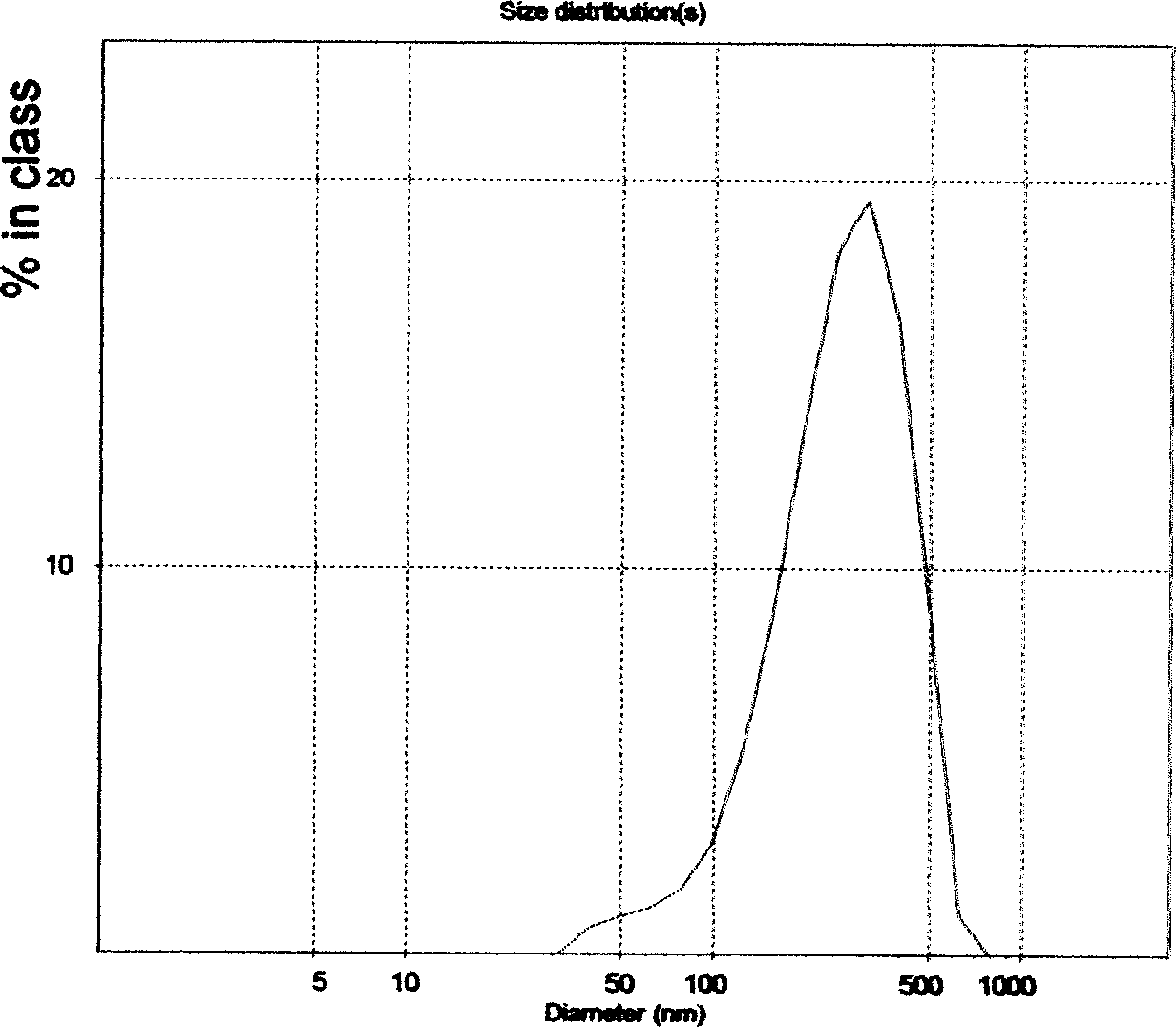
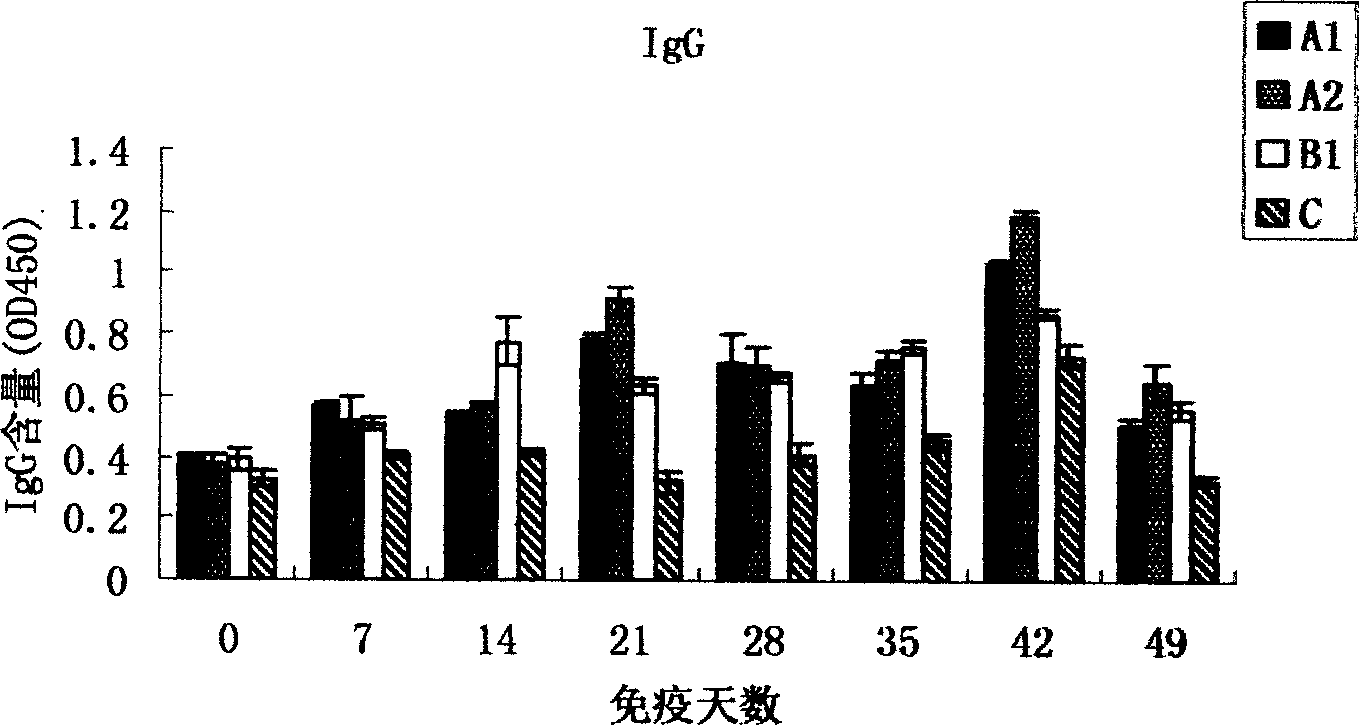
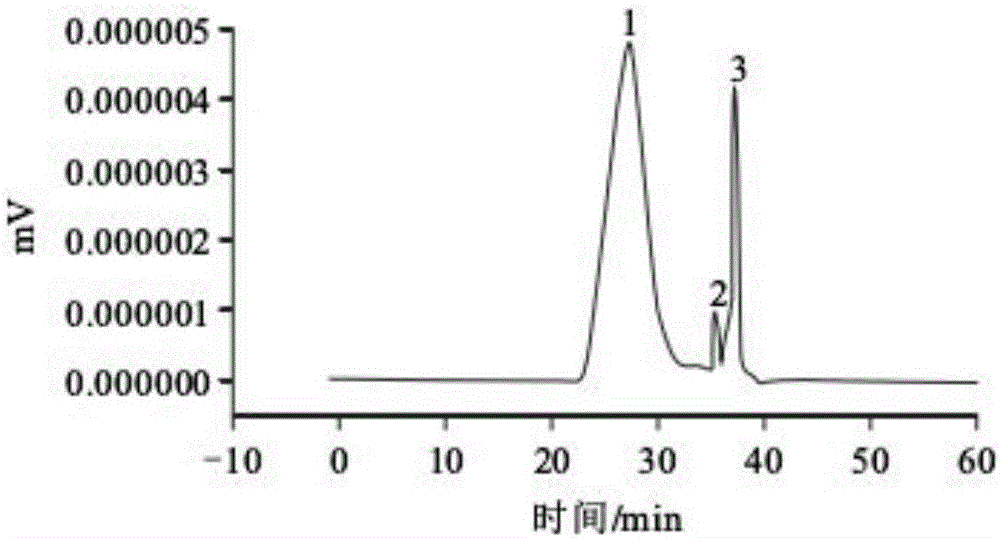
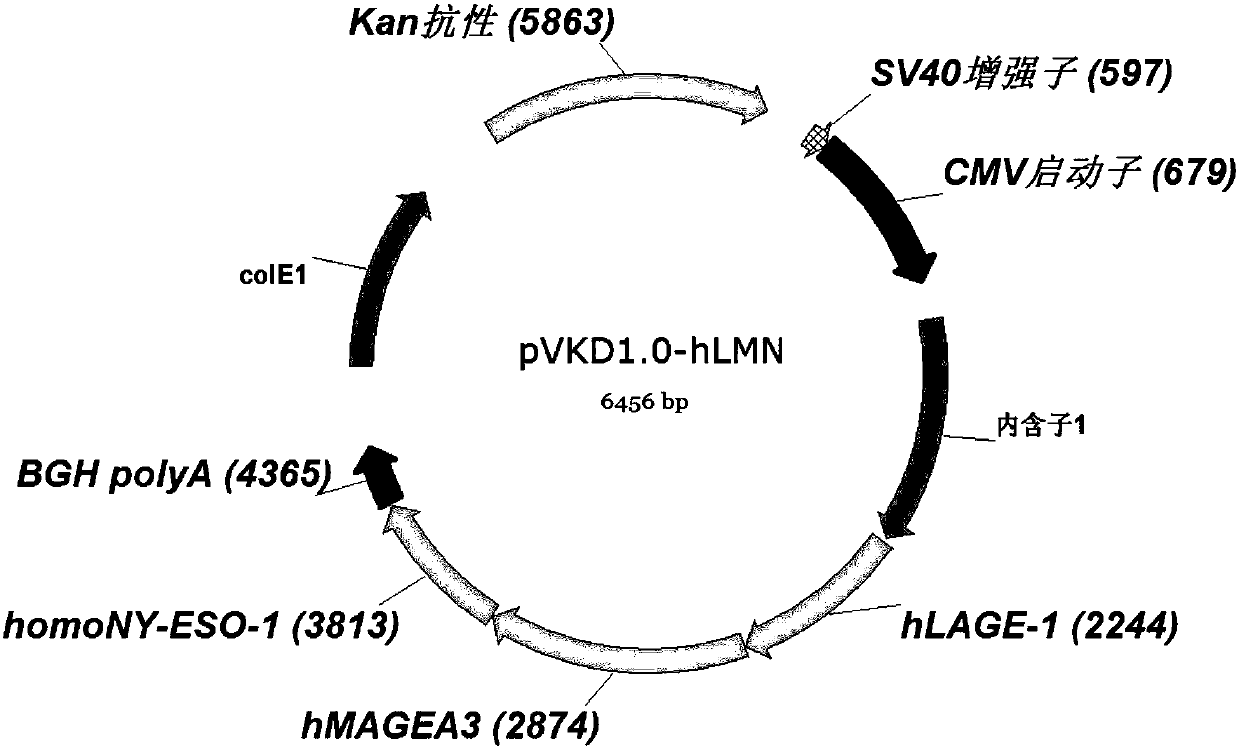
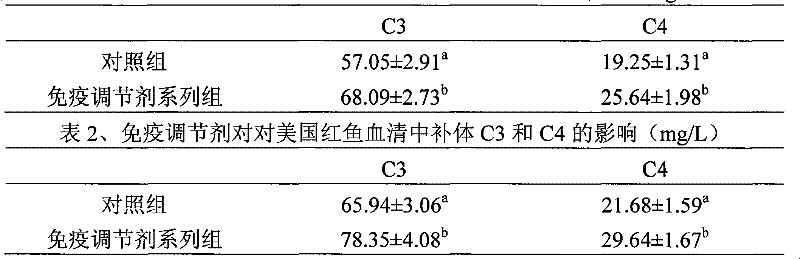
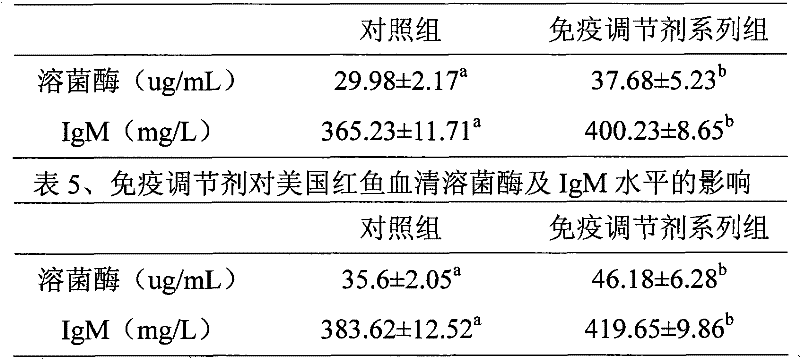
Patents
Literature
58results about How to "Raise the level of immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
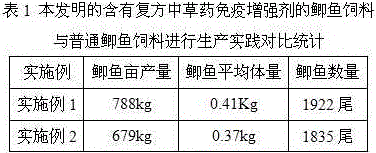
Multifunctional biology aquatic feedstuff additive
ActiveCN101449744APurify the environmentReduce contentFood processingClimate change adaptationBiotechnologyWarm water
The invention provides a multifunctional biological activity aquatic product feed additive, which can be prepared by following steps: (1) uniformly mixing material; (2) adding a little warm water of 30-50 DEG C into molasses and uniformly mixing, adding composite bacterium and uniformly mixing, placing for 1-2 hours, and adding residual water to prepare composite bacteria liquid; (3) adding the composite bacteria liquid into the material, uniformly mixing, and carrying natural aerobic fermentation for 50-60 hours at room temperature; (4) drying the mixture until moisture is less than or equal to 10% under temperature less than 60 DEG C, and grinding package. Content of the multifunctional biological activity aquatic product feed additive provided by the present invention is more than 10<12>cfu / g, the aquatic product feed additive contains a large amount of chitooligosaccharides, alga oligosaccharides and active small peptide, and simultaneously contains a plurality of protease, lipase and amylase. The invention has advantages of simple production method, no need of special heating sterilization and fermentation equipment, less energy consumption and short fermentation period.
