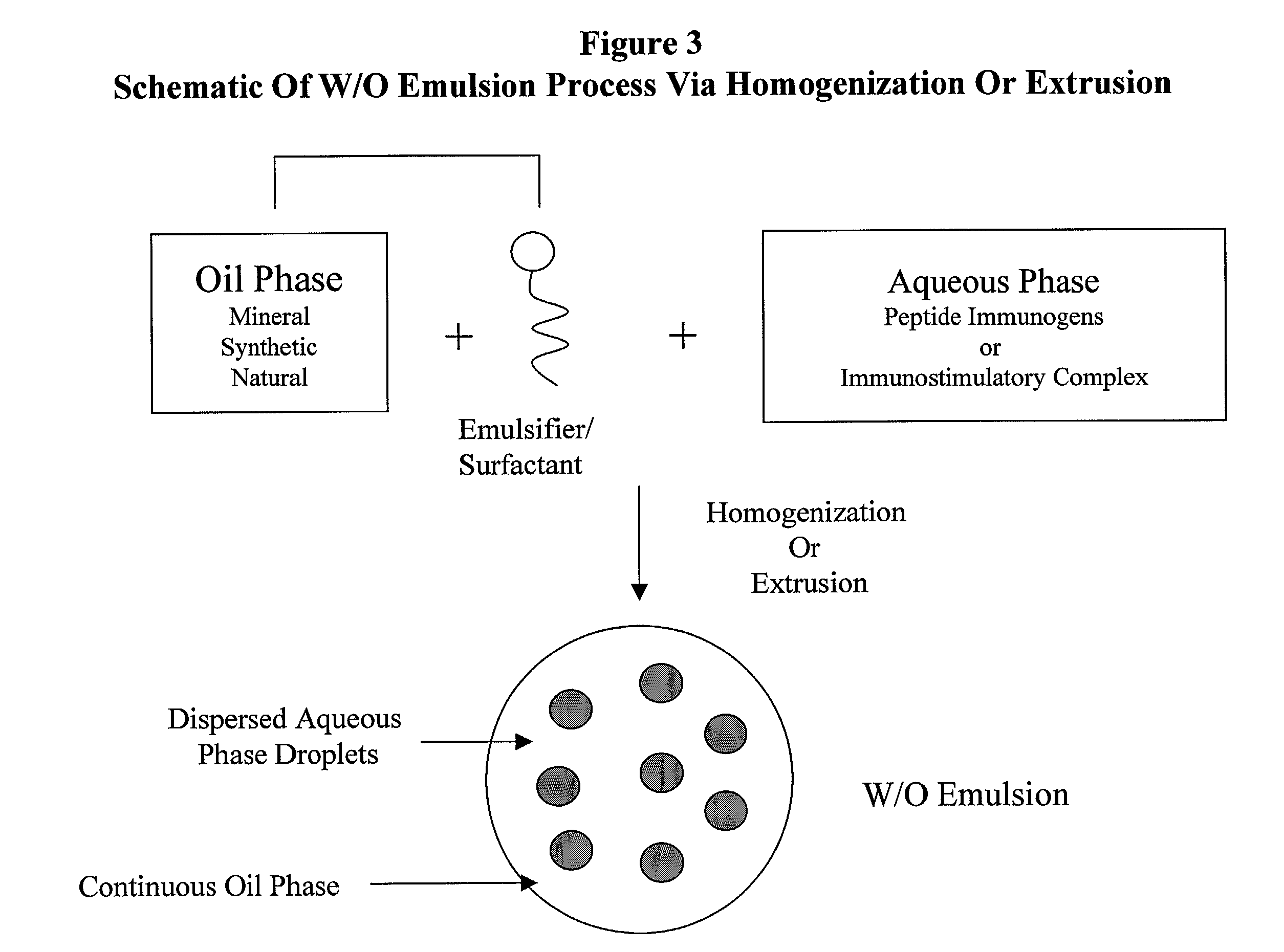
ActiveUS8088388B2Improve propertiesEffectively upregulating immune responseImpression capsPeptide/protein ingredientsSynthetic ImmunogensParticulates
Owner:UNITED BIOMEDICAL INC
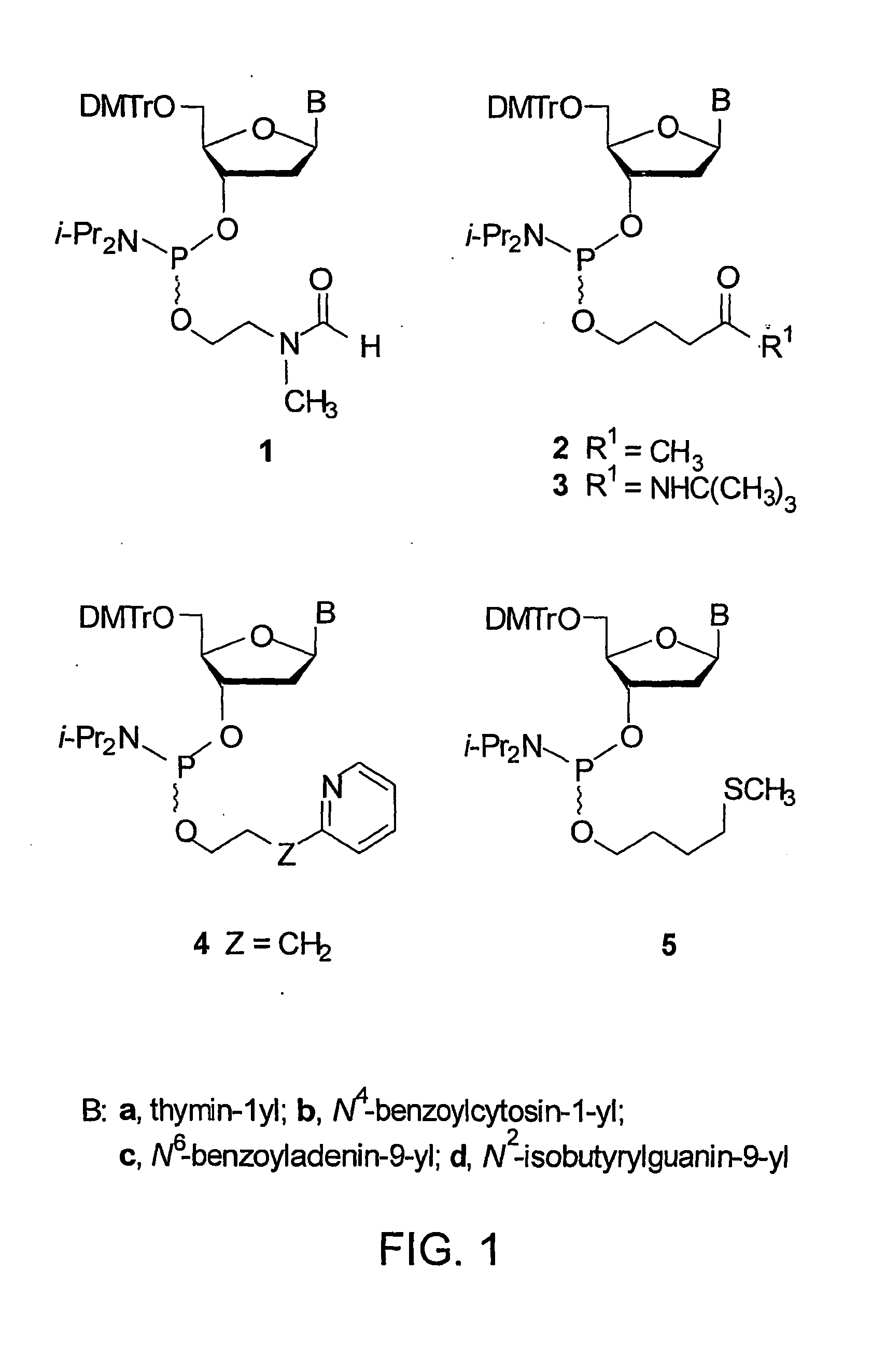
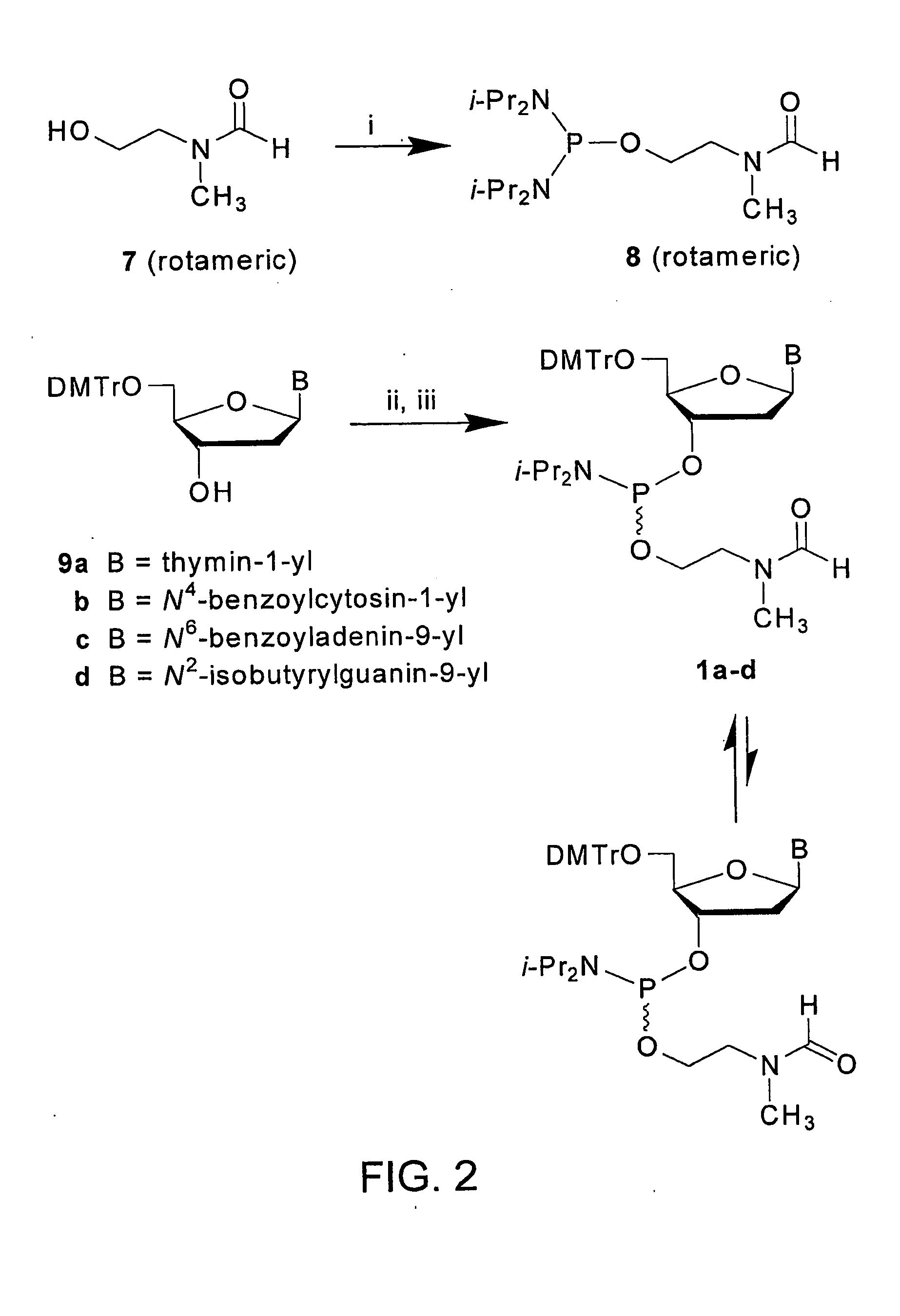
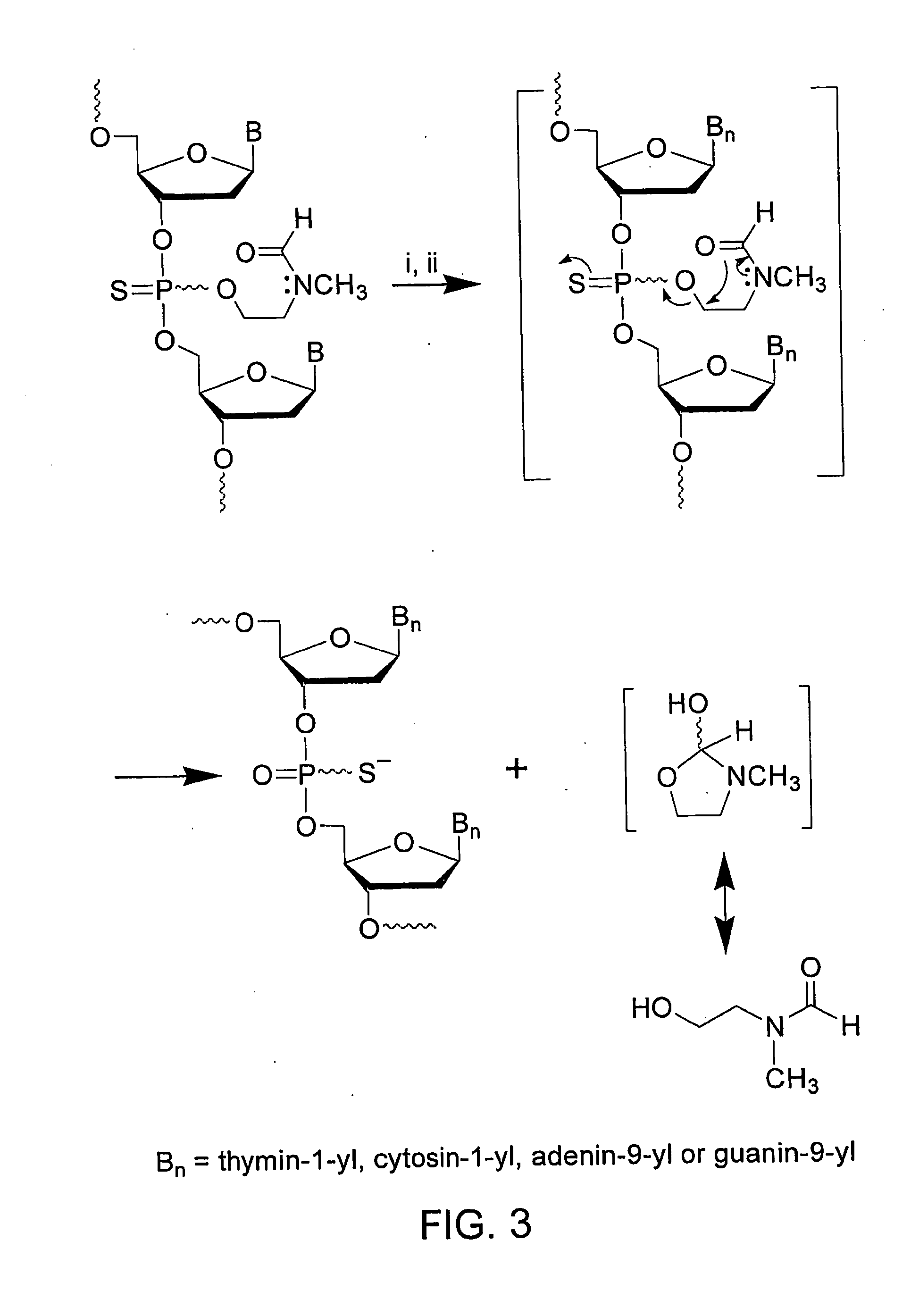
CpG OLIGONUCLEOTIDE PRODRUGS, COMPOSITIONS THEREOF AND ASSOCIATED THERAPEUTIC METHODS
ActiveUS20090263405A1Improve the immunityOrganic active ingredientsSugar derivativesNucleotideCpg oligonucleotides
The present invention provides a CpG oligonucleotide prodrug that includes a thermolabile substituent on at least one nucleotide thereof. The present invention also provides compositions that include a carrier and a therapeutically effective amount of at least one CpG oligonucleotide prodrug. The present invention further provides therapeutic methods of using such thermolabile CpG oligonucleotide prodrugs and compositions thereof. The present invention further provides a method of inhibiting tetrad formation in a CpG oligonucleotide by functionalizing the CpG oligonucleotide with one or more thermolabile substituents.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Compositions having means for targeting at least one antigen to dendritic cells
A composition that can be used as a vaccine containing means for targeting at least one antigen to dendritic cells and as adjuvants a granulocyte macrophage colony stimulating factor and a CpG oligodeoxynucleotide and / or a CpG-like oligodeoxynucleotide. This composition can used to treat cancers, infectious diseases caused by bacterial, viral, fungal, parasitic or protozoan infections, allergies and / or autoimmune diseases.
