Targeted innate immunity
a technology of innate immunity and immune system, applied in the field of cancer therapy agents and methods, can solve the problems of limited immunotherapy attempts, inadequate immune response,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Generation of CpG Immunostimulatory Oligonucleotide Linked chTNT-3 Immunoconjugates
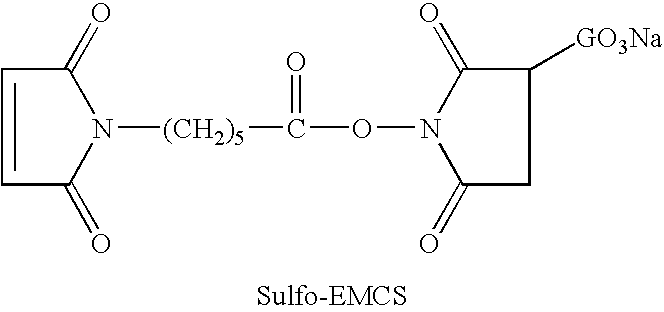
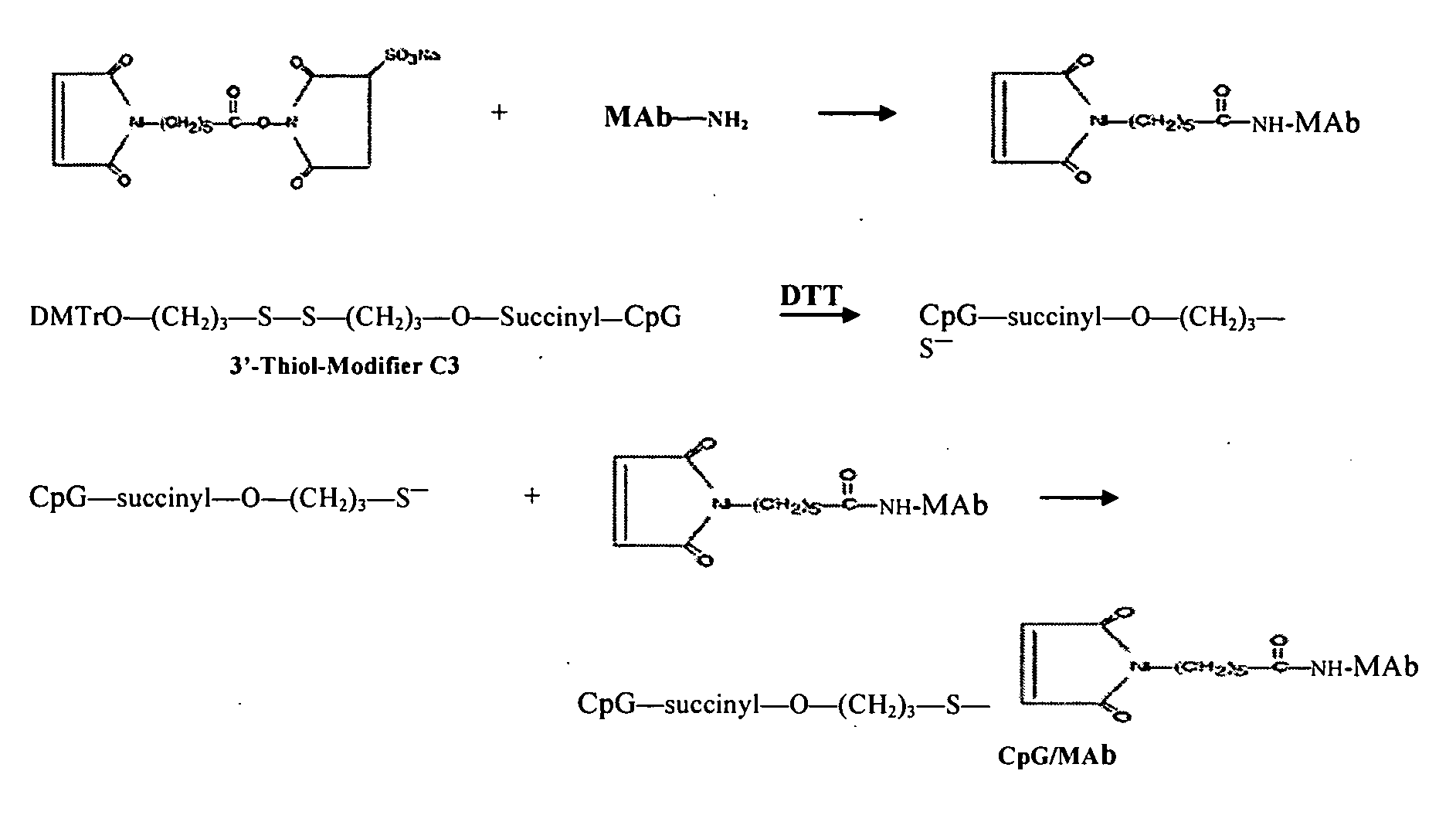
[0124] Chimeric TNT-3 antibody was produced according to published results (Hornick et al., Cancer Biother. & Radiopharm. (1998)13:255-268). cTNT-3 was incubated with the cross-linker N-[ε-Maleimidocaproyloxy]sulfosuccinimide ester (Sulfo-EMCS; Pierce, Ill.) in a 100 mM EDTA-PBS buffer solution (pH 8.3) at a molar ratio of 1:20 for 1 h at room temperature.
[0125] Phosphothioate backbone CpG ODN modified with sulfhydral group at the terminal nucleotide (see FIG. 1), was custom synthesized by Norris Cancer Center Microchemical Core Facility (Los Angeles, Calif.). The phosphothioated sulfhydryl-modified ODN 1826 (SEQ ID NO:1) comprised a CpG motif with the sequence 5′-S-TCCATGACGTTCCTGACGTT-3′. Phosphothioated sulfhydryl-modified ODN 1745 (SEQ ID NO 6):, used as a negative control, 5′-S-TCCAATGAGCTTCCTGAGTCT-3′ for CpG activity along with Phosphothioated sulfhydryl-modified ODN 2006 (SEQ ID NO: 3), 5′-T...
example 2
In Vitro Conjugate Immunoreactivity
[0128] CpG / chTNT-3 preparations were radiolabeled with 125I using a modified chloramine-T method. Immunoreactivity was evaluated by a conventional fixed Raji cell radioimmunoassay. Briefly, Raji lymphoma cells (ATCC: Rockville, Md.) were resuspended in freshly prepared 2% paraformaldehyde in PBS to fix the cells and