Owner:BEIJING GOLDENWAY BIO TECH
Feedstuff additive for reinforcing immunity of sea water fish
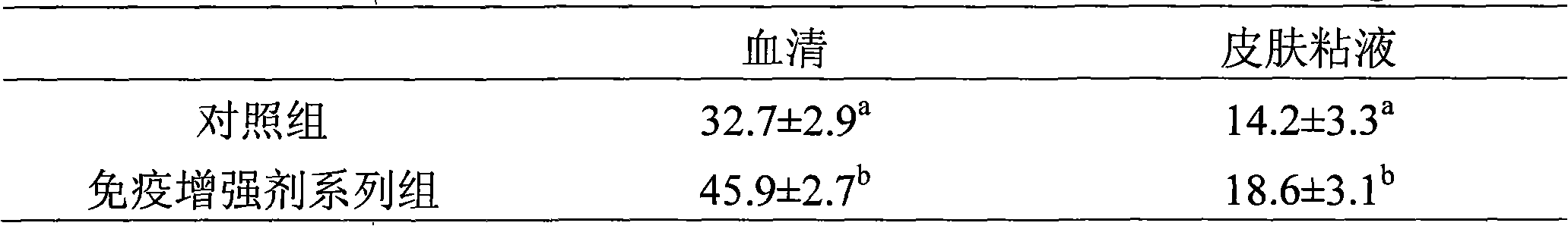
InactiveCN101326964AImprove immune functionRaise the level of immune responseAnimal feeding stuffAccessory food factorsDiseaseWeight gaining
The invention discloses feed additive which improves the immunity of seawater fish. The feed additive consists of three or more than three types in clostridium butyricum, mannan oligosaccharide, xylo-oligosaccharide and fructooligosaccharide by proportion. 0.1 percent to 1 percent of seawater fish immunity intensifier of the invention is added into seawater fish feed, which can obviously improve the level of the muramidase in serum and skin mucus, obviously improve the level of IgM in the serum and the skin mucus, improve the fish immunity response capability, thereby improving the disease resistance of the cultivated fish. 1 percent of the immunity intensifier of the invention is added into the seawater fish feed, which can obviously improve the daily weight gain of the fish and reduce the food coefficient. The feed additive which improves the immunity of the seawater cultivating fish of the invention can obviously improve the immunity response level of the seawater cultivating fish, improve the disease resistance, and is safe without public harm and residue; the seawater fish cultivated by the additive meet the green food sanitation requirements.
