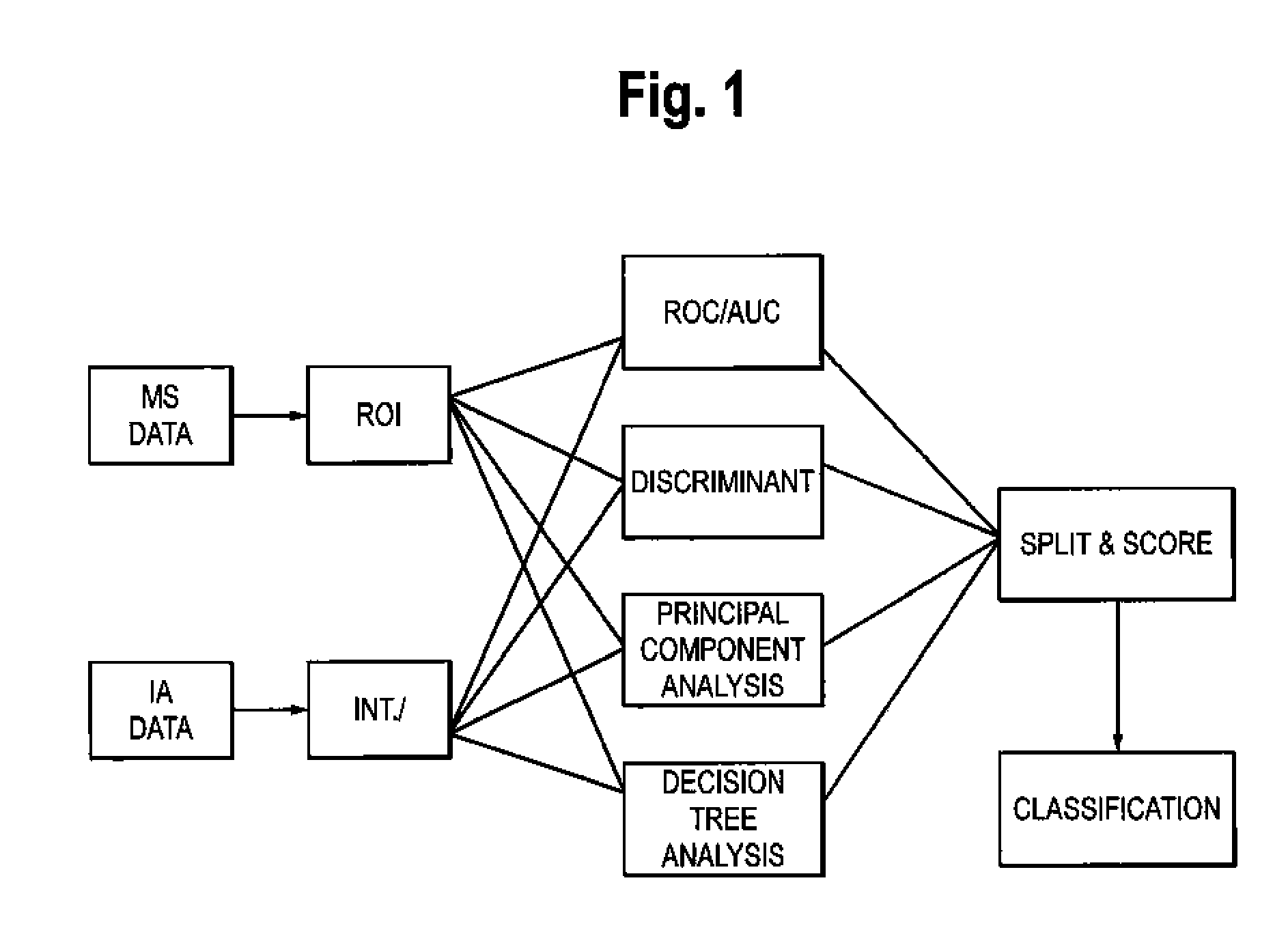
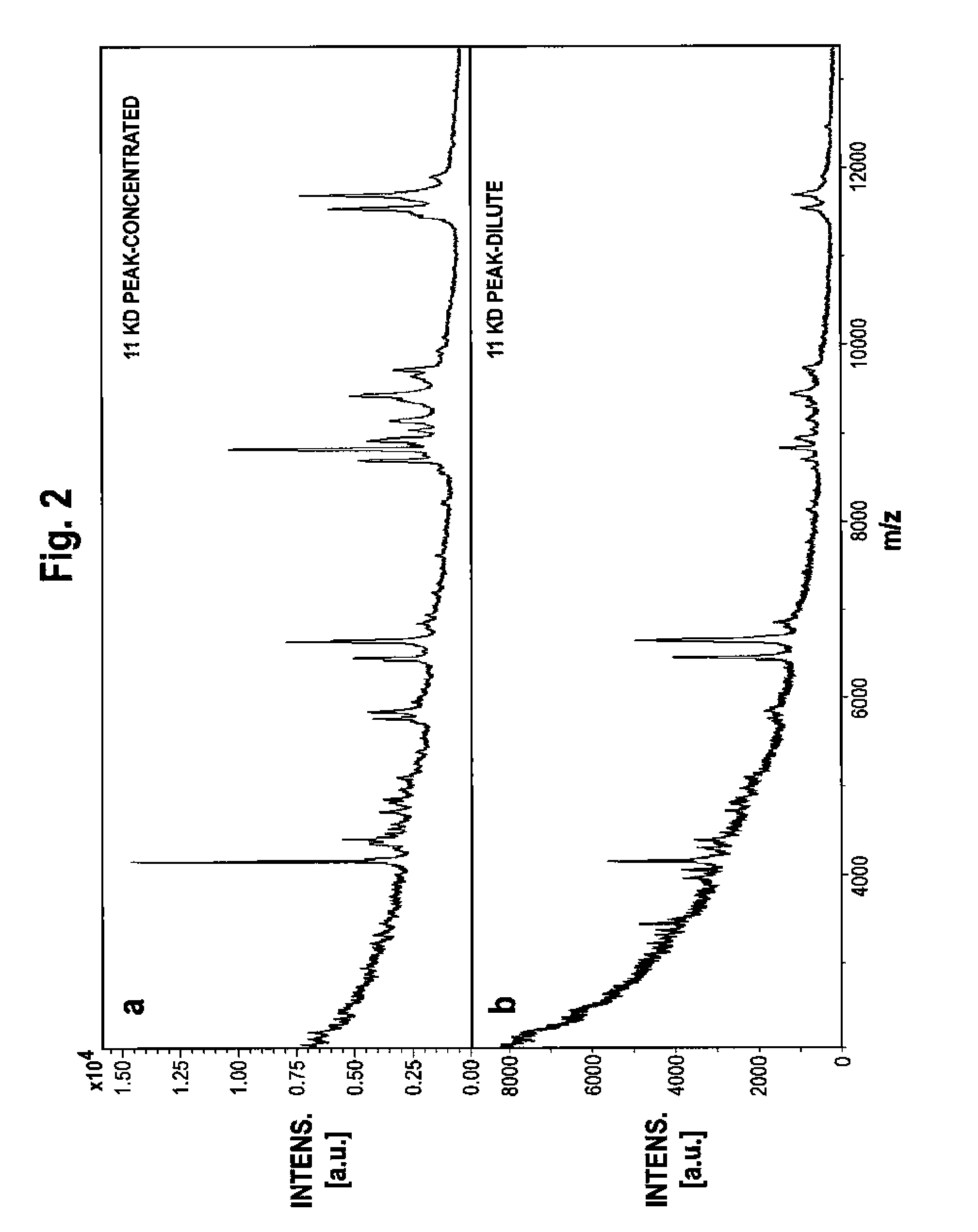
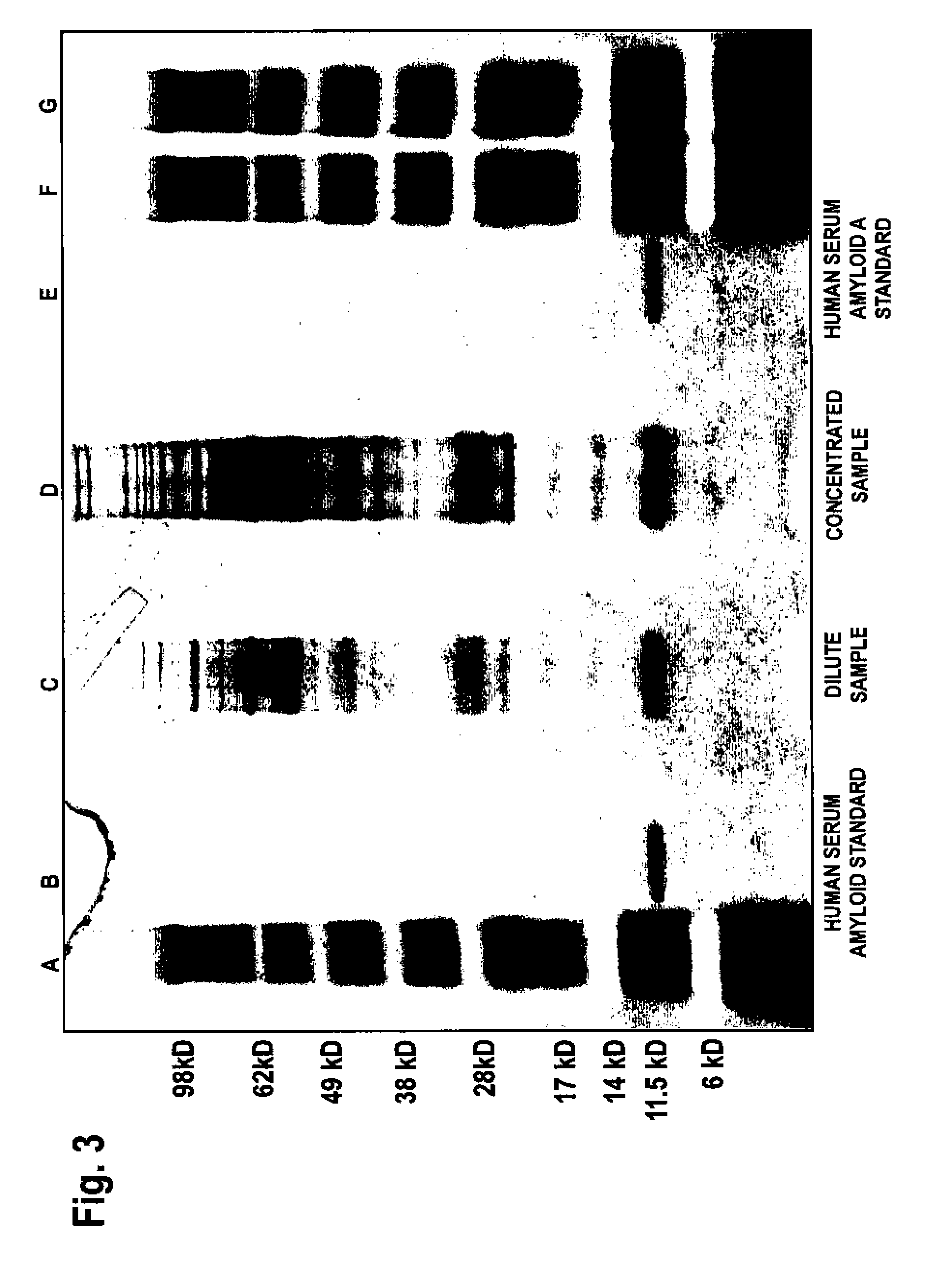
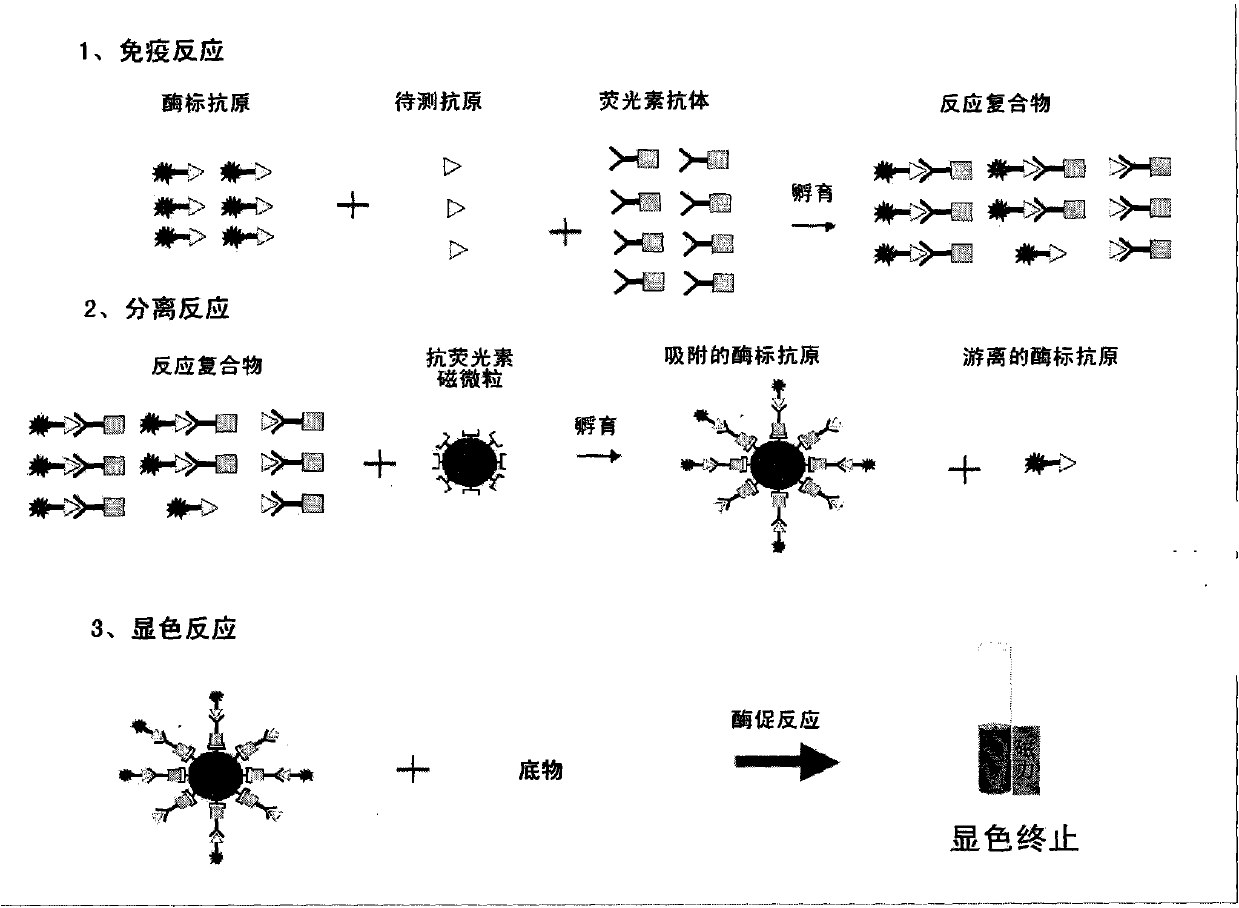
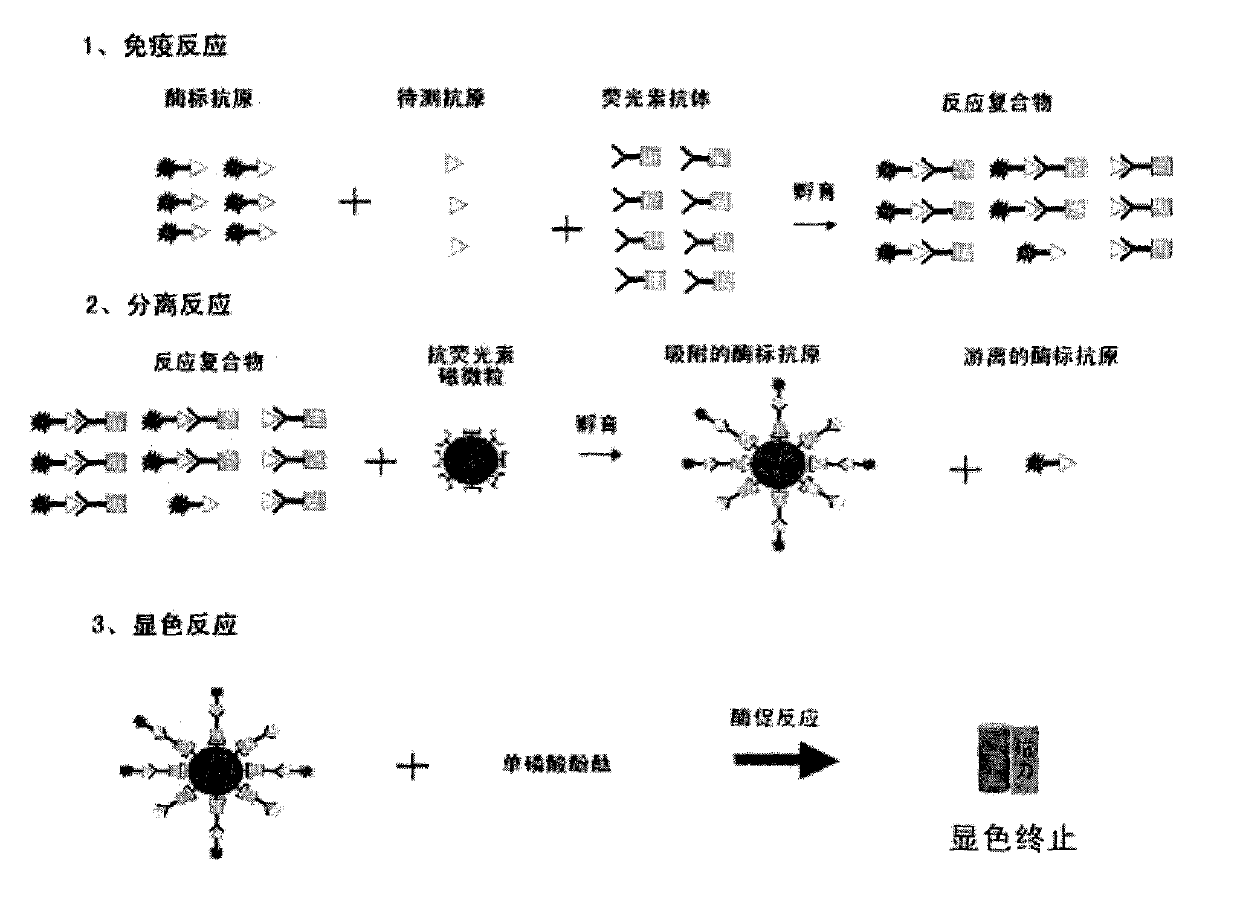
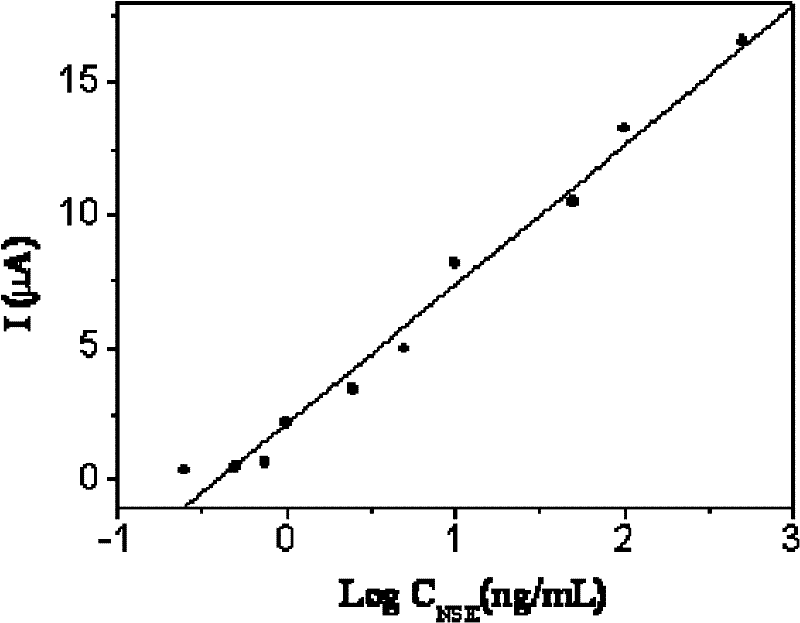
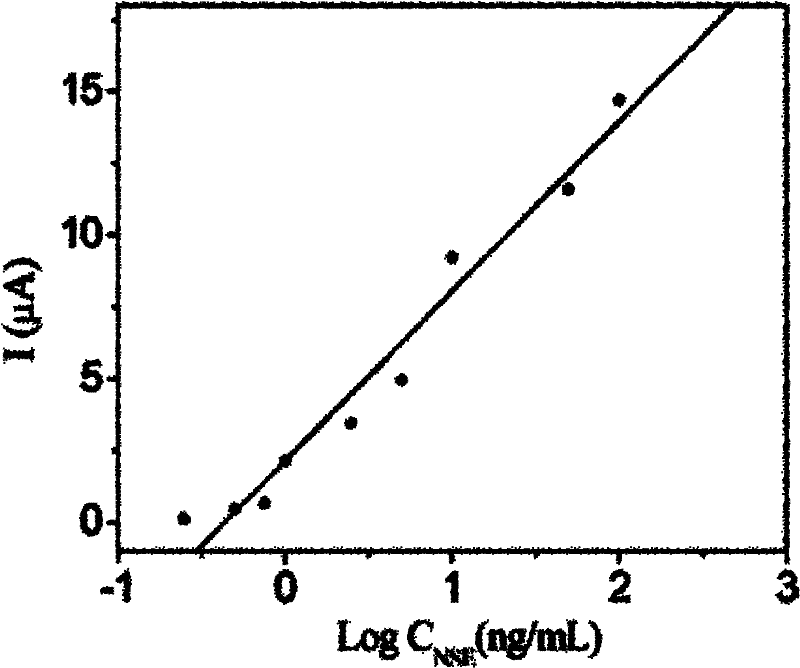
