Preparation of anti-infection CpG oligonucleotide DNA preparation and its application technology
An oligonucleotide and anti-infection technology, which is applied in the direction of anti-infective drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] 1. Design and synthesis of CpG oligonucleotide sequence and its primers
[0054] CpG sequence design and synthesis:
[0055] CpG sequence (see the sequence list on page 12 of the instruction manual)
[0056] The sequence contains 11 CpG motifs, which are characterized by two 5'- AACGTT- 3', 2 x 5'- GTCGTC- 3', 2 x 5'- GACGTT- 3', 2 x 5'- ATCGAT- 3’, 1 5’- GTCGTT- 3’, 1 5’- GGCGTT- 3’, 1 5’- GACGTC- 3' signature sequence.
[0057] Cp sequence primer design and synthesis:
[0058] 5' end: CGCTGCAGAACGTTGTC
[0059] 3' end: CGCTGCAGAACGACATCG
[0060] 2. Preparation of CpG oligonucleotides for immunization
[0061] Using the synthesized CpG sequence as a template, it is amplified by PCR (polymerase chain reaction), and the amplified product is dissolved in sterilized saline, and its concentration is measured by an ultraviolet spectrophotometer.
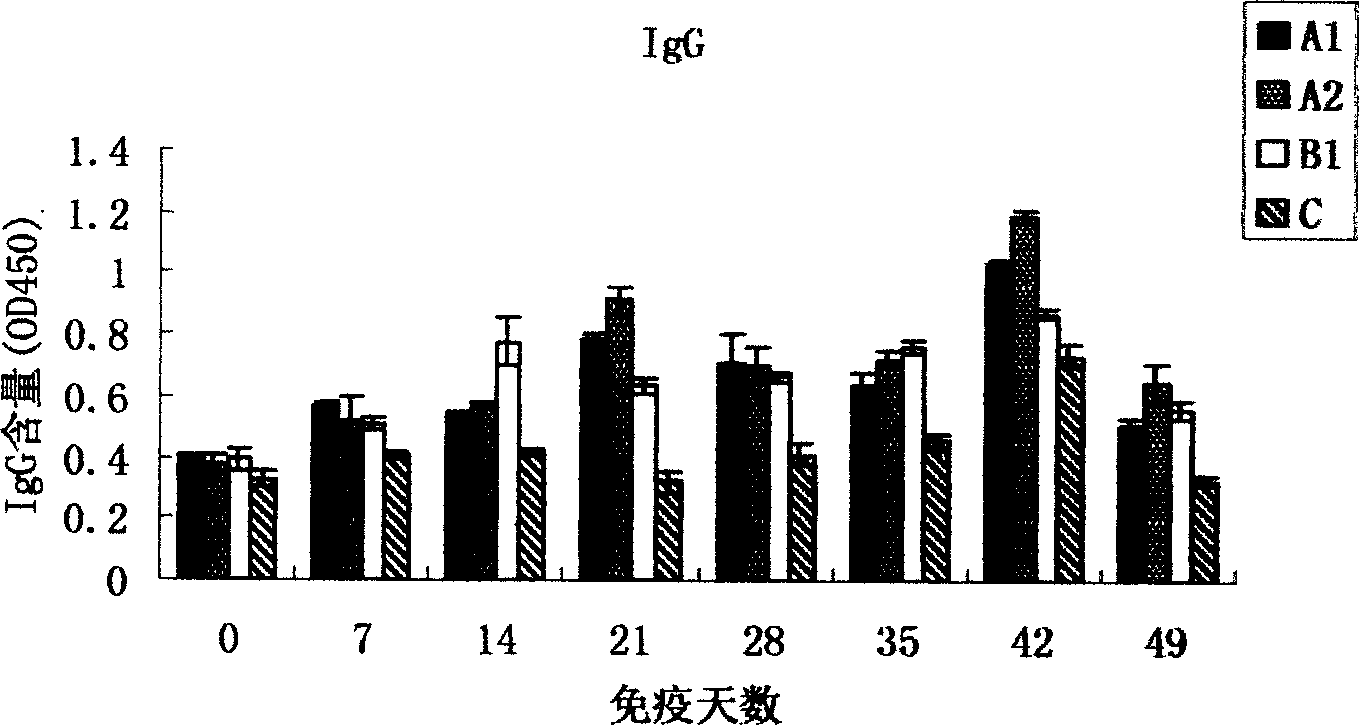
[0062] 3. CpG oligonucleotides stimulate animal blood immune cell proliferation in vitro
[0063] Peripheral ...