Owner:INSTITUT CURIE +4
Modified CpG oligodeoxynucleotide with improved immunoregulatory function
InactiveUS7408050B2Enhance immune regulation functionOrganic active ingredientsIn-vivo radioactive preparationsAnticarcinogenMedicine
The present invention relates to a modified CpG oligodeoxynucleotide (ODN) which is prepared by coupling a consecutive sequence of deoxyribothymine (dT) to the 3′-terminus of CpG ODN having immunoregularory function, thereby improving immunoactivity of splenocytes, macrophages and peripheral mononuclear cells, and therefore, can be effectively used as a vaccine adjuvant for preventing and treating hepatitis B or an anti-cancer agent. Since the phosphorothioate CpG ODN having the consecutive sequence of dT at its 3′-terminus shows high activity inducing Th-1 immune response and does not elicit in vivo toxicity with guaranteeing its safety, it can be effectively used as a vaccine adjuvant.
Owner:YONSEI UNIVERSITY
Preparation and application of CpG DNA molecule anti-infection and immunity prepn
InactiveCN1692943ARaise the level of immune responseReduce plasmid usageSugar derivativesGenetic material ingredientsBiotechnologyMolecular Immunology
An anti-infectious immunopotentiator for pig, ox, yak, etc is prepared through artificially synthesizing the CpG oligonucleotide sequce able to excite the reproductive activity of immune cell, preparing the chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of CpG oligonucleotide. It can be used to immunize the experimental animal by intramuscular injection or oral applialtion.
Owner:SICHUAN UNIV
CpG oligonucleotide possessing immune enhancement activity to aquatic livestock, its preparation method and its application
ActiveCN102399784AImprove efficiencyLess time consumingAnimal feeding stuffDNA preparationBio engineeringFeed additive
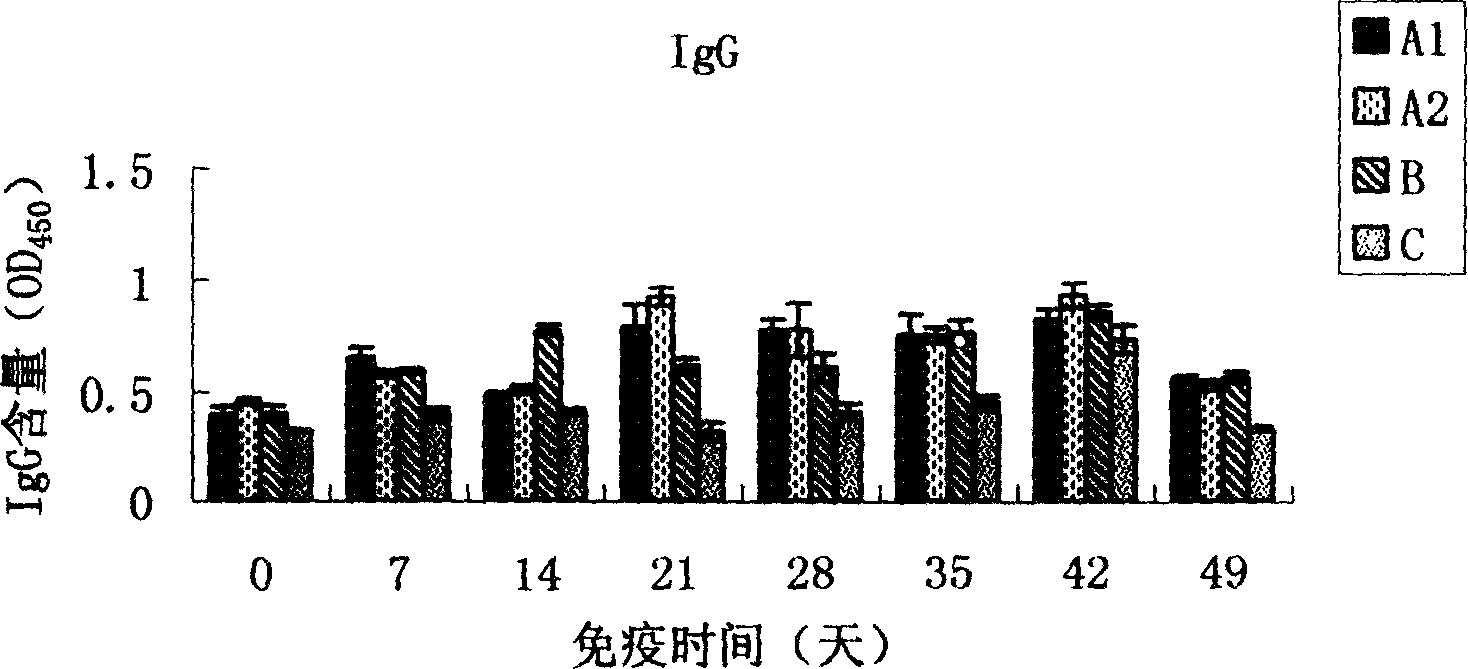
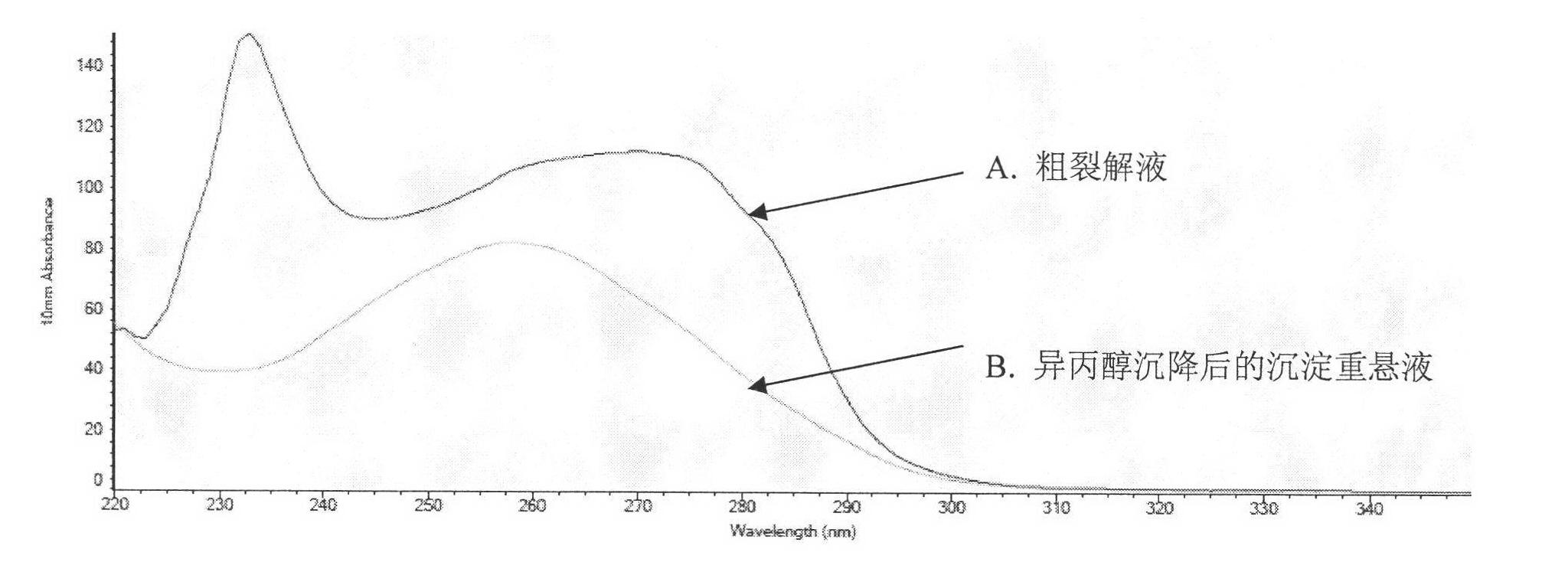
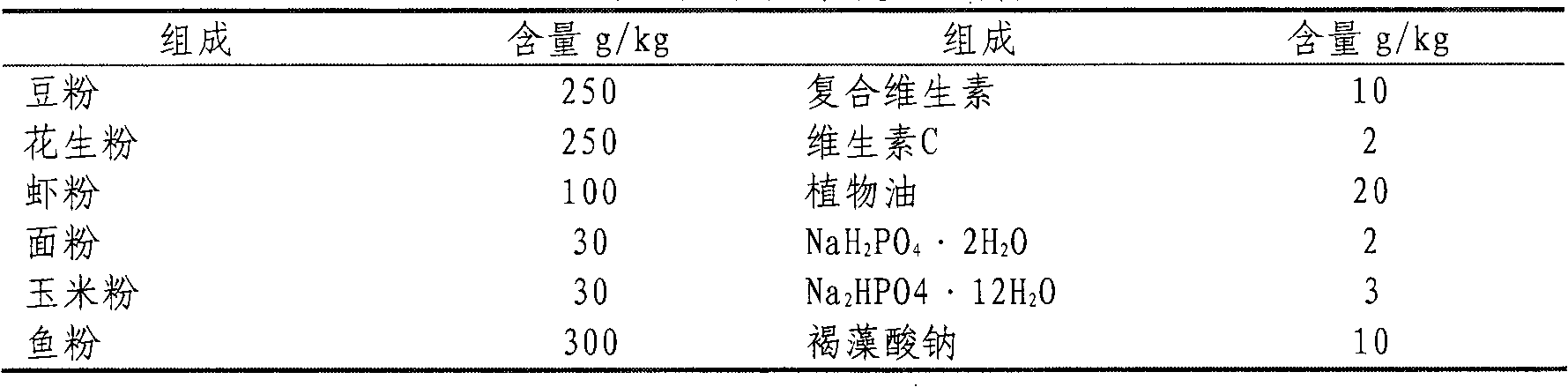
The invention relates to molecular biology, concretely relates to CpG oligonucleotide possessing immune enhancement activity to aquatic livestock such as shrimps and crabs, its preparation method and its application. The CpG oligonucleotide possessing immune enhancement activity has a basic group sequence shown in a sequence table SEQ ID NO.1. The preparation method comprises the following steps: conversing plasmid containing the CpG oligonucleotide sequence into a colibacillus DH5alpha strain, fermenting, collecting the fermented product, separating and purifying CpG ODNs by an isopropanol sedimentation method. The basic group sequence CpG oligonucleotide shown in the sequence table SEQ ID NO.1 can be used as an aquaculture feed additive or an immunopotentiator, such as 10-100mg of the CpG oligonucleotide added