Patents
Literature
360 results about "Immunoresponse" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A marked decrease in contrast enhancement that is not due to actual tumor shrinkage, but that may be due to immunotherapy.
Methods and marker combinations for screening for predisposition to lung cancer
InactiveUS20070178504A1Microbiological testing/measurementBiological testingOncologyLung cancer susceptibility
Owner:ABBOTT LAB INC
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176258A1Improve efficiencyIncrease resourcesBiological material analysisDepsipeptidesImmunodominant EpitopesMonoclonal antibody
Disclosed herein among other MN / CA IX-related inventions are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Subsets of the new antibodies are to either the proteoglycan-like (PG) domain or to the carbonic anhydrase (CA) domain of MN / CA IX, and methods are provided by which antibodies can be prepared to the other MN / CA IX domains. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
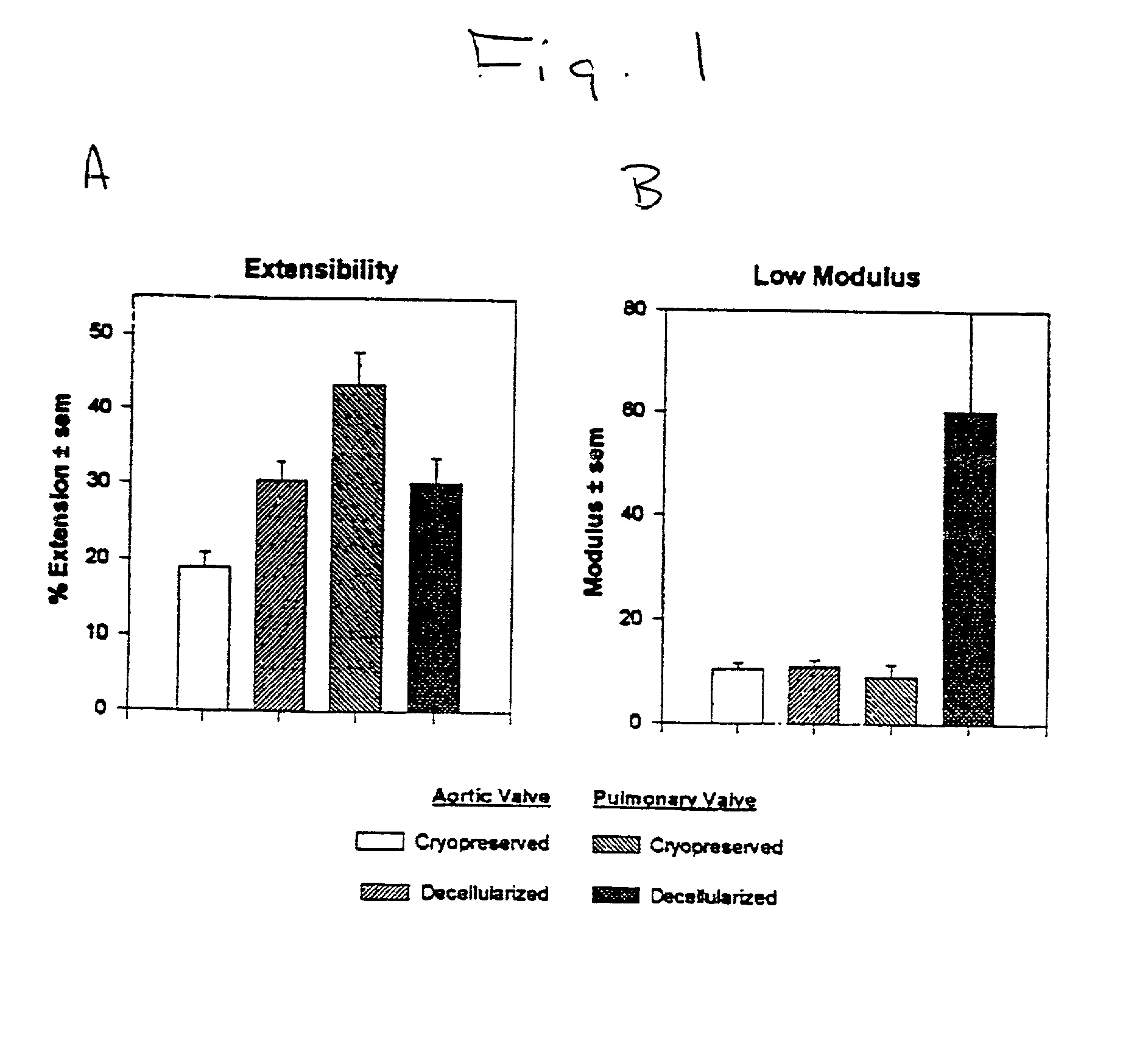