Owner:ZHEJIANG UNIV +2
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Porcine circovirus 2 Cap-cell-penetrating peptide fusion protein gene with high expression and application thereof
ActiveCN107841507ARaise the level of immune responsePolypeptide with localisation/targeting motifViral antigen ingredientsPorcine circovirusRecombinant baculovirus
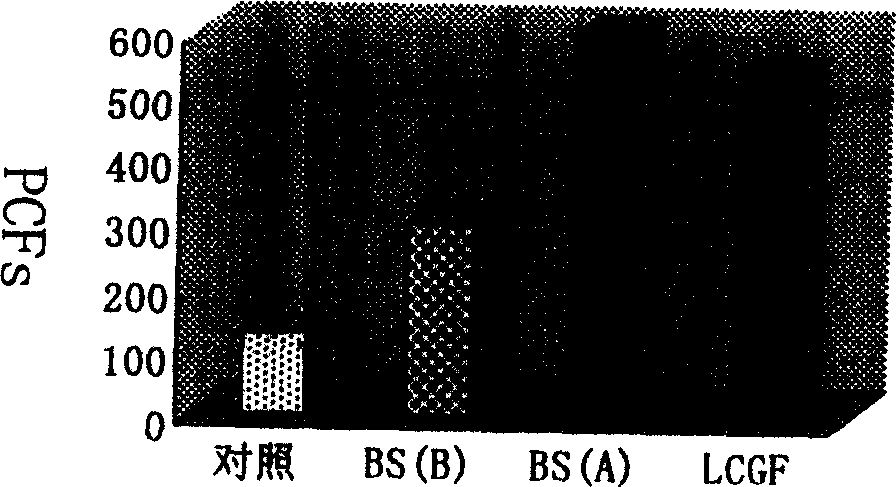
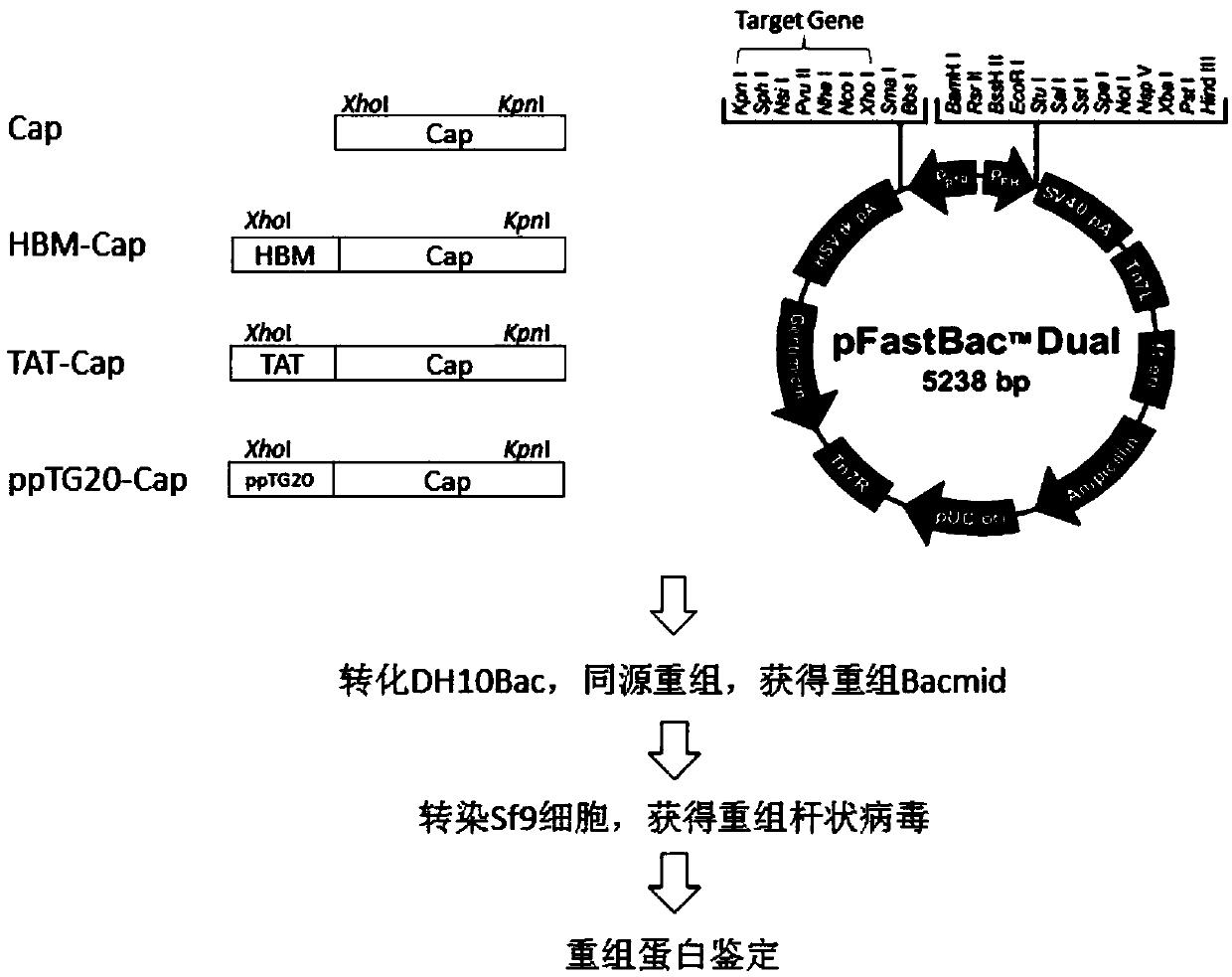
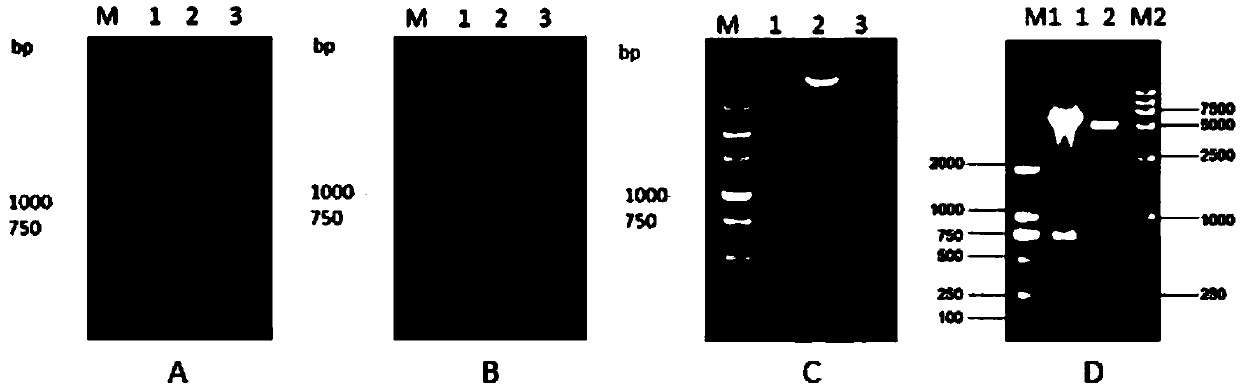
The invention discloses a porcine circovirus 2 (PCV 2) Cap-cell-penetrating peptide fusion protein gene with high expression and application thereof. Coding genes of the fusion gene refer to any kindof genes in (1)-(3): (1) Cap genes of fused cell-penetrating peptide TAT gene sequence; (2) Cap genes of fused cell-penetrating peptide ppTG20 gene sequence; and (3) Cap genes of fused bee signal peptide HBM gene sequence. Study of the research group discovers a DNA vaccine prepared by mixing a cell-penetrating peptide and pVAX-Cap plasmid is capable of improving the in-vivo immune response levelof mice. According to the study, the TAT, ppTG20, HBM and PCV2 Cap protein N end are fused, the recombinant baculovirus is successfully constructed, effective expression of the Cap proteins is realized, and a foundation is laid for researching genetically engineered vaccine PCV 2.
Owner:NANJING AGRICULTURAL UNIVERSITY
Preparation and application of CpG DNA molecule anti-infection and immunity prepn
InactiveCN1692943ARaise the level of immune responseReduce plasmid usageSugar derivativesGenetic material ingredientsBiotechnologyMolecular Immunology
An anti-infectious immunopotentiator for pig, ox, yak, etc is prepared through artificially synthesizing the CpG oligonucleotide sequce able to excite the reproductive activity of immune cell, preparing the chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of CpG oligonucleotide. It can be used to immunize the experimental animal by intramuscular injection or oral applialtion.
Owner:SICHUAN UNIV
Snail feed nutrient
The invention relates to a snail feed nutrient. The snail feed nutrient comprises the following components in part by weight: 3-5 parts of amino acid, 2-4 parts of lecithin, 4-6 parts of vitamin, 2-4 parts of sodium selenite, 5-7 parts of ferrous sulfate, 15-22 parts of soybean protein isolate powder, 18-20 parts of earthworm powder, 8-15 parts of eggshell powder, 10-20 parts of kelp powder and 2-4 parts of composite bacterial agent. The snail feed nutrient provided by the invention has the advantages of improving the appetite, promoting the growth of an animal, promoting the feed digestion, enhancing immune function of the organism, preventing and treating snail rotten foot diseases and shell top dropping diseases as well as increasing the commodity value, and has the characteristics of no toxic and side effect and no drug residue. The snail feed nutrient provided by the invention is a green and safe feed nutrient.
Owner:常熟市润丰农业有限公司
Crucian carp feed containing compound Chinese herbal medicine immunity enhancer
InactiveCN106212880AIncrease the rate of weight gainSuitable for growth and development requirementsFood processingClimate change adaptationRapeseedCrucian carp
The invention discloses a crucian carp feed containing a compound Chinese herbal medicine immunity enhancer. The crucian carp feed comprises the raw materials of corn flour, wheat bran, bean pulp, wheat, bagasse, fish bone powder, vinegar dregs, corn straw, rice bran, rapeseed dregs, silkworm chrysalis meal, Chinese chives, spinach leaves, the compound Chinese herbal medicine immunity enhancer and phagostimulant. The compound Chinese herbal medicine immunity enhancer is prepared from folium cortex eucommiae, isatis roots, honeysuckle flowers, dried tangerine peel, folium artemisiae argyi, hawthorn fruit, Chinese mugwort, tea, milkvetch roots, lignum sappan, common andrographis herb and cortex cinnamomi according to certain proportions. According to the crucian carp feed containing the compound Chinese herbal medicine immunity enhancer, raw material matching and proportioning of the crucian carp feed are optimized, the feed is high in use ratio and good in palatability, the weight increasing speed of crucian carps is significantly increased, and the added compound Chinese herbal medicine immunity enhancer can effectively enhance the immunity function of the crucian carps and improve the disease resistance of the crucian carps, so that the survival rate of the bred crucian carps is greatly increased. Therefore, application of antibiotics can be reduced, the yield of the crucian carps can be increased, the quality of the crucian carps can be improved, and important industrial value is achieved.