in one kilogram feed is capable of obviously promoting the growth of the breeding animals. According to the invention, a biological engineering fermentation technology and a simplified extraction method are used for amplifying and extracting CpG oligonucleotide with large amount, so that the time and the labor can be saved by comparing with that under the experiment condition, and the efficiency is higher. The method for preparing the CpG oligonucleotide is convenient for industrial extension.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
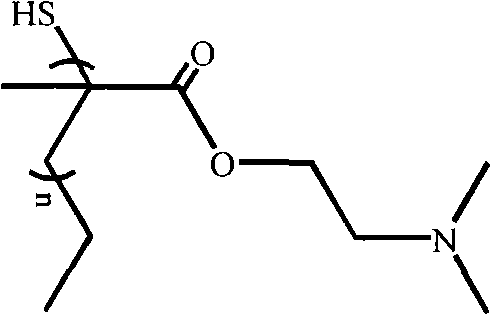
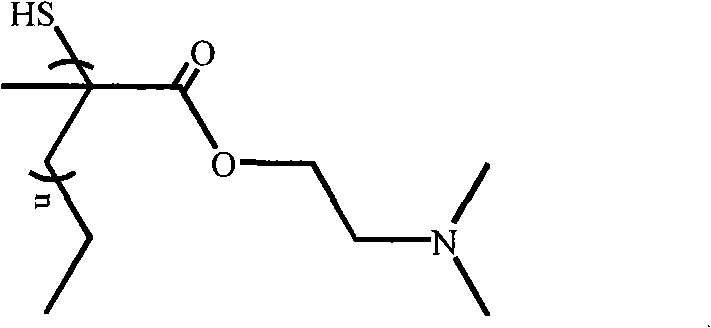
Methacrylic ester polymer, compounds thereof as well as preparation methods and application of all
InactiveCN101659722AEfficient transfectionHas in vitro immune efficacyVector-based foreign material introductionPlasmid dnaIn vivo
The invention discloses a mercaptyl-terminated (2-dimethylamino ethyl) polymethacrylic ester polymer, a self-assembled and modified nano-gold compound (PDMAEMAGNPs) of the polymer as well as a compound of the polymer and plasmid DNA; and the invention also discloses preparation methods of the polymer and the compounds as well as the application of all in carrier as genes. When the concentration ofthe compounds of PDMAEMAGNPs and GFP DNA (green fluorescent protein expression DNA) is 30Mug / mL, the transfection efficiency to HEK 293T cell is 30 percent, and the survival rate to HEK 293T cell, HeLa cell and 293 cell is more than 80 percent. The PDMAEMAGNPs / CpG ODN (human CpG oligodeoxynucleotide) compound has remarkable effects in gene therapy in vitro and in vivo. The suppression ratio to tumor growth of the PDMAEMAGNPs / p53 DNA (tumor suppressor gene) is 30-37 percent.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
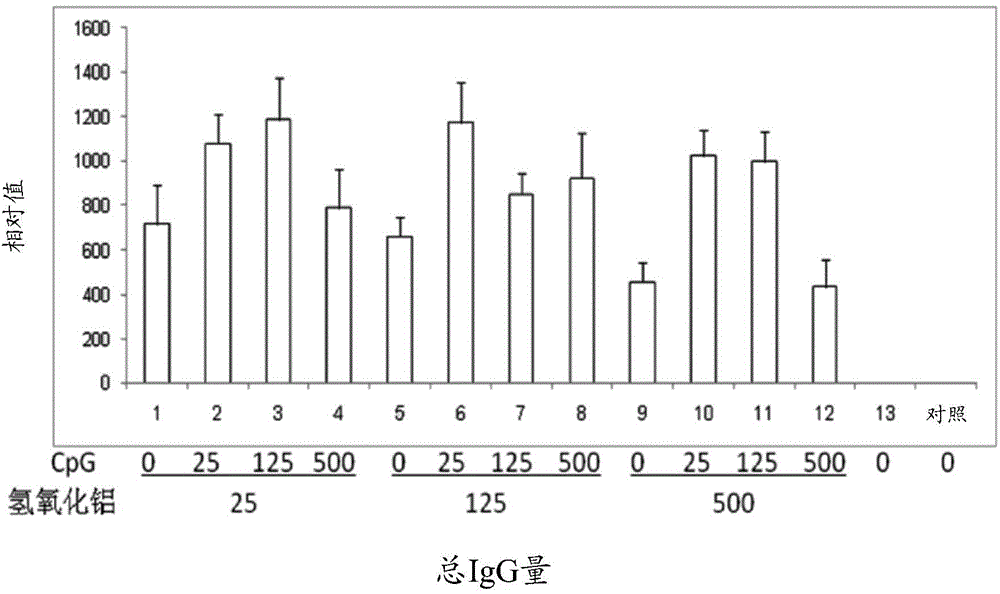
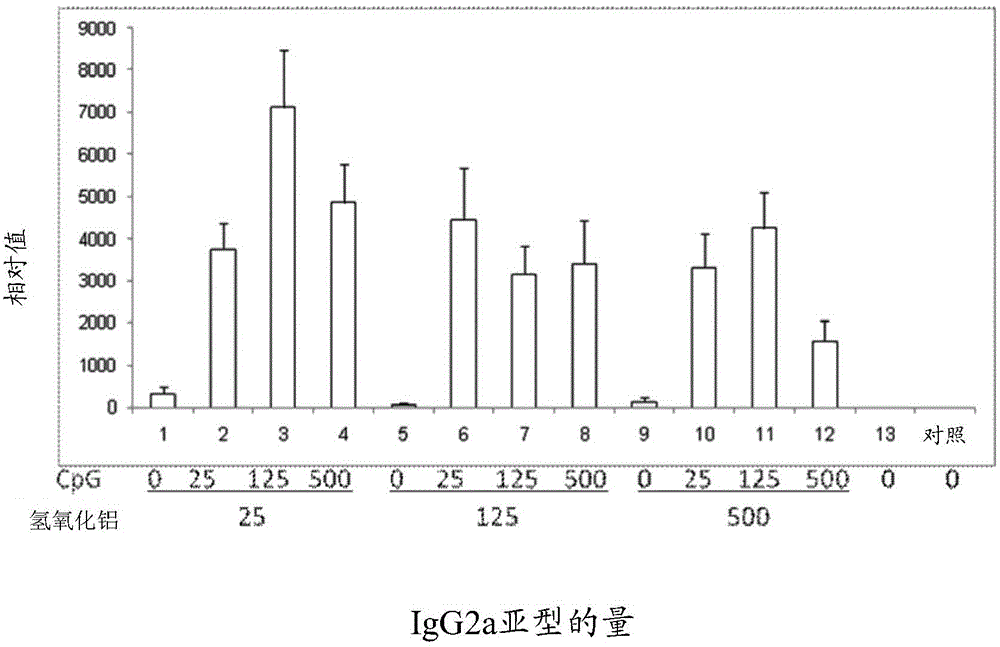
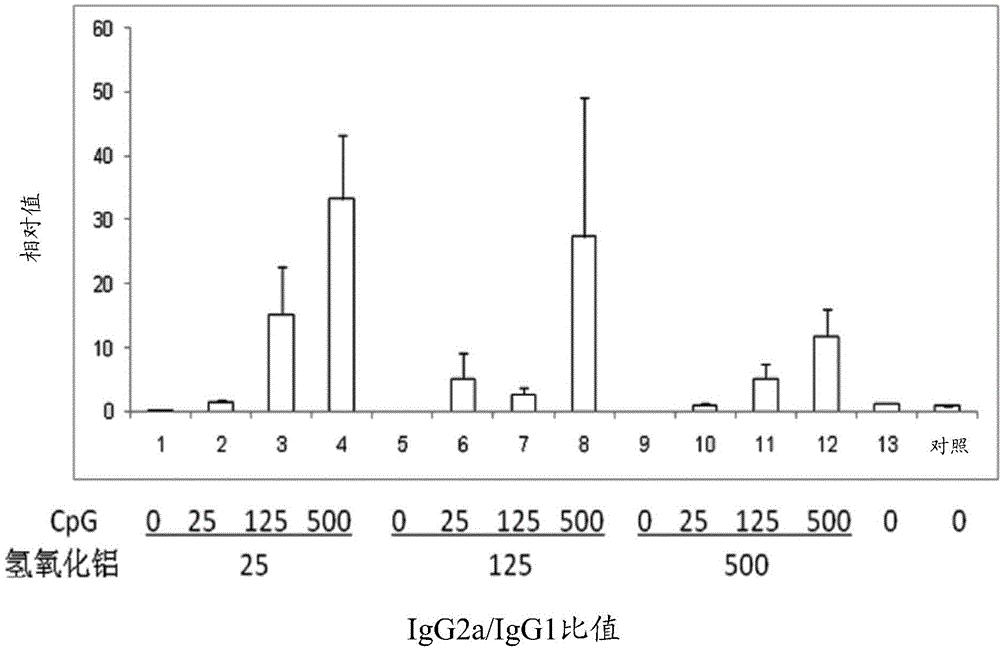
Pharmaceutical composition containing CpG oligonucleotide
InactiveCN105214083AShorten the duration of full immunizationReduce the number of dosesViral antigen ingredientsAntiviralsAdjuvantPharmaceutical drug
The invention provides a pharmaceutical composition for inducing immunoreaction in a subject's body. The pharmaceutical composition comprises 1 Mug / ml to 100 Mug / ml of an antigen (a), 25 Mug / ml to 500 Mug / ml of CpG oligonucleotide containing a sequence 5'-tcgacgttcgtcgttcgtcgttc-3', and 25 Mug / ml to 500 Mug / ml of an aluminum adjuvant (c). The pharmaceutical composition may induce or enhance the immunoreaction for antigens, in the subject's body.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif: X1X2X3X4X5X6X7X8, wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Compound adjuvant for tuberculosis subunit vaccine, tuberculosis subunit vaccine from same and preparation method and application thereof
