Protein peptide vaccine carrying system and preparing method thereof
A protein polypeptide and drug-carrying system technology, applied in the field of biomedicine, can solve problems such as high toxicity, and achieve the effect of improving expression or secretion and improving immune response effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
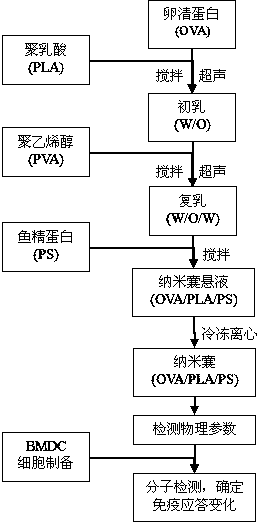
[0043] Embodiment 1: OVA / PLA / PS nanocapsule (containing 25mg / ml OVA) preparation
[0044] 1. Weigh 250mg OVA, dissolve it in PBS with pH 7.0, 0.01mol / L, and set the volume to 10.0ml, as the inner water phase.
[0045] 2. Weigh 125mg of PLA, dissolve it in 10ml of dichloromethane, stir well until uniform, and use it as the organic phase.
[0046] 3. Take 3ml of the organic phase prepared in step 2, add 450 μl of the internal aqueous phase prepared in step 1 dropwise to the organic phase, and stir to form a suspension.
[0047] 4. Using the JY99-2D ultrasonic cell breaker produced by Ningbo Xinzhi Biotechnology Research Institute, insert an ultrasonic probe into the suspension prepared in step 3, set the ultrasonic breaker to a success rate of 200W, an ultrasonic time of 3 seconds, and an interval of 5 seconds , sonicated 7 times to prepare colostrum.
[0048] 5. Weigh 100-300 mg of polyvinyl alcohol (PVA), dissolve it in pH 7.0, 0.01 mol / L phosphate buffered aqueous solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com