cause disruption of the cell membrane. Radioiodinated immunoconjugates (approximately 100,000 cpm / tube) were then incubated in triplicate with 106 fixed Raji cells for 1 h. Both the chTNT-3 parental antibody and chTNT-3 / CpG immunoconjugates showed immunoreactivities of 70% or greater.
[0129] The biological activity of the CpG motif in the immunoconjugates was assessed using the murine macrophage cell lines J7-74 and J77743A, available from the ATCC (Rockville, Md.) as described by Kandimalla et al. Bioconjug. Chem. (2002) 13:966-974. Briefly, cells were plated in 24 well dishes using 106 cells / ml. CpG alone (positive control) and the C...
example 3
In Vivo CpG Immunostimulatory Oligonucleotide Immunoconjugate Cancer Therapy Evaluation
[0131] In vivo cancer therapy studies were performed in tumor-bearing BALB / c mice. Groups of mice (n=5) were transplanted with the Colon 26 colon carcinoma in the left flank by the injection of 5×106 cells subcutaneously using a 0.2 ml inoculum. When the tumors reached 0.5 cm in diameter, treatment was initiated and consisted of 4 daily intravenous injections of chTNT-3 / CpG (1826) or control chTNT-3 for each group. In addition, one group was treated with 4 daily intratumoral injections of unconjugated CpG 1826. Tumor volumes were calculated every other day by caliper measurement.
[0132] As shown in FIG. 3, tumor reduction of about 50% was observed at day 21 post-treatment in the chTNT-3 / CpG 1826 group compared to control treated mice. The amount of tumor reduction by chTNT-3 / CpG 1826 at a 30 ug dose was comparable to that achieved with the 5 ug intratumoral administration of free CpG 1826 even th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com