InactiveCN103203018AImproving immunogenicityHelps induce Th1 type anti-TB immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantNucleotide
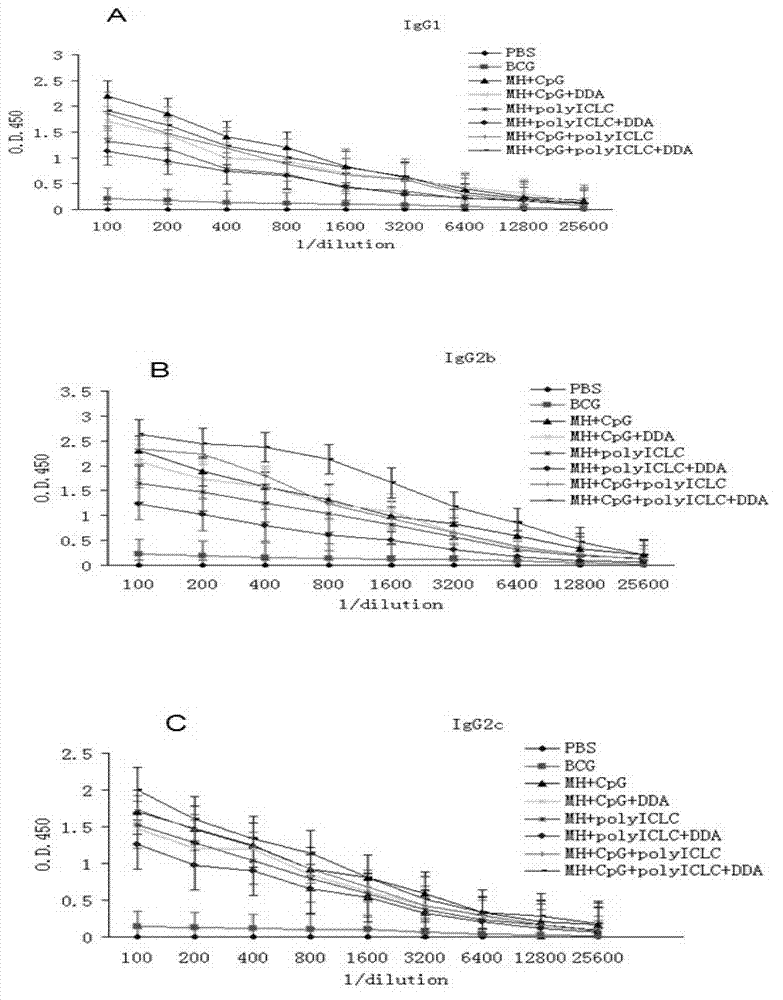
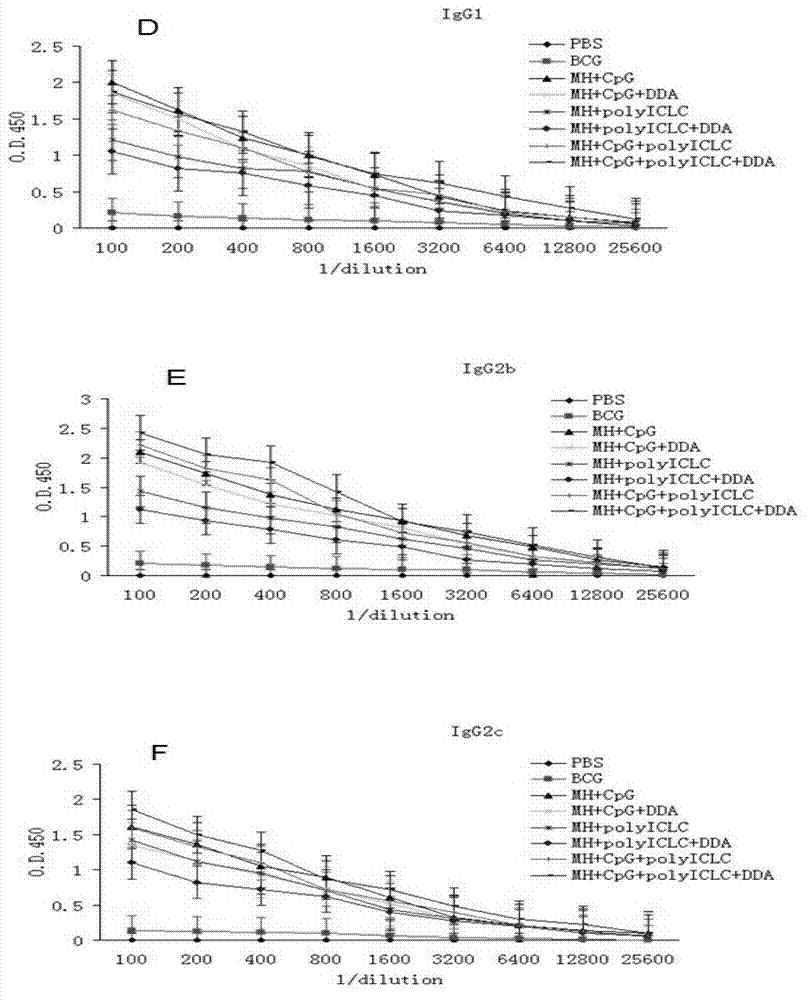
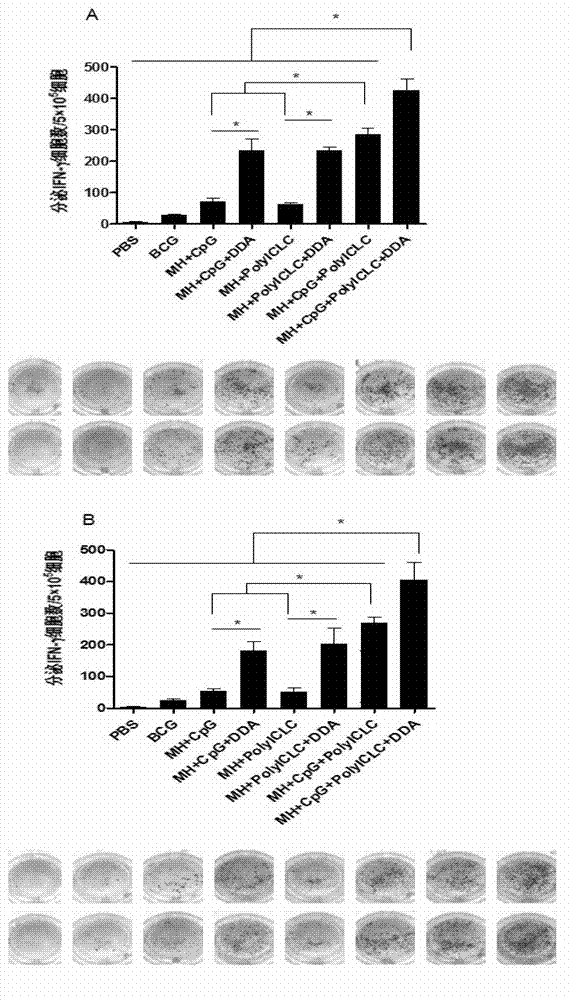
The present invention discloses a compound adjuvant for tuberculosis subunit vaccine, containing cationic liposome dimo-thylidioctyl ammonium bromide (DDA), TLR9 ligand CpG oligodeoxynucleotides 2395 and TLR3 ligand PolyICLC, and also provides the tuberculosis subunit vaccine and preparation method and application thereof. The beneficial effect of the invention is that the compound adjuvant for tuberculosis subunit vaccine provided by the present invention enhances the immunogenicity of the fusion protein of Mycobacterium tuberculosis, contributing to the induction of the tuberculosis fusion protein to Th1-type anti-tuberculosis immune response.
Owner:LANZHOU UNIVERSITY
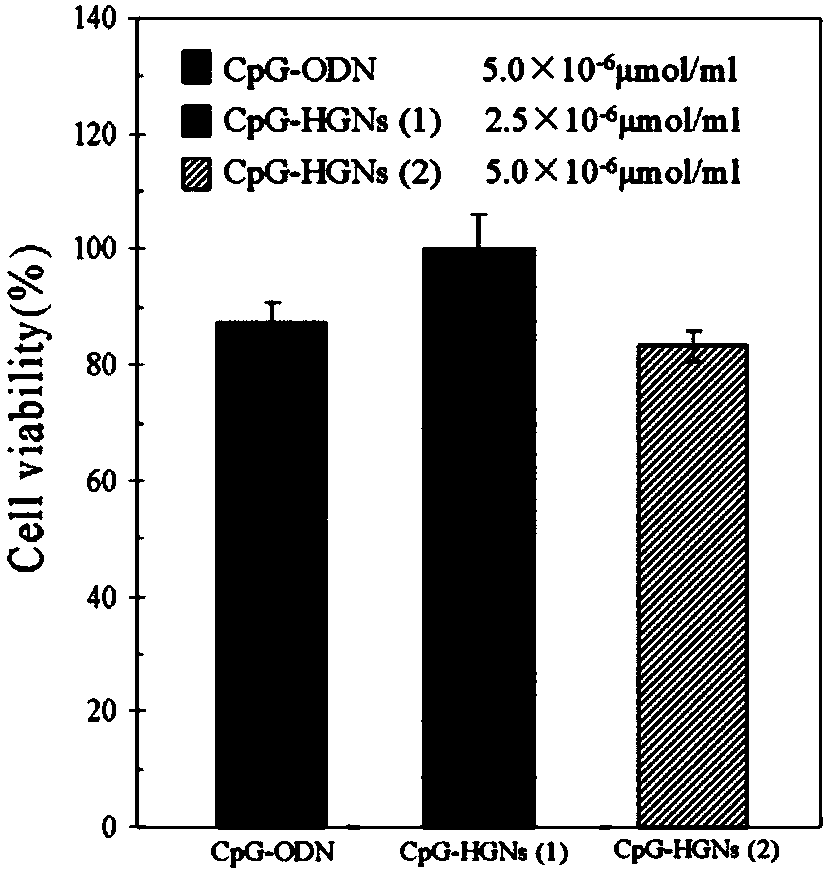
Hollow gold nanospheres modified by CpG oligodeoxynucleotide as well as preparation method and applications
InactiveCN108578694AImprove intake capacityIncrease loadEnergy modified materialsCancer antigen ingredientsAntitumor immunityActive agent
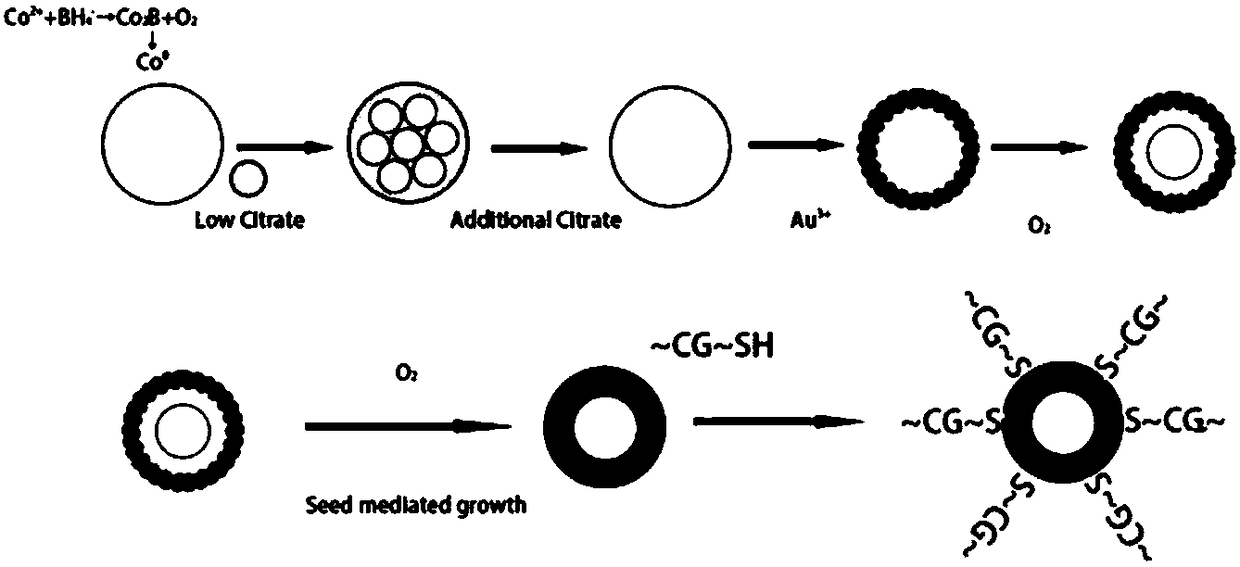
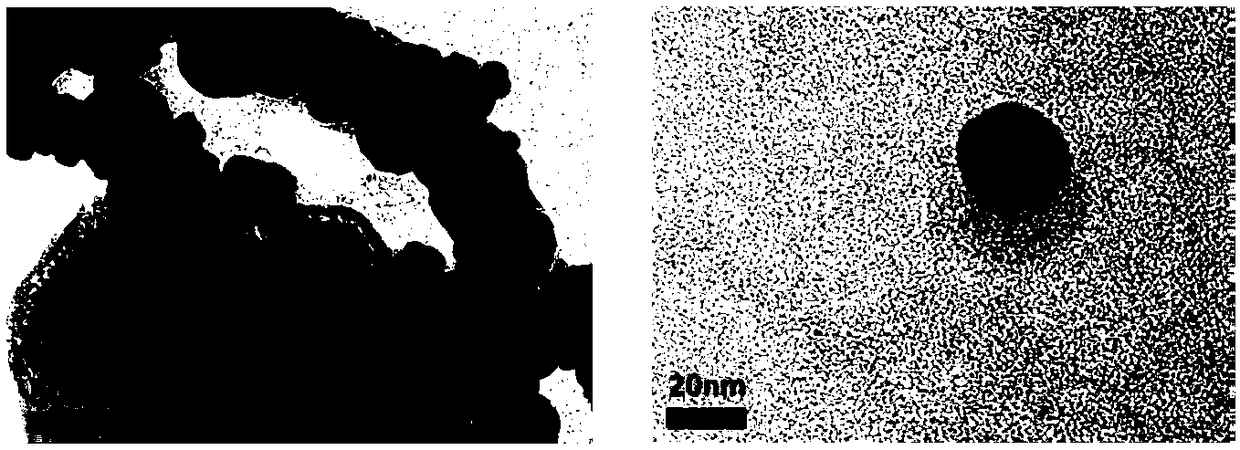
The invention discloses a preparation method and applications of hollow gold nanospheres modified by CpG oligodeoxynucleotide. Cobalt chloride is subjected to reduction by adopting sodium borohydride,thus cobalt nanoparticles are obtained, cobalt nanoparticles are adopted for carrying out reduction on chloroauric acid, and thus hollow gold nanospheres are prepared; CpG oligodeoxynucleotide, a surfactant and a buffer solution are added into a solution of the hollow gold nanospheres, shaking culture is carried out, and thus the hollow gold nanosphere material modified by CpG oligodeoxynucleotide is obtained. According to the technical scheme, by utilizing the property that the hollow gold nanospheres have the large loading capacity, CpG is loaded through the self-assembly effect, the synthesis method is simple, and the method is suitable for large-scale production and application; the cell uptake capacity of CpG oligodeoxynucleotide can be enhanced, and the biocompatibility is good; thehollow gold nanospheres modified by CpG oligodeoxynucleotide have the near-infrared surface plasma adsorbing effect, the primary tumor is eliminated by utilizing the photothermal ablation effect, a tumor antigen is generated in situ, under the promoting of CpG oligodeoxynucleotide, the antitumor immunity of the whole body is induced, and the distant metastasis tumor is further treated.