Owner:宋晓燕
Veterinary vaccine immunologic adjuvant as well as preparation and application method thereof
InactiveCN105797153AFacilitates multiple antigen and antibody bindingEnhance immune response level and antigen presentation abilityImmunological disordersAntibody medical ingredientsIonAntibody
Owner:ZHEJIANG FORESTRY UNIVERSITY
Immunoenhancer and application thereof
ActiveCN108117583ARaise the level of immune responseEase of mass productionPeptide/protein ingredientsViral antigen ingredientsChemical synthesisCtl epitope
The invention provides an immunoenhancer, a swine foot and mouth disease vaccine composition containing the immunoenhancer and application. The immunoenhancer is prepared from three swine foot and mouth disease virus CTL epitope polypeptides, i.e., one or a random combination of two or more of a polypeptide shown as a sequence 1 in a sequence table, a polypeptide shown as a sequence 2 in the sequence table and a polypeptide shown as a sequence 3 in the sequence table. The swine foot and mouth disease vaccine composition provided by the invention is prepared from the immunoenhancer and a swinefoot and mouth disease antigen. The immunoenhancer provided by the invention can be prepared in a large batch through a solid-phase chemical synthesis method and is mixed with an inactivation antigenaccording to a certain ratio, so that the vaccine composition can be produced and prepared in a large scale; the vaccine composition can be used for inducing organisms to generate a body fluid immuneresponse level and also can be used for enhancing a cell immune response level of the organisms and obtaining a relatively good immune effect. The immunoenhancer provided by the invention is safe andreliable and has a wide application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Preparation method of immune-enhanced recombinant PRRSV virus-like particle subunit vaccine
ActiveCN109402145ABroad-spectrum cross-immunogenicityImproving immunogenicityViral antigen ingredientsVirus peptidesBaculovirus expressionVirus-like particle
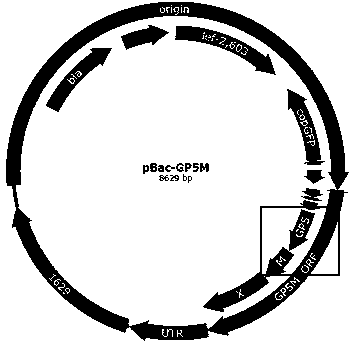
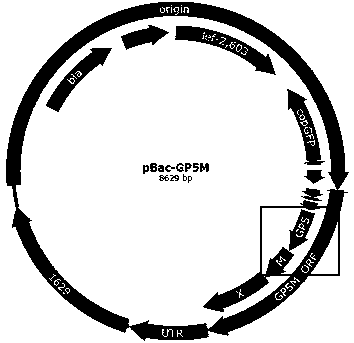
The invention discloses a preparation method of an immune-enhanced recombinant PRRSV virus-like particle subunit vaccine. The subunit vaccine is prepared from PRRSV virus-like particles and compound immunological adjuvants. By a genetic engineering means, a PRRSV GP5-M gene is modified, and PRRSV virus-like particles with high immunogenicity are prepared by constructing a rhabdovirus expression vector. In addition, by improving the immunological adjuvants, the immuno-enhanced recombinant PRRSV virus-like particle subunit vaccine is obtained, and the subunit vaccine has better immunization effects.
Owner:陕西诺威利华生物科技有限公司
Peptide feedstuff additive for regulating immunity function of sea water fish
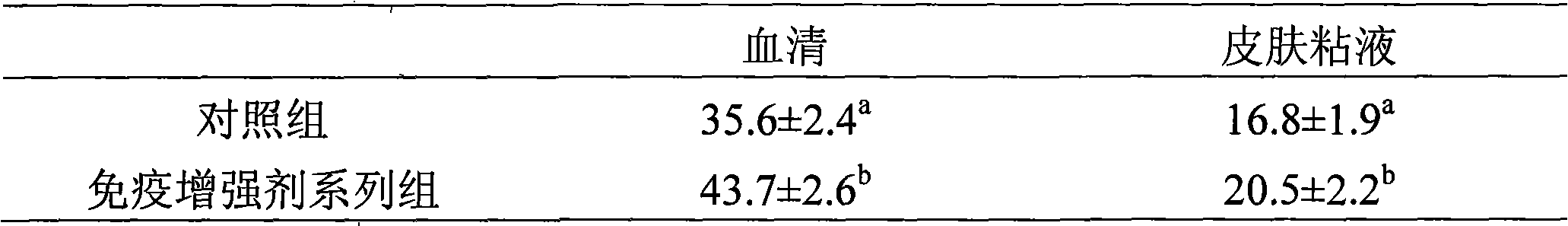
InactiveCN101326958AEnhance the ability to resist pathogenic infectionIncrease IgM level and blood cell respiratory burst activityAnimal feeding stuffAccessory food factorsSeawaterEnzymatic hydrolysis
The invention discloses peptide feed additive used to modulate the immune functions of seawater fish. The peptide feed additive consists of three or more than three types in peptide which is produced by enzymatic hydrolysis of cod fish, skin and internal organs with the molecular weight ranges respectively 100Da to 500Da, 500Da to 1500Da, 1500Da to 3000Da, 3000Da to 5000Da, and 5000Da to 8000Da. 1 percent to 5 percent of immune modulator of the invention is added into feed, which can obviously improve the level of IgM and muramidase in the serum of the seawater fish; the immune modulator of the invention can also improve the liver SOD level of the cultivated fish, thereby improving the oxidation resistant capability of the cultivated fish. The immune modulating peptide feed additive of the invention can also obviously improve the daily weight gain of the fish and reduce the liver index. The peptide feed additive used to modulate the immune functions of the seawater fish is safe without public harm, and the fish cultivated by the additive meet the green food sanitation requirements.
Owner:ZHEJIANG UNIV +2
Immune-enhanced recombinant PRRSV (Porcine Reproductive and Respiratory Syndrome Virus)-like particle subunit vaccine
ActiveCN109395073ABroad-spectrum cross-immunogenicityHigh immune protection rateViral antigen ingredientsVirus peptidesImmunogenicitySubunit vaccines
The invention discloses an immune-enhanced recombinant PRRSV (Porcine Reproductive and Respiratory Syndrome Virus)-like particle subunit vaccine. The immune-enhanced recombinant PRRSV virus-like particle subunit vaccine consists of PRRSV-like particles and a compound immunologic adjuvant. The immune-enhanced recombinant PRRSV-like particle subunit vaccine disclosed by the invention has the beneficial effects that by the means of gene engineering, PRRSV GP5-M gene is modified, and the PRRSV-like particles with high immunogenicity are prepared by constructing rhabdovirus expression vectors. In addition, by improvement on the immunologic adjuvant, the immune-enhanced recombinant PRRSV-like particle subunit vaccine is obtained and the subunit vaccine has a better immune effect.
Owner:陕西诺威利华生物科技有限公司
Prepn. and application tech. for pig interleukin-4 gene anti-diseases prepns.
InactiveCN1692942ARaise the level of immune responseImprove immunityGenetic material ingredientsAntiinfectivesDiseaseTibetan pig
An anti-infectious immunopotentiator for animals is prepared through cloning the interleukin-4 gene of Tibetan pig, configuring its eukaryon expression plasmid VTPIL-4, preparing chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of plasmid VTPIL-4. It can be used for immunizing experimental animal by intramuscular injection.
Owner:SICHUAN UNIV
A compound immune adjuvant and its preparation method and application
ActiveCN106798920BDiureticHigh puritySsRNA viruses positive-senseViral antigen ingredientsMedicineGinkgo biloba
The invention discloses a complex immunity adjuvant. The adjuvant is prepared from the following components in percentage by weight: 1.5 to 3.5 percent of horttuyia cordala thunb polysaccharide, 0.2 to 0.55 percent of alanine, 0.8 to 2.2 percent of ginkgo leaf flavonoid, 5 to 8 percent of polyethylene castor oil, 5 to 8 percent of Span 80, 2.5 to 5 percent of polyethylene glycol, 6 to 10 percent of squalene, 30 to 35 percent of soybean oil for injection and 35 to 50 percent of water for injection, and the sum of the percentage by weight of the various components is 100 percent. The complex immunity adjuvant is safe and effective, can effectively enhance the immunostimulation capacity of PRRSV vaccines, and is suitable for immunoprophylaxis of porcine reproductive and respiratory syndrome (PRRS).