PUM
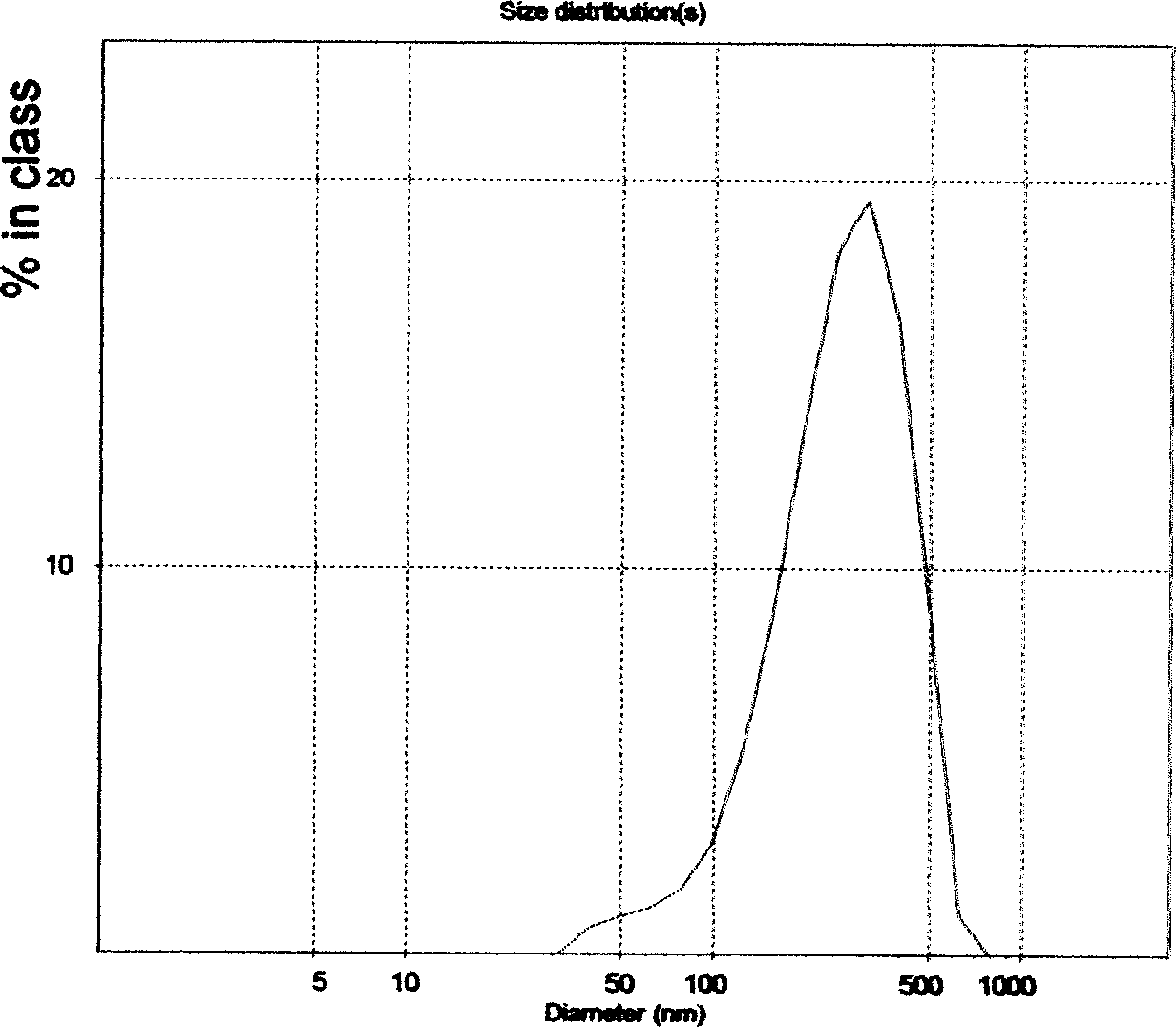
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com