Owner:WUHAN UNIV OF SCI & TECH
Method for quickly screening stichopus japonicus immune enhancers with high throughput
The invention discloses a method for quickly screening stichopus japonicus immune enhancers with high throughput, which is characterized by comprising the following steps: firstly determining beta-glucan, peptidoglycan, chitosan, mannan-oligosaccharide, CpG-oligodeoxynucleotide, soy peptide, lactoferrin, levamisole, vitamin C and vitamin E as the screened immune enhancers, then carrying out primary culture for stichopus japonicus coelom cells, detecting four sensitive immune indexes of the immune enhancers on stichopus japonicus by the cultured stichopus japonicus coelom cells, namely effects of phagotrophy rate, superoxide anion content, superoxide dismutase activity and nitric oxide synthase activity, and determining a screening result for the effects of the four indexes according to the immune enhancers. The method can screen the immune enhancers on large scale and in short time, and simultaneously can research the action mechanisms of the various immune enhancers and lay a foundation for the deep study of the immune enhancers.
Owner:OCEAN UNIV OF CHINA
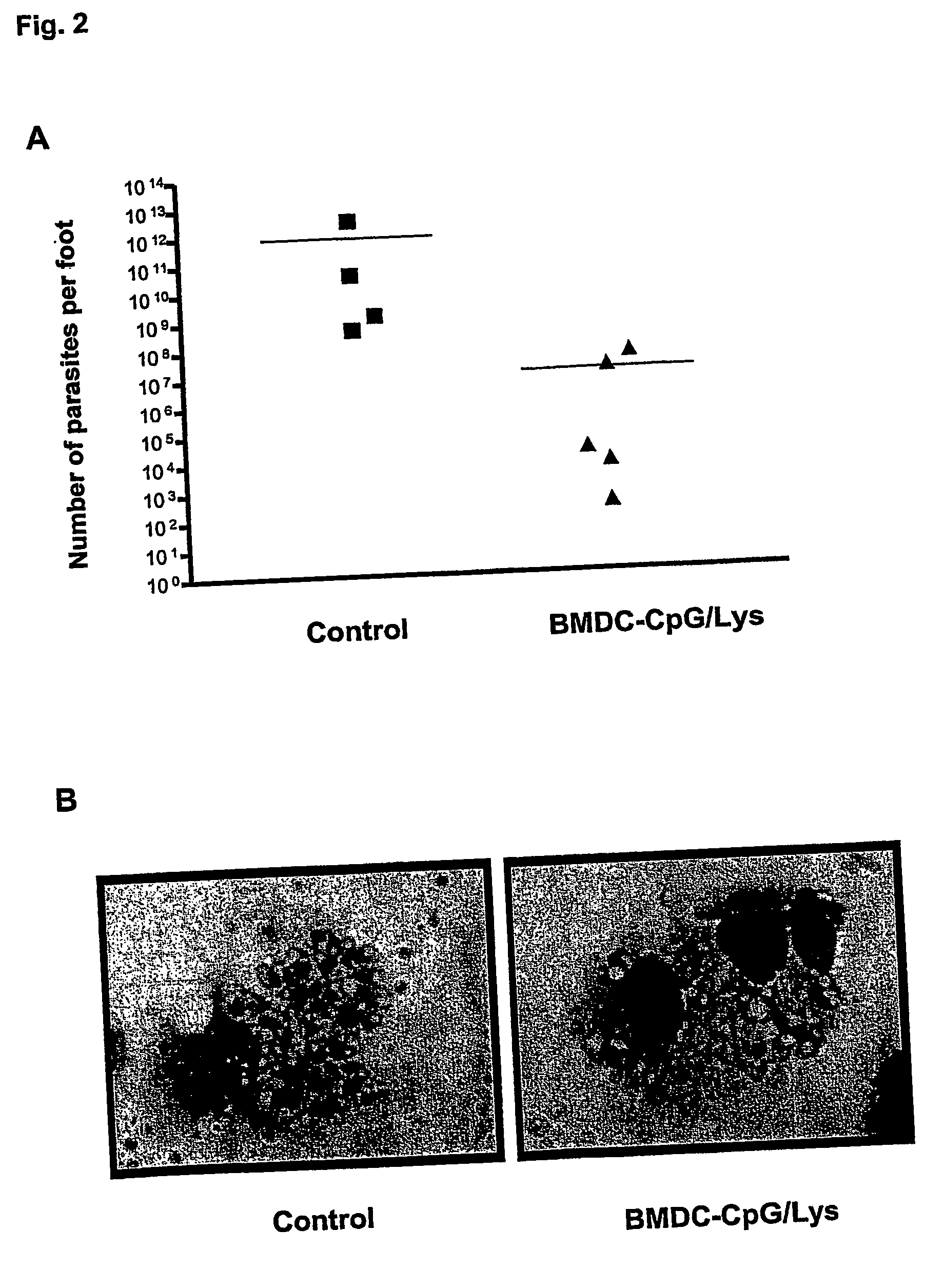
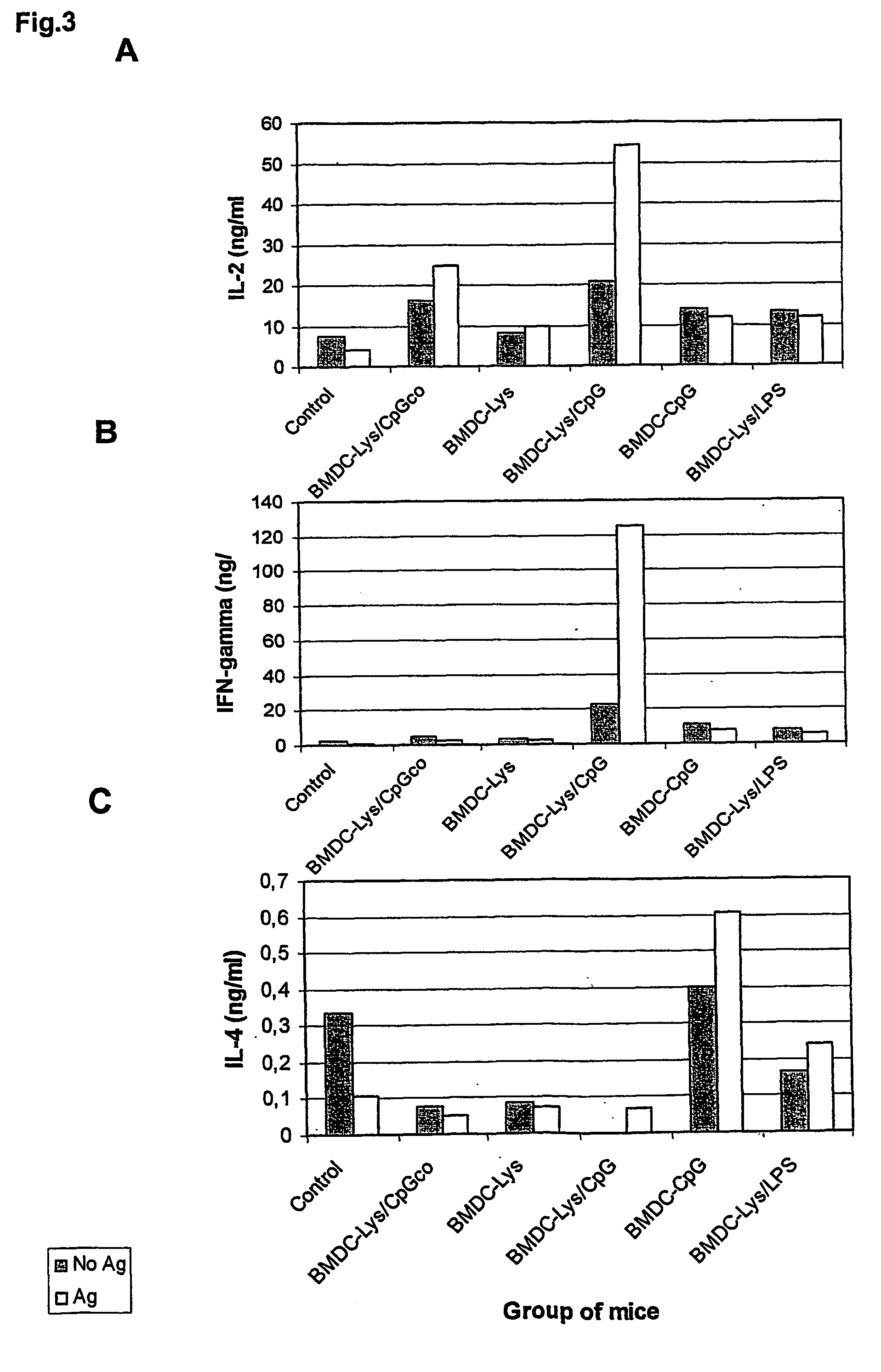
Method for generating antigen-presenting cells
Described is a method for the generation of antigen-presenting cells (APC), preferably bone marrow-derived dendritic cells (BMDC) or peripheral blood-derived dendritic cells, as antigen carrier having immunostimulatory properties for anti-infective treatment comprising the steps of (a) pulsing the APC with antigen and (b) treating the APC with a CpG oligonucleotide. Said APC are useful as an immune prophylactic or immune therapeutic agent against diseases like AIDS, tuberculosis, malaria or leishmaniasis.
Owner:MERCK PATENT GMBH
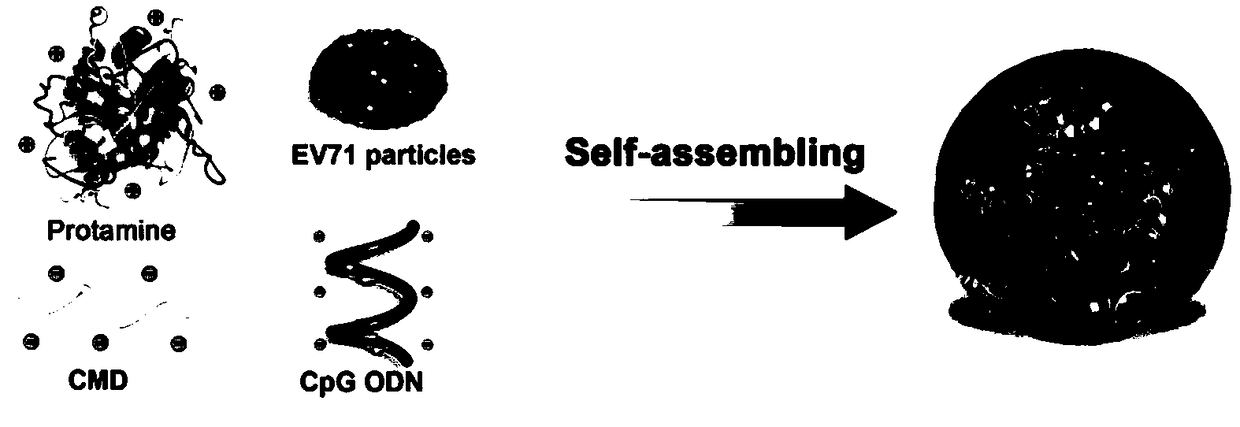
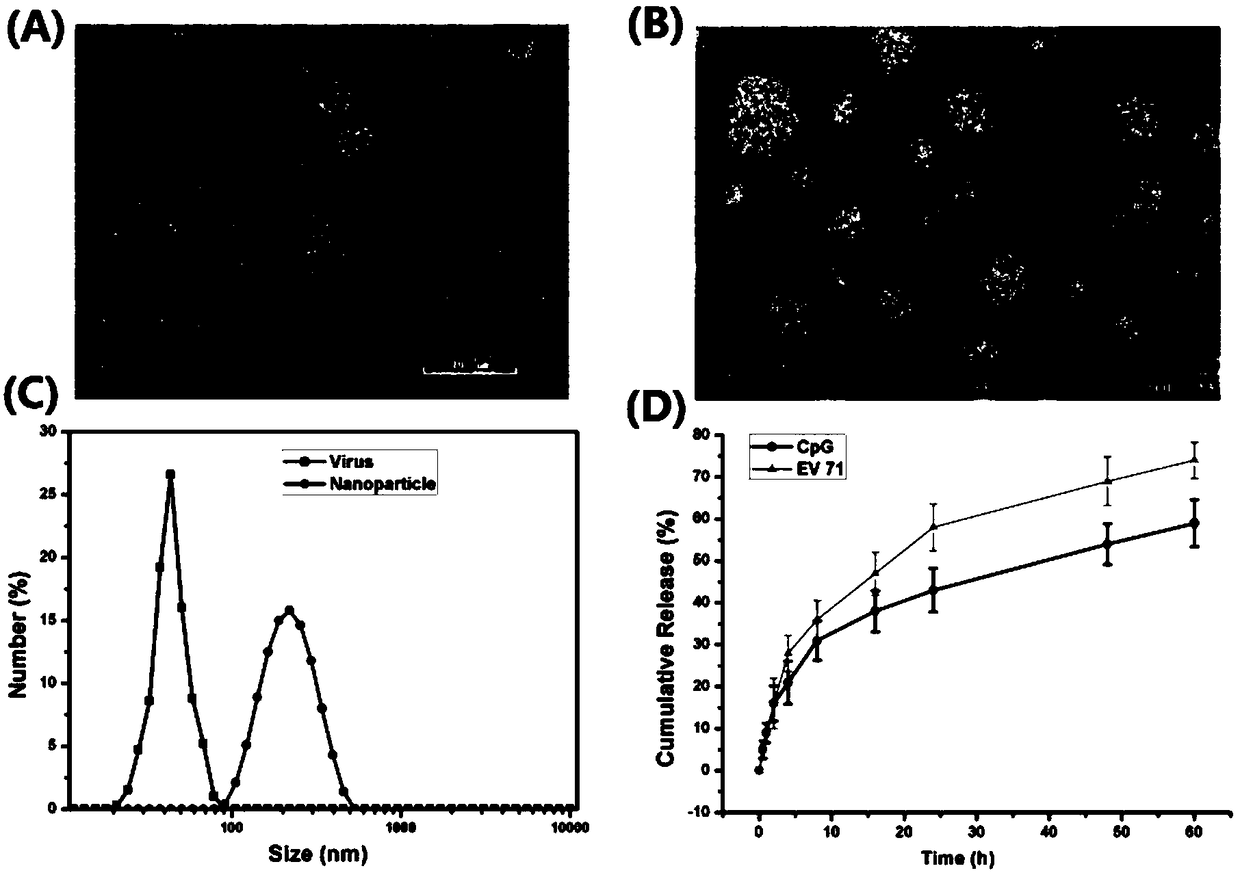
Self-assembly nano adjuvant and preparation method of nano vaccine formed by self-assembly nano adjuvant and application
InactiveCN108714213AEfficient packagingSolve the problem of easy degradation in the body and low bioavailabilitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantMedicine
The invention relates to a self-assembly nano adjuvant and a preparation method of a nano vaccine formed by the self-assembly nano adjuvant and application. The nano adjuvant comprises self-assembly materials of protamine sulfates and carboxymethyl glucan, the self-assembly materials and CpG oligodeoxynucleotides self-assembly form the nano adjuvant, and the nano vaccine is formed by the nano adjuvant and the virus antigen. The nano adjuvant increases the bioavailability of the CpG oligodeoxynucleotides, degradation in the body is avoided, and the B type CpG is added the function of A type CpG. The nano vaccine not only can induce the humoral immune response of TH1 type, but also induce relatively high cellular immune response. The unvaccinated experiments in mice body proves that the nanovaccine has good protection function, which provides help in the application of nano vaccine in the future.