Owner:北京康宝利华生物科技有限公司
Low-protein antibiotic-free ration for weaned pigs and application of low-protein antibiotic-free ration
InactiveCN108419938AImprove the level ofReduce diarrhea rateFood processingAnimal feeding stuffIntestinal structureDL-methionine
The invention relates to a low-protein antibiotic-free ration. The low-protein antibiotic-free ration comprises L-lysine hydrochloride, DL-methionine, L-threonine, L-tryptophan and branched chain amino acids. Under the condition of being free from antibiotic growth promoting agents, the low-protein ration is prepared, through adjustment of proportion and quantity of the lysine, the methionine, thethreonine, the tryptophan, three branched chain amino acids and the like, so that the diarrhea rate of the weaned pigs after weaning can be reduced; the metabolic capability of the weaned pigs for protein and the deposit capacity of the weaned pigs for nitrogen are improved; the digestive absorption level of amino acids by the weaned pigs is increased; the development of intestines of the weanedpigs is promoted; the digestive ability of the weaned pigs for lactose, cane sugar and maltose is improved; the digestive ability of the weaned pigs for protein, energy, and dry matter is improved; and the absorptive capacity of the weaned pigs for nutrient substances is improved.
Owner:CHINA AGRI UNIV
Preparation of anti-infection CpG oligonucleotide DNA preparation and its application technology
InactiveCN1720922ARaise the level of immune responseReduce plasmid usagePowder deliveryOrganic active ingredientsProliferation activityExperimental animal
The invention provides a fragment of CpG oligodeoxynucleotides sequence capable of stimulating proliferation activities of animal immunologic cells, and a method for packaging this fragment of CpG oligodeoxynucleotides to prepare anti-infection DNA nano granular formulation, the use of the CpG nano particles as novel anti-infection molecular immune enhancement agent is also disclosed. The method comprises packaging CpG oligodeoxynucleotides with chitosan nano particles, immunizing experimental animals with the prepared DNA nano granular formulation separately or synergically through intramuscular injection or oral administration, finally detecting the cell and body fluid immunity indexes of the experimental animal.
Owner:SICHUAN UNIV +1
Complex immunity adjuvant, and preparation method and application thereof
ActiveCN106798920ADiureticHigh puritySsRNA viruses positive-senseViral antigen ingredientsAdjuvantGinkgo biloba
The invention discloses a complex immunity adjuvant. The adjuvant is prepared from the following components in percentage by weight: 1.5 to 3.5 percent of horttuyia cordala thunb polysaccharide, 0.2 to 0.55 percent of alanine, 0.8 to 2.2 percent of ginkgo leaf flavonoid, 5 to 8 percent of polyethylene castor oil, 5 to 8 percent of Span 80, 2.5 to 5 percent of polyethylene glycol, 6 to 10 percent of squalene, 30 to 35 percent of soybean oil for injection and 35 to 50 percent of water for injection, and the sum of the percentage by weight of the various components is 100 percent. The complex immunity adjuvant is safe and effective, can effectively enhance the immunostimulation capacity of PRRSV vaccines, and is suitable for immunoprophylaxis of porcine reproductive and respiratory syndrome (PRRS).
Owner:北京康宝利华生物科技有限公司
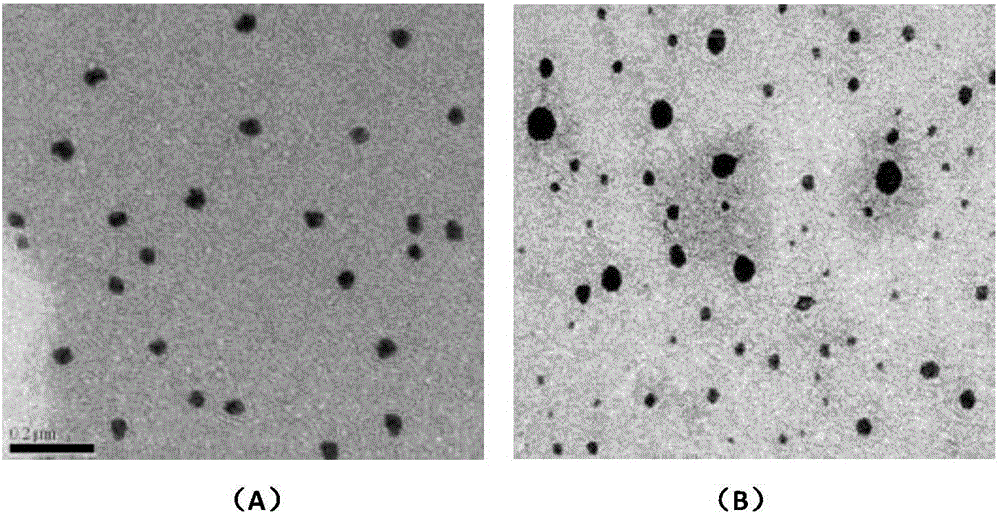
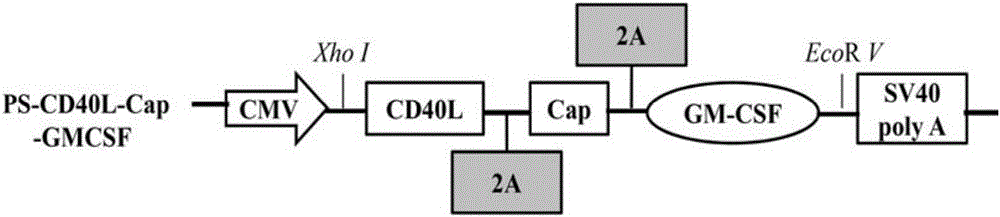
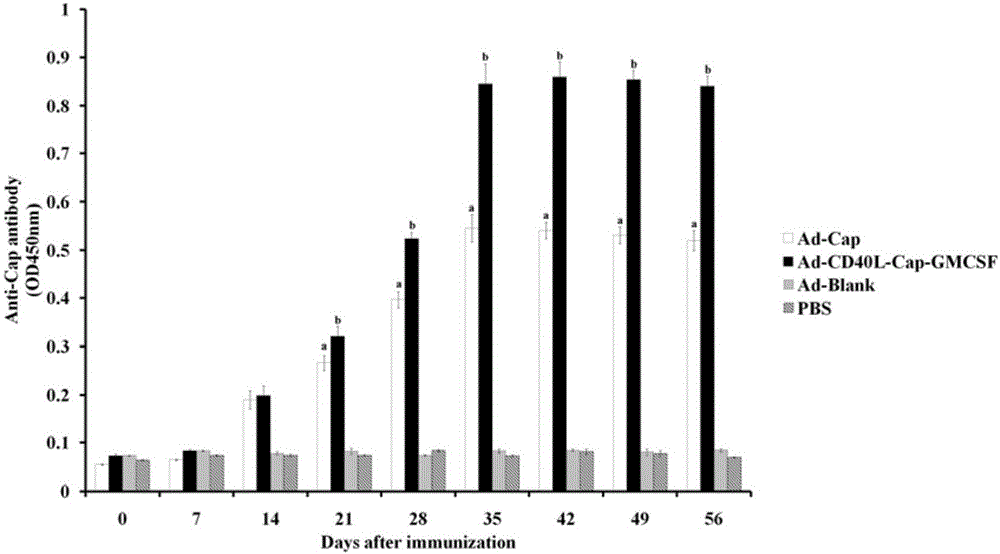
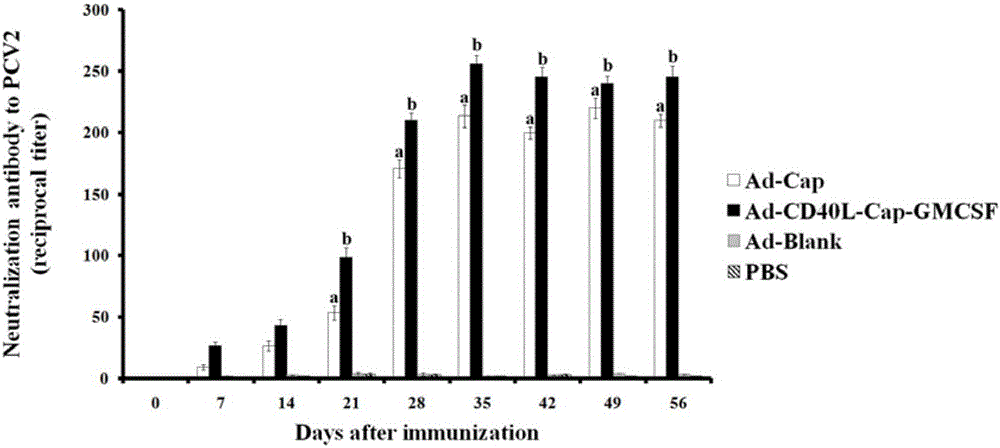
Construction, amplification and purification method of porcine CD40L/GMCSF/PCV2Cap recombinant adenovirus
InactiveCN105838684AImprove the level ofPromote secretionMicrobiological testing/measurementVirus peptidesEnzyme digestionPurification methods
The invention relates to a construction, amplification and purification method of a porcine CD40L / GMCSF / PCV2Cap recombinant adenovirus. The method comprises the steps of: S1. connecting CD40L, GM-CSF and Cap to a vector pUC57 in order, and naming the product as pUC-CD40L-Cap-GMCSF; S2. connecting CD40L, Cap and GM-CSF to pShuttle-CMV, converting DH5a, conducting bacteria picking, bacteria shaking and plasmid extraction, and naming the product as PS-CD40L-Cap-GMCSF; S3. linearizing the constructed PS-CD40L-Cap-GMCSF, then conducting electric transformation on BJ5183 with the linearized PS-CD40L-Cap-GMCSF and skeleton plasmid pAdEasy-1, and then carrying out bacteria picking, bacteria shaking and plasmid extraction, and performing single enzyme digestion identification; S4. when the identification result is right, using a kit to extract plasmid, transfecting HEK293A cell, when cell lesion appears, collecting cells, performing centrifugation, then resuspending the precipitate in autoclaving PBS; S5. carrying out repeated freezing and thawing, performing centrifugation to extract the supernatant, thus obtaining recombinant adenovirus; 6. amplifying the obtained recombinant adenovirus; and 7. purifying the recombinant adenovirus. The method provided by the invention for the first time adds porcine tumor necrosis factor related activation protein gen and porcine granulocyte-macrophage colony stimulating factor into the adenovirus vector simultaneously to improve expression of the PCV2 Cap recombinant adenovirus immunogenicity.