Tissue decellularization
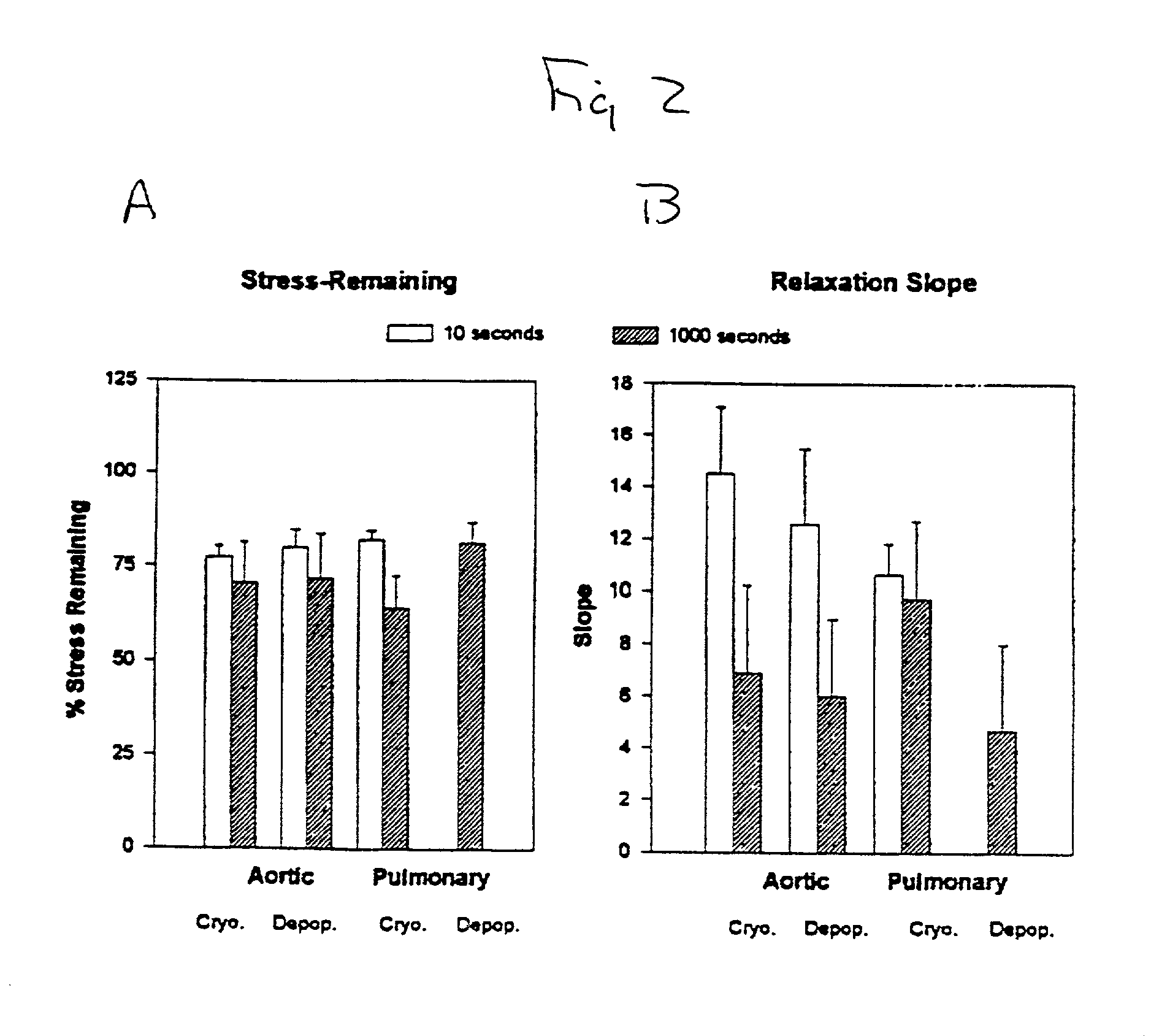
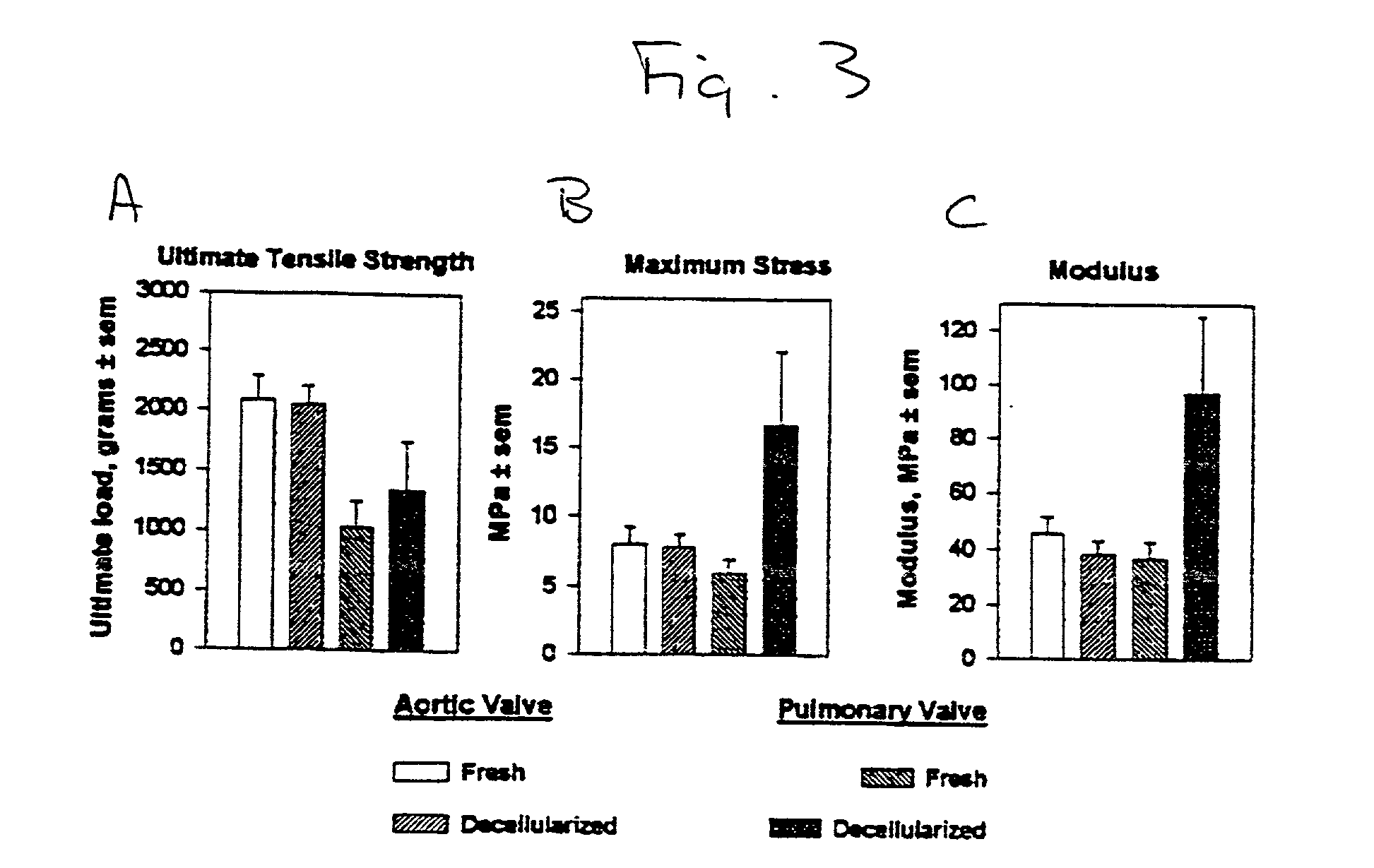
InactiveUS20010000804A1Increased durabilityDecreased immunoreactivitySuture equipmentsHeart valvesTissue DecellularizationLigament structure
The present invention relates, in general, to tissue decellularization and, in particular to a method of treating tissues, for example, heart valves, tendons and ligaments, so as to render them acellular and thereby limit mineralization and / or immunoreactivity upon implementation in vivo.