Owner:BEIJING UNIV OF TECH
Modified CpG oligodeoxynucleotide with improved immunoregulatory function
InactiveUS20050152921A1Improve survival rateSurvival rateOrganic active ingredientsGenetic material ingredientsAnticarcinogenCpG Oligodeoxynucleotide
The present invention relates to a modified CpG oligodeoxynucleotide (ODN) which is prepared by coupling a consecutive sequence of deoxyribothymine (dT) to the 3′-terminus of CpG ODN having immunoregularory function, thereby improving immunoactivity of splenocytes, macrophages and peripheral mononuclear cells, and therefore, can be effectively used as a vaccine adjuvant for preventing and treating hepatitis B or an anti-cancer agent. Since the phosphorothioate CpG ODN having the consecutive sequence of dT at its 3′-terminus shows high activity inducing Th-1 immune response and does not elicit in vivo toxicity with guaranteeing its safety, it can be effectively used as a vaccine adjuvant.
Owner:YONSEI UNIVERSITY
Treatment with an oncolytic virus and an immunostimulant for in vivo enhancement of immune system recognition of neoplasms
InactiveCN101304761ANo or no toxicityOrganic active ingredientsSugar derivativesDendritic cellOncolytic adenovirus
Owner:ONCOLYTICS BIOTECH
Method capable of renforving gene gun inoculation DNA Vaccine inducel cell immune response
InactiveCN1513559AEnhance the level of Th1 type immune responseGenetic material ingredientsGene therapyAntigenAdjuvant
A method for inducing the Th1-type immune response for cells after the gene gun is used to inoculate DNA vaccine features that when the plasmid DNA vaccine is inoculated by gene gun, the synthetic oligonucleotide CpG DNA (CpG ODN) as adjuvant is proportionally introduced. A process for preparing the plasmid DNA-CpG oligonucleotide coated gold particle is also disclosed. It can be used for treating tumor and durable virus infection.
Owner:FUDAN UNIV +1
Nucleic acid derivative having immunostimulatory activity
ActiveUS20180264105A1High activityAntibacterial agentsCancer antigen ingredientsAdjuvantCpg oligonucleotides
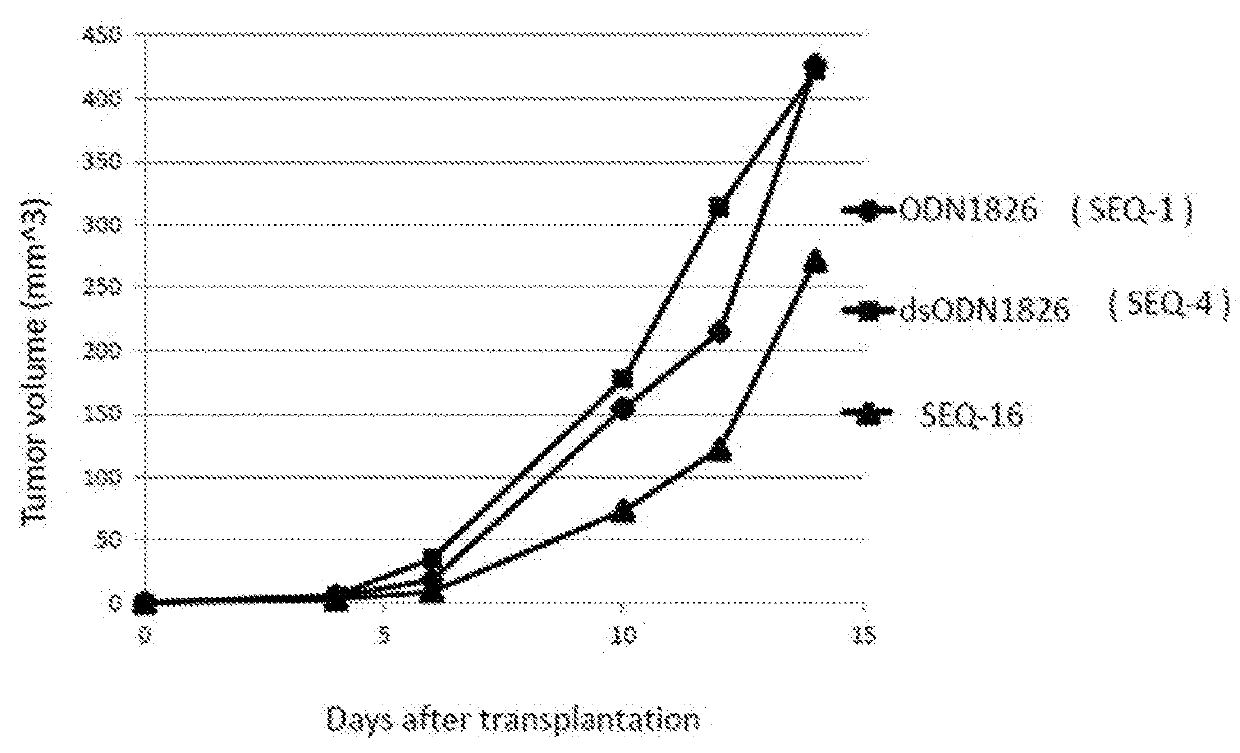
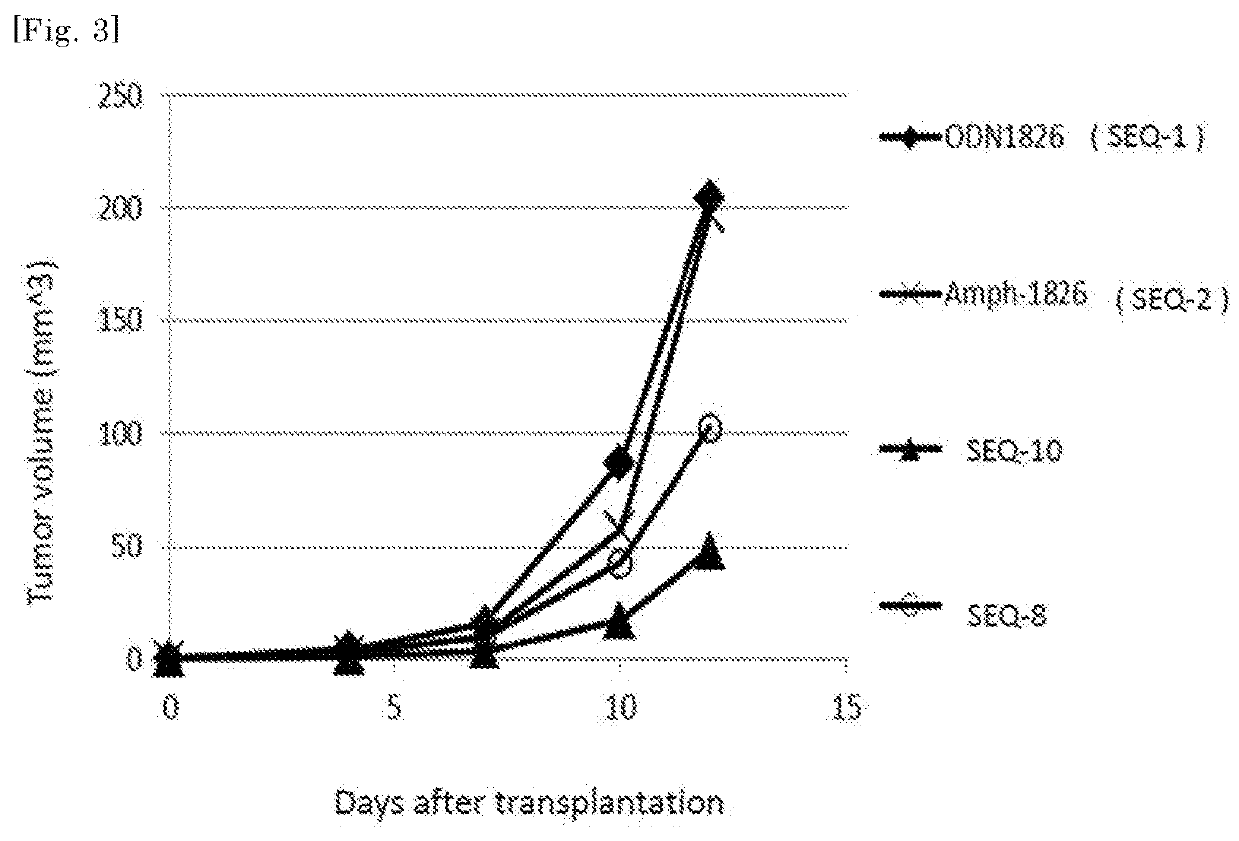
The purpose of the present invention is to provide double-stranded oligonucleotides comprising the CpG oligonucleotide mentioned below, as a nucleic acid derivative having an immunostimulatory activity.An adjuvant comprising a double-stranded oligonucleotide, whereina first strand is a CpG oligonucleotide consisting of 8 to 50 nucleotides,a second strand is an oligonucleotide consisting of 8 to 60 nucleotides and comprisinga sequence capable of hybridizing with the first strand, and a lipid binds to the second strand through a linker.