Owner:NORTHWEST A & F UNIV
Recombinant serum amyloid A capable of enhancing immune response of crassostrea gigas, and preparation method thereof
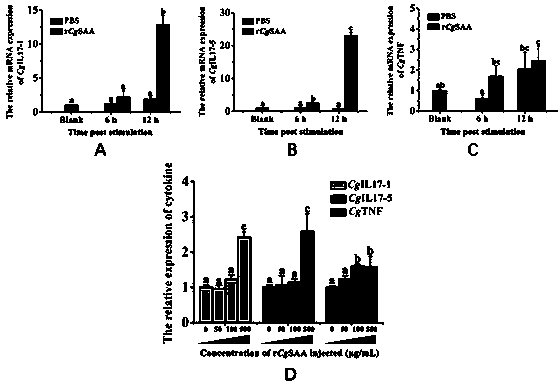
InactiveCN110845594ARaise the level of immune responsePeptide/protein ingredientsPeptide preparation methodsImmunologic preparationOstrea gigas
The invention discloses recombinant serum amyloid A capable of enhancing immune response of crassostrea gigas. The recombinant serum amyloid A is characterized that the amino acid sequence is represented by SEQ ID NO. 1. Gene expression of cytokines CgIL17-1, CgIL17-5, and CgTNF in blood lymphocytes can be remarkably promoted inside and outside bodies of the crassostrea gigas, the immune responselevel of the crassostrea gigas can be enhanced, and the recombinant serum amyloid A has application value in preparation of novel immune preparations and other drugs for shellfishes, especially breeding shellfishes.
Owner:DALIAN OCEAN UNIV
Protein peptide vaccine carrying system and preparing method thereof
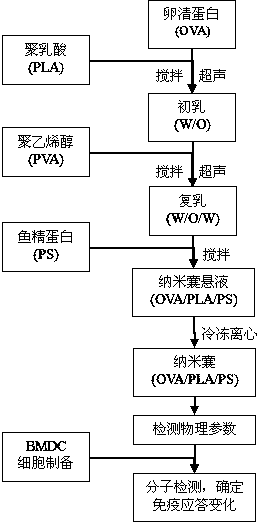
InactiveCN104189900AEnhance immune responseNo toxicityPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDendritic cellMethylene Dichloride
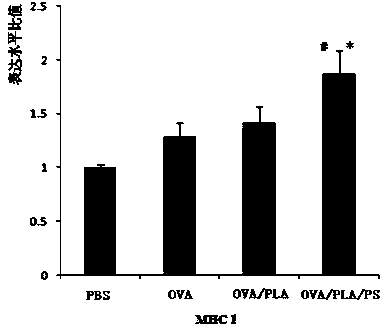
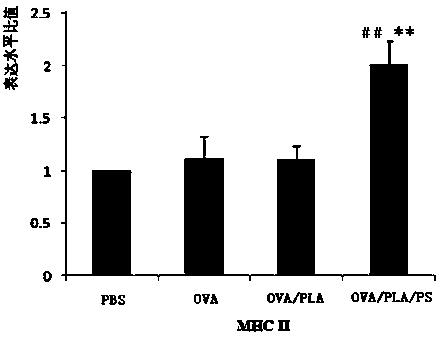
The invention provides a polylactic acid-cladded protein peptide vaccine, a protein peptide vaccine carrying system for a protamine-cladded polylactic acid nanocapsule and a preparing method of the carrying system. Ovalbumin (OVA) is used as a model protein peptide vaccine, and an OVA solution is dispersed in a PLA-containing methylene dichloride organic phase by a probe ultrasonography method to form a primary emulsion. The primary emulsion is added into a polyvinyl alcohol containing external aqueous phase and treated by probe ultrasonography to form a multiple emulsion. A cured OVA / PLA microcapsule is obtained after the methylene dichloride organic phase is fully volatilized through agitating. Protamine (PS) is added into an OVA / PLA microcapsule suspension and agitated to obtain an OVA / PLA / PS microcapsule. The OVA / PLA / PS microcapsule has stable nanometer grain size, stable dispersing coefficient and surface potential suitable for transfect cells. The extracting capability of bone marrow derived dendritic cells (BMDC) for the OVA / PLA / PS microcapsule can be effectively improved. The expression of BMDC surface molecules MHCI, MHCII, CD83 and CD86 is obviously improved, and the secretion of a BMDC polarizing factor IL-12p70 is increased.