Owner:SHIONOGI & CO LTD
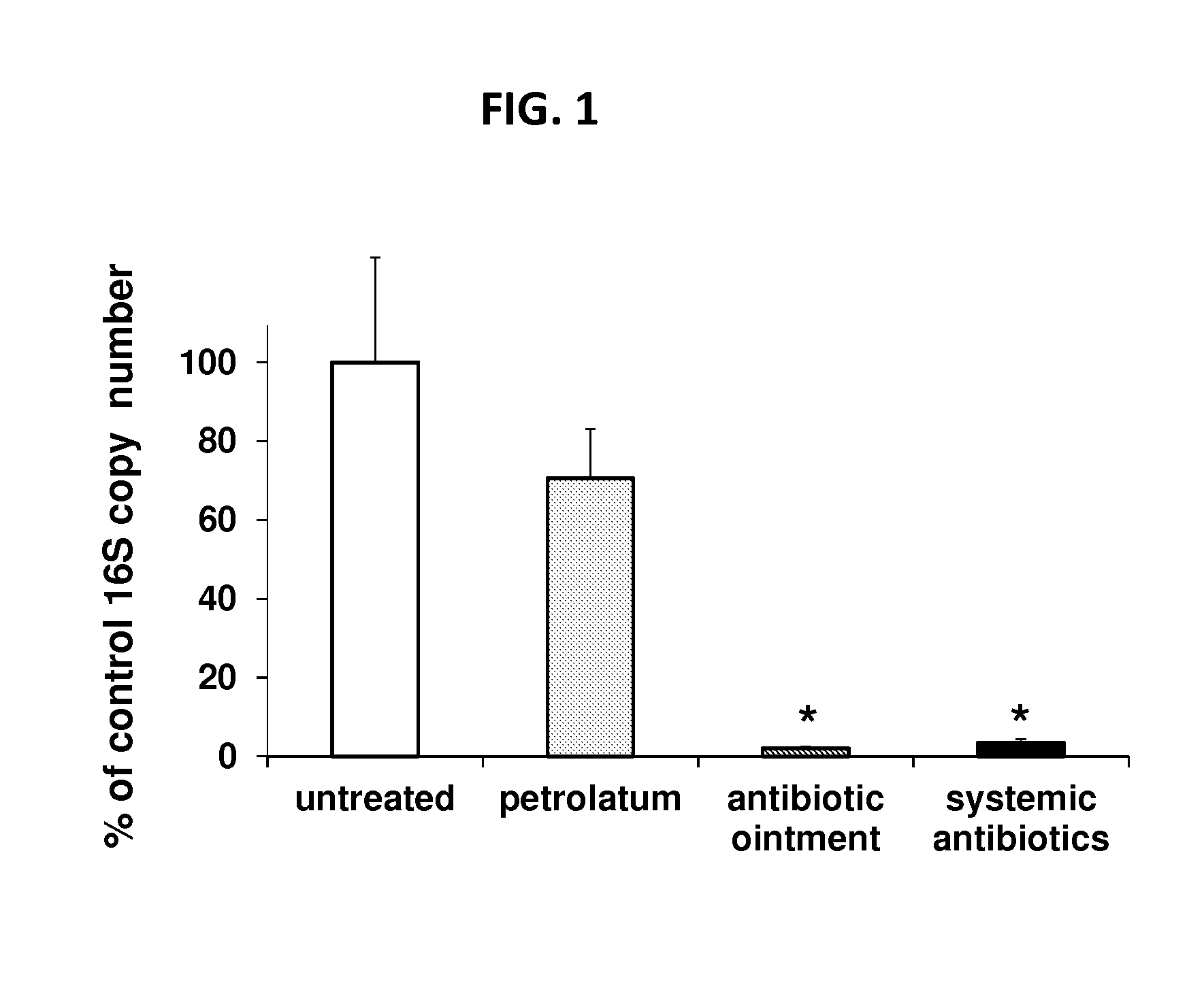
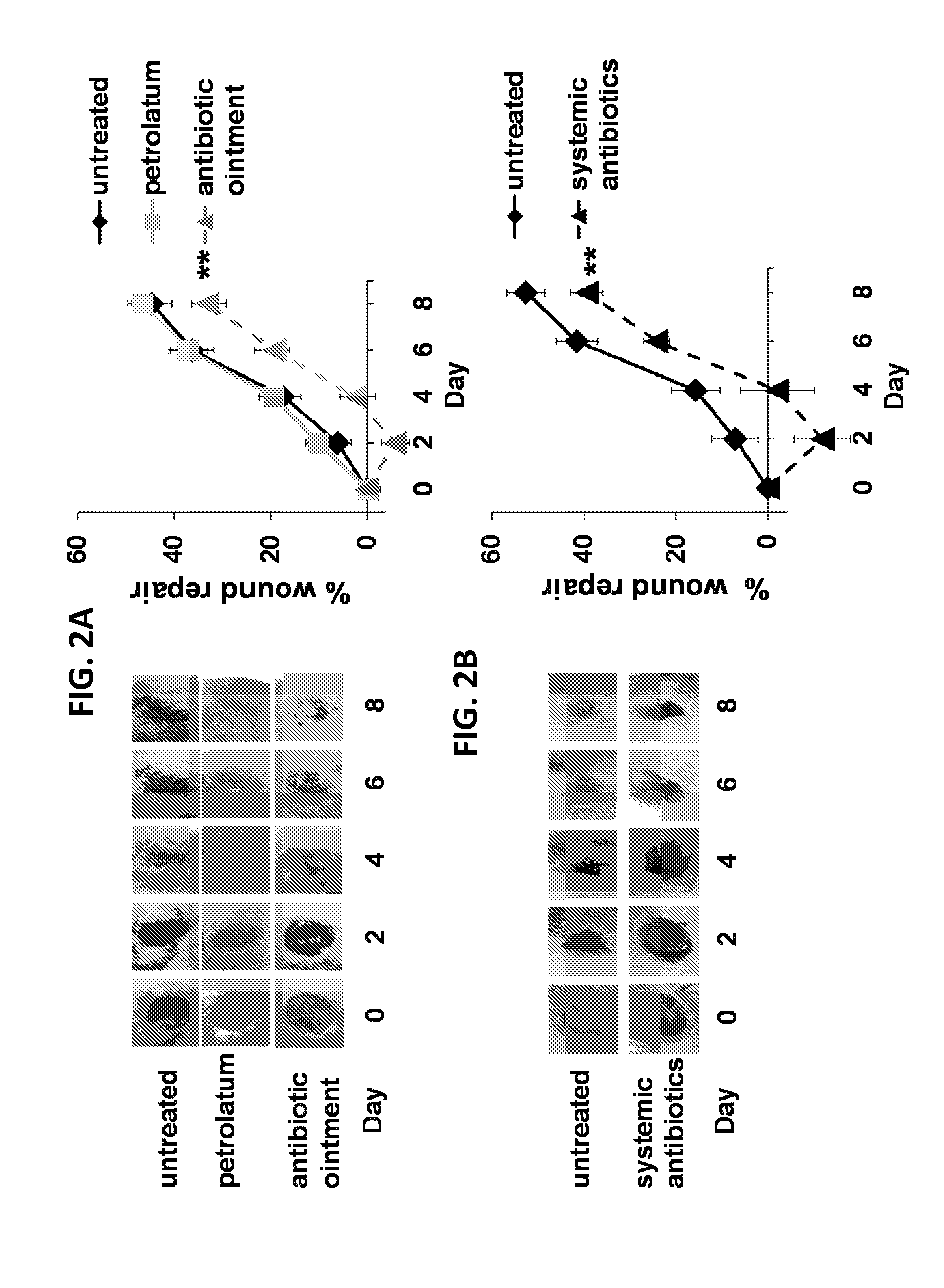
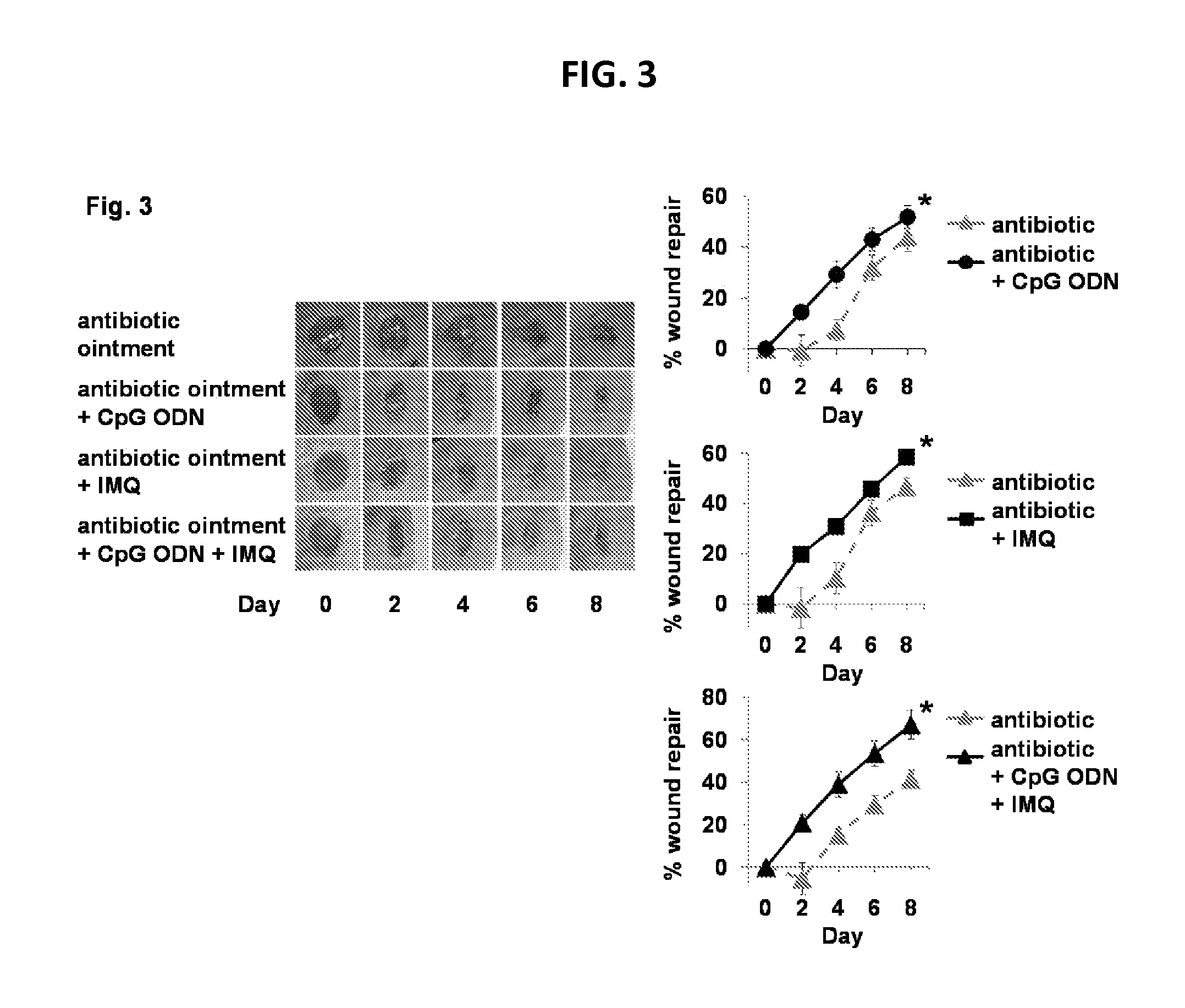
Use of cpg oligonucleotides co-formulated with an antibiotic to accelerate wound healing
ActiveUS20150104482A1Delay wound healingDelayed resistanceOrganic active ingredientsOintment deliveryReceptorNucleotide
Pharmaceutical compositions are provided that include an antibiotics, but that include ingredients that counteract the effect of that antibiotic on wound healing, without altering the bactericidal properties of the antibiotic. These pharmaceutical compositions include an effective amount of 1) an imidazoquinoline having toll-like receptor 7 (TLR7) ligand activity, 2) an immunostimulatory K-type CpG oligodeoxynucleotide (ODN) comprising an unmethylated CpG motif, 3) an antibiotic, and 4) a surfactant, wherein the composition is formulated for topical administration. Methods for accelerating wound healing are also provided. These methods include topically administering the disclosed compositions. The wound can be in the skin or in the eye.
Owner:UNITED STATES OF AMERICA
Immunostimulatory composition and use thereof
PendingCN112972670AStrong immune responseEnhance immune stimulationAntibacterial agentsOrganic active ingredientsDiseaseAdjuvant
The invention provides an immunostimulatory composition, the immunostimulatory composition comprises saponin and CpG oligodeoxynucleotide, or the immunostimulatory composition is composed of an adjuvant containing the saponin, and the CpG oligodeoxynucleotide, wherein the CpG oligodeoxynucleotide sequence is provided with two or more copies of a 5'-TTCGTT-3' sequence motif or a 5'-TCGTCGTCG-3 'sequence motif. The invention provides the use of the immunostimulatory composition in the preparation of a medicament for the treatment of diseases.
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
SPECIFIC VIRUS-LIKE PARTICLE-CpG OLIGONUCLEOTIDE VACCINES AND USES THEREOF
InactiveUS20170035864A1Viral antigen ingredientsCancer antigen ingredientsMedicineCpg oligonucleotides
The invention provides vaccines containing, as its only active ingredient, a VLP having a CpG oligonucleotide attached thereto and a non-toxic pharmaceutically acceptable carrier or diluent and uses thereof. The invention further provides a pharmaceutical composition comprising a vaccine consisting of a VLP having a CpG oligonucleotide, one or more non-toxic pharmaceutically acceptable carrier or diluent, and a therapeutic agent admixture therewith and uses thereof.