Owner:WUHAN INST OF BIOTECH +1
CD4 helper T cell epitope fusion peptide and vaccines thereof
InactiveCN109575141ARaise the level of immune responseOvercoming immune toleranceSsRNA viruses negative-senseAntibacterial agentsImmune toleranceTumor antigen
The invention provides CD4 helper T cell epitope fusion peptide, coding nucleic acid of the CD4 helper T cell epitope fusion peptide and an immune composition containing the CD4 helper T cell epitopefusion peptide. The epitope fusion peptide comprises cytomegalovirus epitope and influenza virus epitope. The epitope fusion peptide can greatly improve the cellular immune response level of target immunogens, especially weak immunogens, is an effective means for overcoming the immune tolerance of an immune system to antigens, especially tumor antigens or infection-related antigens, and is suitable for effectively enhancing the efficacy of vaccines.
Owner:VACDIAGN BIOTECH
Three recombinant lactic acid bacteria for expressing avian influenza virus proteins as well as construction method and application thereof
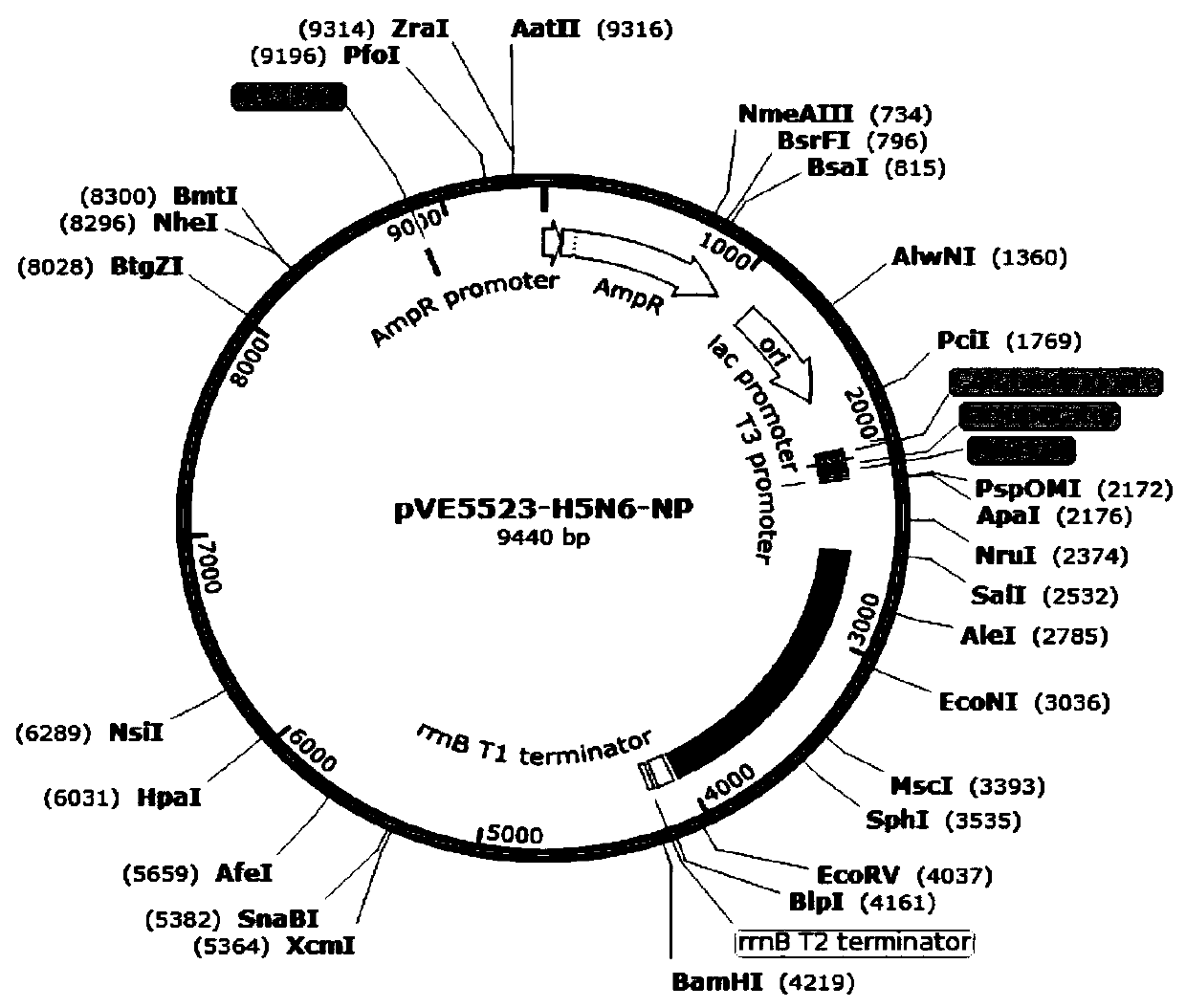
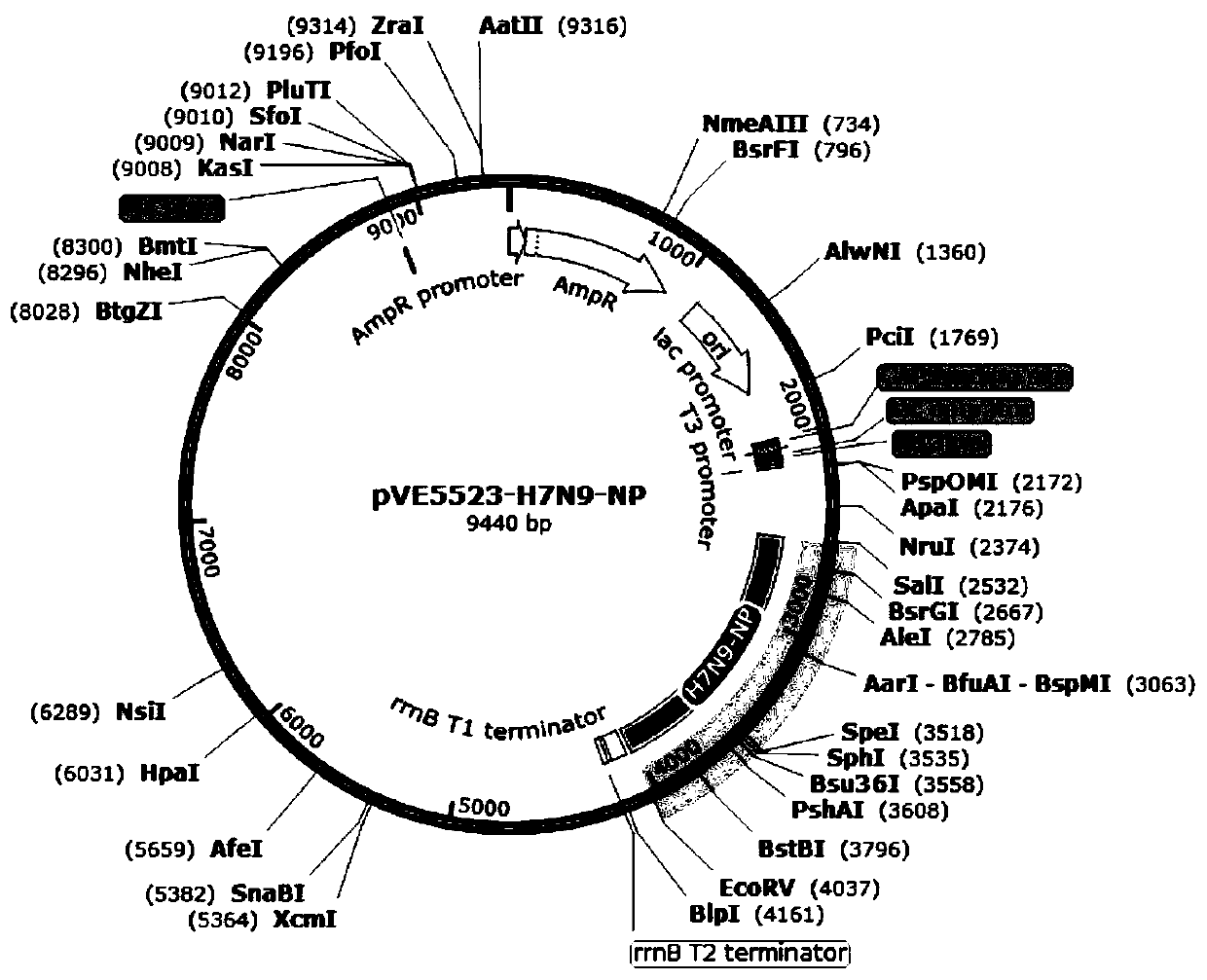
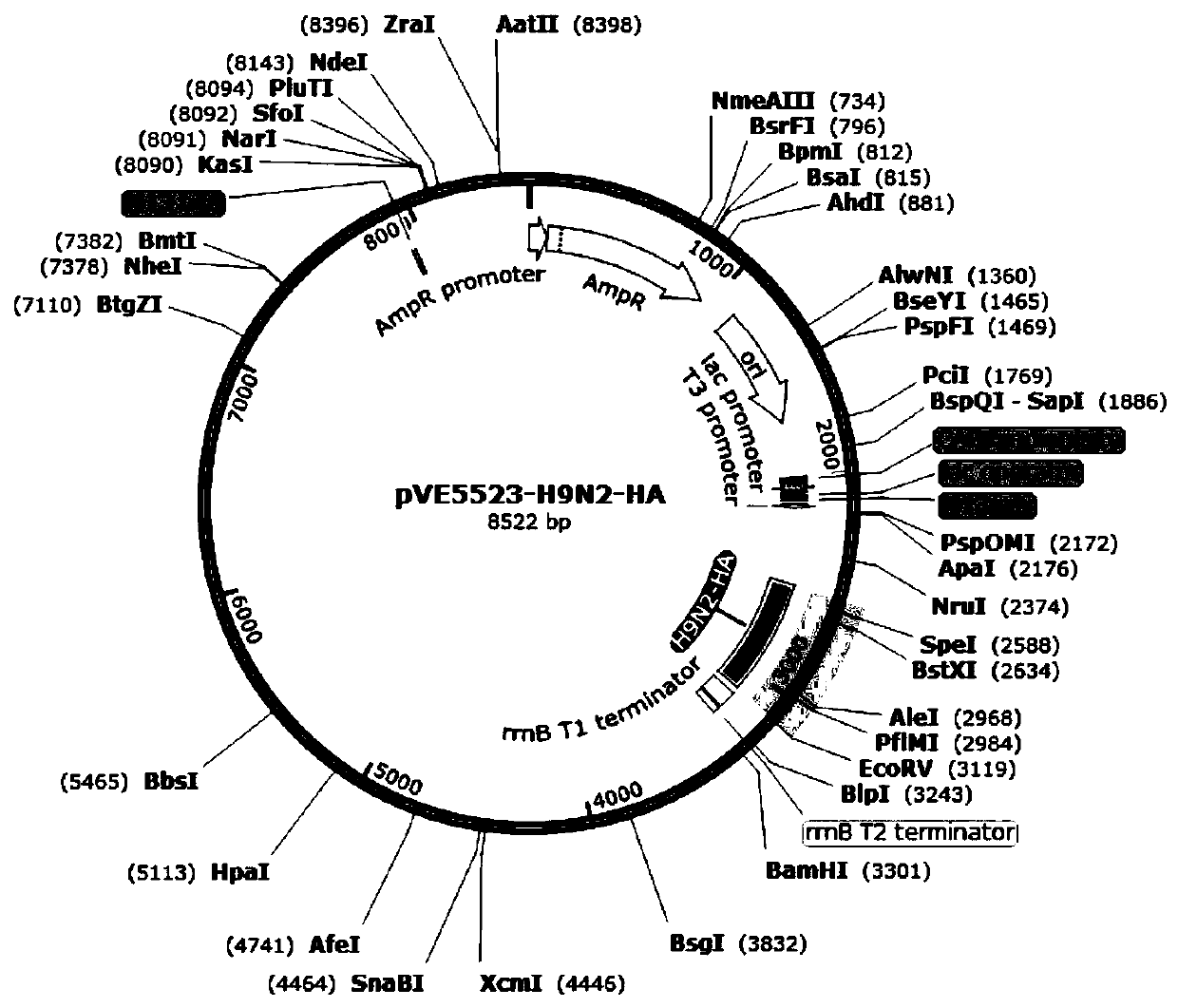
InactiveCN111500512AEnhance immune responseRaise the level of immune responseBacteriaViral antigen ingredientsTGE VACCINEBacilli
The invention relates to three recombinant lactic acid bacteria for expressing avian influenza virus proteins as well as a construction method and application thereof. The recombinant lactobacillus strain for expressing avian influenza virus protein contains any one of a gene sequence for encoding H5N6 avian influenza virus NP protein, a gene sequence for encoding H7N9 avian influenza virus NP protein or a gene sequence for encoding H9N2 avian influenza virus HA protein. The construction method mainly comprises the following steps: bonding a target gene with a lactic acid bacteria expression vector; and introducing recombinant plasmid into the competence of ATCC393 lactobacillus casei to obtain a recombinant lactic acid bacteria strain. The recombinant lactic acid bacteria can express specific protein in an animal body, stimulate the body to generate an antibody with a protective effect, and have important significance for preventing and treating H5, H7 and H9 avian influenza. The invention also provides an oral vaccine or medicine for preventing and treating avian influenza, which can effectively prevent and treat H5, H7 and H9 avian influenza.