Owner:BULLET BIOTECH
Antigen and adjuvant co-delivery nanometer vaccine applied to liver cancer immunization therapy
ActiveCN110585426AIncrease endocytosisImprove the effect of immunotherapyPowder deliveryLiver cancer vaccineDendritic cellTreatment effect
The invention relates to an antigen and adjuvant co-delivery nanometer vaccine applied to liver cancer immunization therapy. Sodium alginate and polymine are used as carrier materials, liver cancer specific polypeptide antigen phosphatidylinositol proteoglycan 3127-136 peptides(GPC3127-136, AMFKNNYPSL) are used as immunizing antigens, CpG oligodeoxynucleotide is used as an adjuvant, and through static electricity interaction, the antigen and adjuvant co-delivery nanometer vaccine is prepared, wherein the mass ratio of the carrier materials to the immunizing antigens to the adjuvant is 1: (1-10): (0.3-0.8). The nanometer vaccine disclosed by the invention can increase the endocytosis quantity of dendritic cells (dendritic cell, DC) to the antigen and the adjuvant, and through raising costimulatory molecules on the surfaces of the DCs and promoting the secretion of cytokine of TNF-a, IL-6 and the like, arousing organisms to produce effective immune response is facilitated; and besides, the raw materials are cheap and easy to obtain, the preparation method is easy and easily repeated, large-scale processing and producing are easy, the liver cancer immunization therapy effects can be strengthened, and the antigen and adjuvant co-delivery nanometer vaccine has favorable application prospects.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Preparation of anti-infection CpG oligonucleotide DNA preparation and its application technology
InactiveCN1720922ARaise the level of immune responseReduce plasmid usagePowder deliveryOrganic active ingredientsProliferation activityExperimental animal
The invention provides a fragment of CpG oligodeoxynucleotides sequence capable of stimulating proliferation activities of animal immunologic cells, and a method for packaging this fragment of CpG oligodeoxynucleotides to prepare anti-infection DNA nano granular formulation, the use of the CpG nano particles as novel anti-infection molecular immune enhancement agent is also disclosed. The method comprises packaging CpG oligodeoxynucleotides with chitosan nano particles, immunizing experimental animals with the prepared DNA nano granular formulation separately or synergically through intramuscular injection or oral administration, finally detecting the cell and body fluid immunity indexes of the experimental animal.
Owner:SICHUAN UNIV +1
Complex containing oligonucleotide having immunopotentiating activity and use thereof
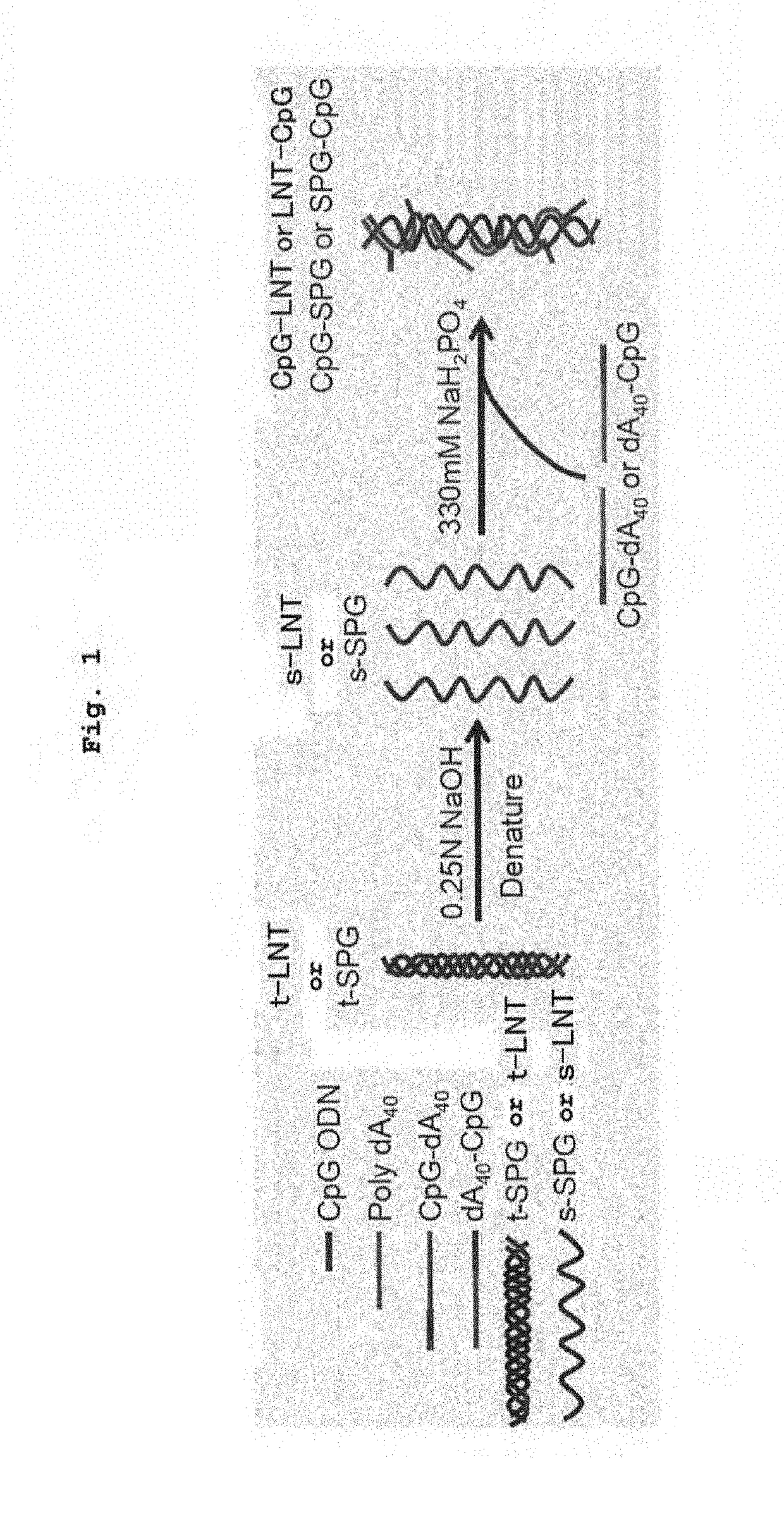
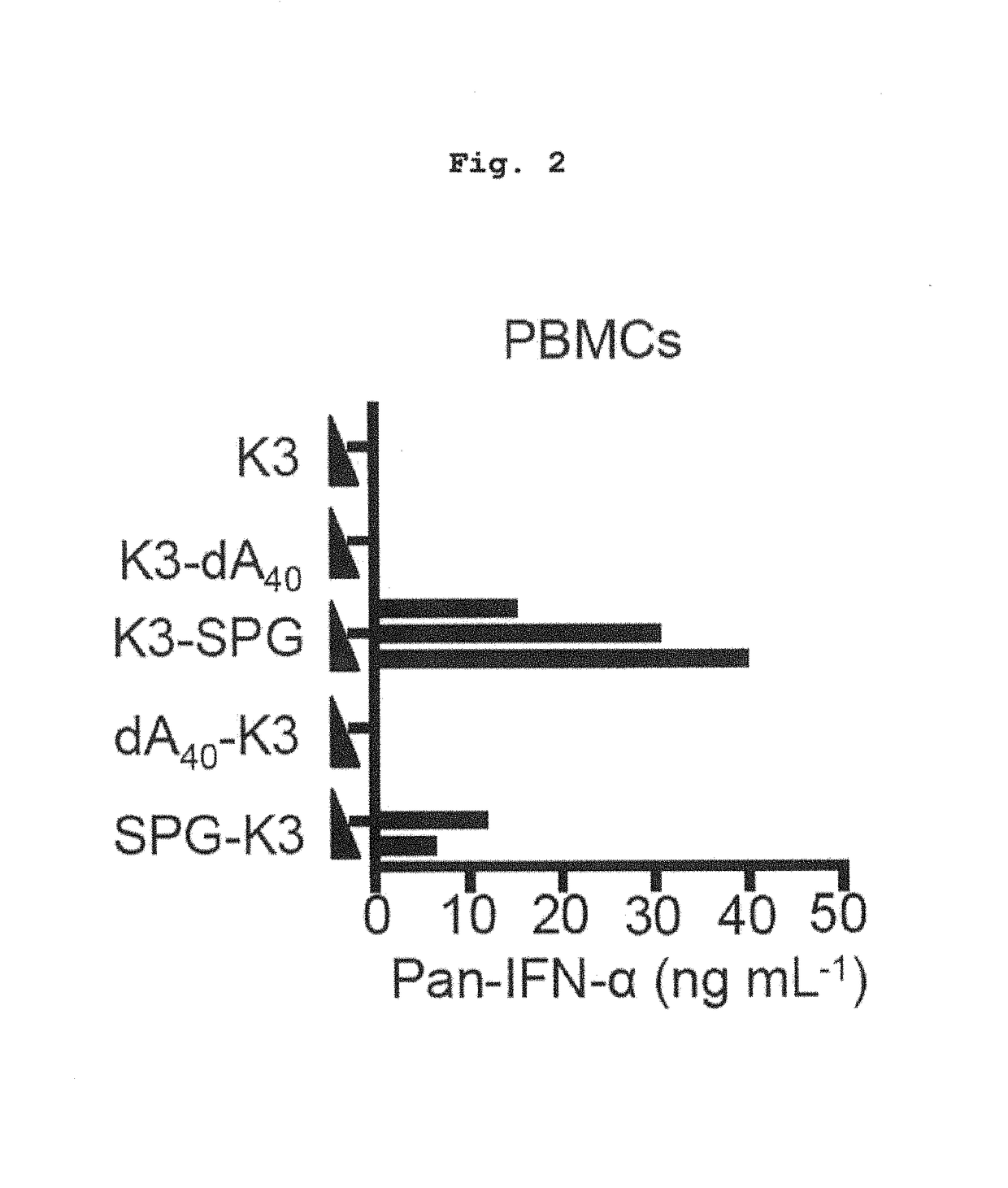
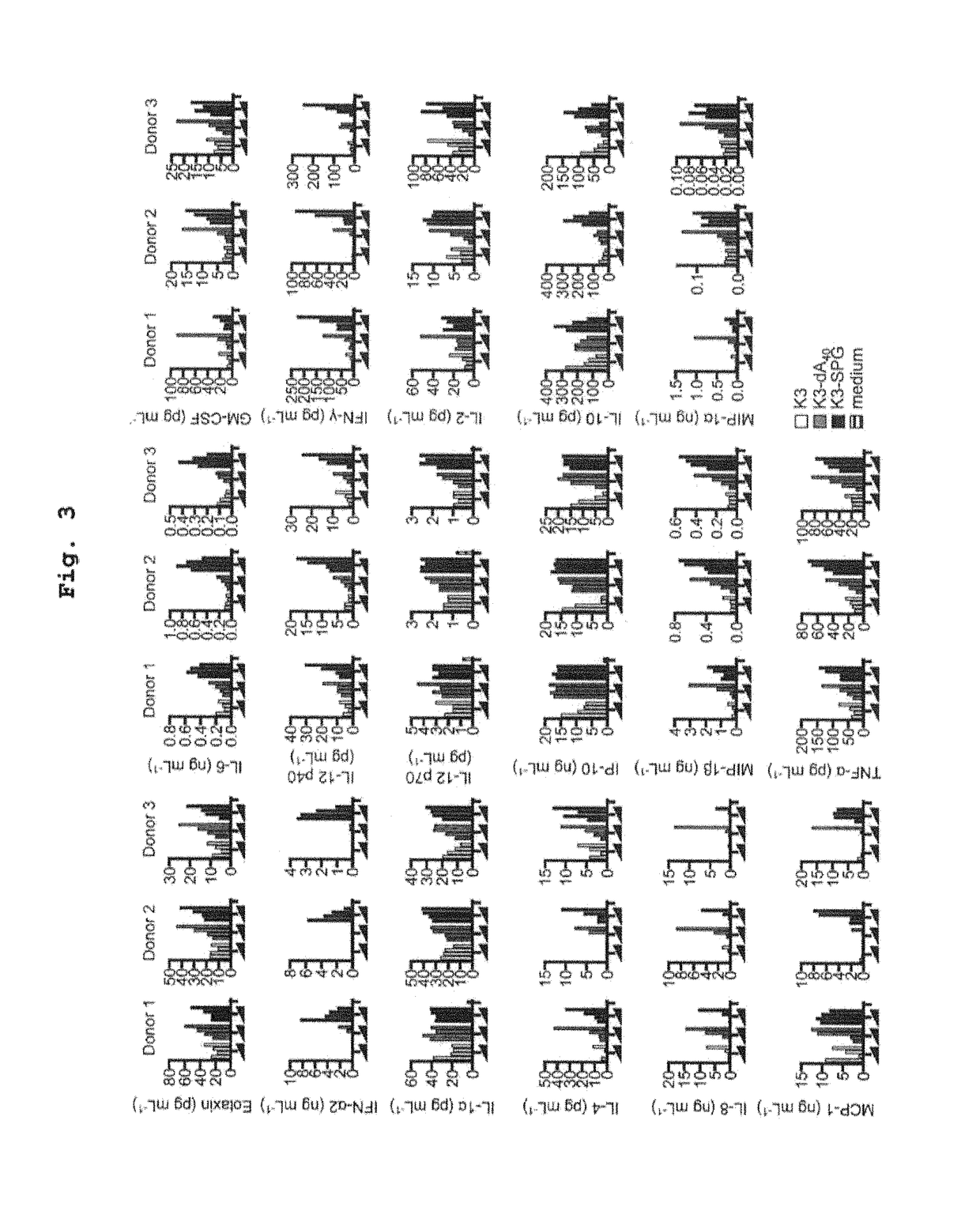
ActiveUS10202606B2High activityImprove protectionAntibacterial agentsSsRNA viruses negative-senseCpG OligodeoxynucleotideDeoxyadenosine
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com