Owner:张大生
Recombinant adenovirus high-expression vector for promoting effective presentation of Hantavirus fusion protein G2S0.7
InactiveCN102618578ARaise the level of immune responseFermentationGenetic engineeringTransfer vectorDigestion
The invention relates to a recombinant adenovirus high-expression vector for promoting effective presentation of Hantavirus fusion protein G2S0.7. A G2S0.7-pCAG transfer vector containing G2S0.7 mosaic gene of Hantavirus 76-118 strain and a CAG promoter / enhancer is mainly transformed to promote the effective presentation of the high-expression Hantavirus fusion protein Hantavirus in an organism. By a gene recombination technology, the recombinant adenovirus transfer vector G2S0.7-pCAG containing the mosaic gene G2S0.7 is transformed, and Ub gene is connected to the vector. The recombinant transfer vector and the adenovirus vector are respectively subjected to double digestion, and a recombinant fragment Ub-G2S0.7-pCAG is integrated into the DNA of the adenovirus vector; and after packaging, purification and titer detection, rAd-Ub-G2S0.7-pCAG and recombinant adenovirus rAd-G2S0.7-pCAG containing the G2S0.7 mosaic gene and the CAG promoter / enhancer, which is constructed early by the inventor are respectively used for immunizing C57BL / 6 mice, immunological characteristics are researched, and results show that compared with the conventional recombinant adenovirus, the recombinant adenovirus can effectively improve partial humoral immune response level and partial cellular immune response level of organisms of experimental animals.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Anti-disease prepn. contg. piginterleukin-4,6 fused gene
InactiveCN1692945AReduce dosageRaise the level of immune responseGenetic material ingredientsAntiinfectivesDiseaseChitosan nanoparticles
An anti-disease preparation for animals is prepared through cloning the interleukin-4 and -6 gene of pig, fusing them, configuring the eukaryon expression plasmid VPIl 46 of fusion gene, preparing the chitosan nanoparticles by ion exchange method, dissolving the plasmid in the solution of tripolyphosphate and the deacetyl chitosan solution in acetic acid, and proportional mixing. It can be used to immunize the experimental animal by oral application.
Owner:SICHUAN UNIV
Novel retinoic acid nanoemulsion adjuvant for efficiently enhancing humoral immune response and mucosal immune response and preparation method and application of adjuvant
PendingCN113713093APromote aggregationEnhance immune responseImmunological disordersAntibody medical ingredientsMucosal Immune ResponsesAdjuvant
The invention discloses a novel retinoic acid nanoemulsion adjuvant for efficiently enhancing humoral immune response and mucosal immune response and a preparation method and application of the adjuvant. The retinoic acid nanoemulsion adjuvant is prepared from retinoic acid, an oil phase, a surfactant, a cosurfactant and a water phase, wherein the retinoic acid is wrapped by a formed oil-in-water type nanoemulsion adjuvant system. The retinoic acid nanoemulsion adjuvant can be delivered together with multiple antigens (a model antigen OVA, a staphylococcus aureus recombinant protein antigen and a neocoronavirus recombinant protein antigen) respectively, the immune response level of a vaccine antigen can be enhanced after injection immunization, the adjuvant has a good immune protection effect, can induce humoral immune response of a system and efficiently activate immune response of mucosal parts (intestinal mucosa, vaginal mucosa, lung mucosa, gastric mucosa and nasal mucosa) and has great market application value and broad application prospect.
Owner:ARMY MEDICAL UNIV
Preparation and application of pig interleukin-2 gene anti-infectious preparation
InactiveCN1720998AEnhanced upregulationRaise the level of immune responseGenetic material ingredientsImmunological disordersIndividual animalExperimental animal
The invention discloses the preparation and application of porcine interleukin-2 gene anti-infectious preparation, which comprises, preparing plasmid chitosan nano particles with ion cross-linking method, dissolving porcine Interleukin-2 gene eucaryon plasmid (VPIL2) into tripolyphosphate solution and chitosan acetic acid solution with a molecular weight of 150000 and deacetylated degree of over 95%, water-bathing at 50 deg. C, keeping constant temperature for 20 minutes, homogeneously mixing plasmid solution and chitosan solution for 5 minutes by right proportion, obtaining VPIL2 plasmid chitosan nano particle liquid with a grain diameter of 40-60mn, Zeta electric potential of +25.6. The preparation has no cell toxicity and can resist the degradation to DNA by nuclease. The DNA nano granular formulation can be used for immunizing experimental animals separately through intramuscular injection or in combination with vaccines.
Owner:SICHUAN UNIV +1
Prepn. and application of nanometer molecule prepn. with fused immunity pig gene used for anti-infection
InactiveCN1692944ARaise the level of immune responseStrong immune responsePowder deliveryGenetic material ingredientsIon exchangeInterleukin 4
An anti-infectious nano-class molecular preparation for animals is prepared through fusing the interleukin-4 gene of pig with the interleukin-6 gene of pig, configuring its eukaryon expression plasmid VPIL 46, preparing chitosan nanoparticles by ion exchange method, dissolving said plasmid in the solution of tripolyphosphate and deacetyl chitosan solution in acetate acid, and proportional mixing. It can be used to immunize the experimental animal by intramuscular injection.
Owner:SICHUAN JINZHAO BIOTECH
Recombinant adenovirus high expression vector for promoting effective presentation of hantavirus fusion protein G1S0.7
InactiveCN102676583ARaise the level of immune responseFermentationVector-based foreign material introductionCell immunityTransfer vector
The invention relates to a ubiquitin Ub-containing recombinant adenovirus high expression vector for promoting effective presentation of a hantavirus fusion protein G1S0.7. Primarily, G1S0.7 mosaic genes of hantavirus-containing 76-118 strains and a G1S0.7-pCAG transfer vector of a CAG (Cytotoxin Associated Gene) promoter / enhancer are constructed for promoting effective presentation of highly expressed hantavirus fusion protein G1S0.7 by an organism. Compared with the recombinant adenovirus, partial humoral immunity response level and partial cell immunity response level of an animal organism can be effectively improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Peptide feedstuff additive for regulating immunity function of sea water fish
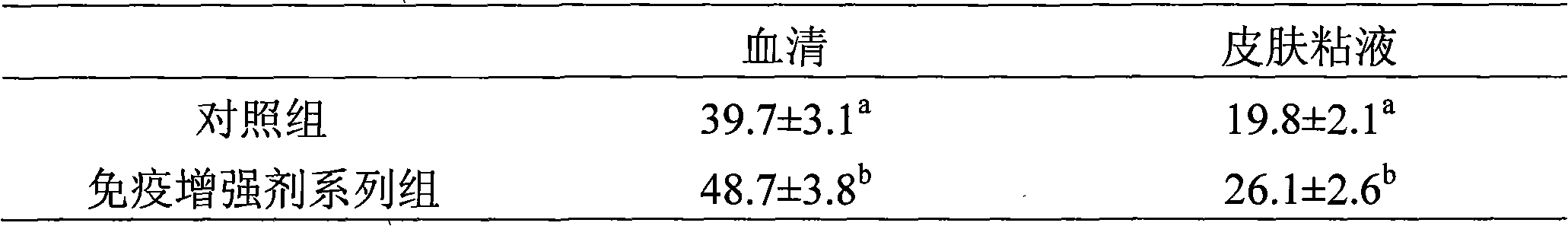
InactiveCN101326958BEnhance the ability to resist pathogenic infectionRaise the level of immune responseAnimal feeding stuffAccessory food factorsOxidation resistantFeed additive
The invention discloses peptide feed additive used to modulate the immune functions of seawater fish. The peptide feed additive consists of three or more than three types in peptide which is produced by enzymatic hydrolysis of cod fish, skin and internal organs with the molecular weight ranges respectively 100Da to 500Da, 500Da to 1500Da, 1500Da to 3000Da, 3000Da to 5000Da, and 5000Da to 8000Da. 1 percent to 5 percent of immune modulator of the invention is added into feed, which can obviously improve the level of IgM and muramidase in the serum of the seawater fish; the immune modulator of the invention can also improve the liver SOD level of the cultivated fish, thereby improving the oxidation resistant capability of the cultivated fish. The immune modulating peptide feed additive of the invention can also obviously improve the daily weight gain of the fish and reduce the liver index. The peptide feed additive used to modulate the immune functions of the seawater fish is safe without public harm, and the fish cultivated by the additive meet the green food sanitation requirements.
Owner:ZHEJIANG UNIV +2
Prepn. and application of pig interleukin-6 gene anti-infection immunopotentiator
InactiveCN1692941ARaise the level of immune responseEnhance immune responseGenetic material ingredientsAntiinfectivesChitosan nanoparticlesWhite blood cell
An anti-infectious immunopotentiator for animals is prepared through cloning the interleukin-6 gene of Chenghua pig, configuring its eukaryon expression plasmid VPIL-6, preparing chitosan nanoparticles from deacetyl chitosan and using said chitosan nanoparticles for molecular packaging of plasmid VPIL-6. It can be used to immunize the experimental animal by intramuscular injection or oral taking.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com