Owner:CRYOLIFE
Immunoassay apparatus for diagnosis
InactiveUS6689317B1Easy to manufactureKeep for a long timeAnalysis using chemical indicatorsAnalysis by subjecting material to chemical reactionAnalyteAllergy
Immunoassay analytical test apparatus for allergy diagnosis, which apparatus comprises a zone for receiving a sample containing an analyte, a zone for receiving a mobile phase (the zone may be the same as the sample receiving zone, or different thereto), a detection means for permitting detection of the analyte by immunoreaction, a first flow path for flow of the analyte in the mobile phase from the sample receiving zone to the detection means, and a second flow path permitting flow of a mobile phase to the detection means.
Owner:MCCULLOCH PETER FREDERICK
Method of purifying polypeptides by simulated moving bed chromatography
InactiveUS20040241878A1Reduce linear flow rateSlow velocityIon-exchange process apparatusComponent separationBiotechnologyChemical compound
Provided are methods of separating an immunoreactive compound from at least one immaterial component, using a simulated moving bed ("SMB") system and a SMB apparatus for use in these methods. Also provided are purified immunoreactive compounds prepared using the SMB methods and apparatus and methods of treatment with the purified immunoreactive compounds.
Owner:BIOGEN INC
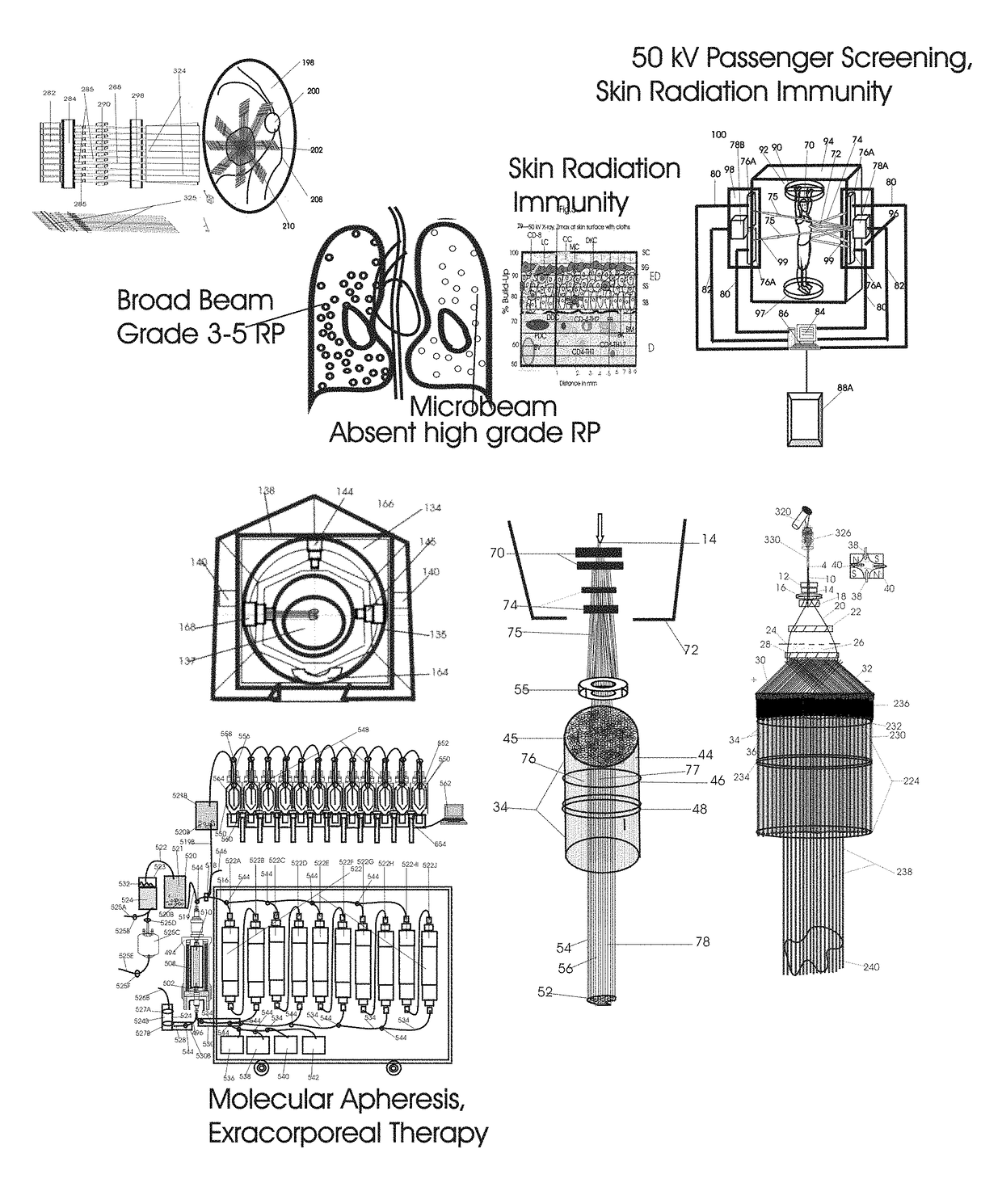
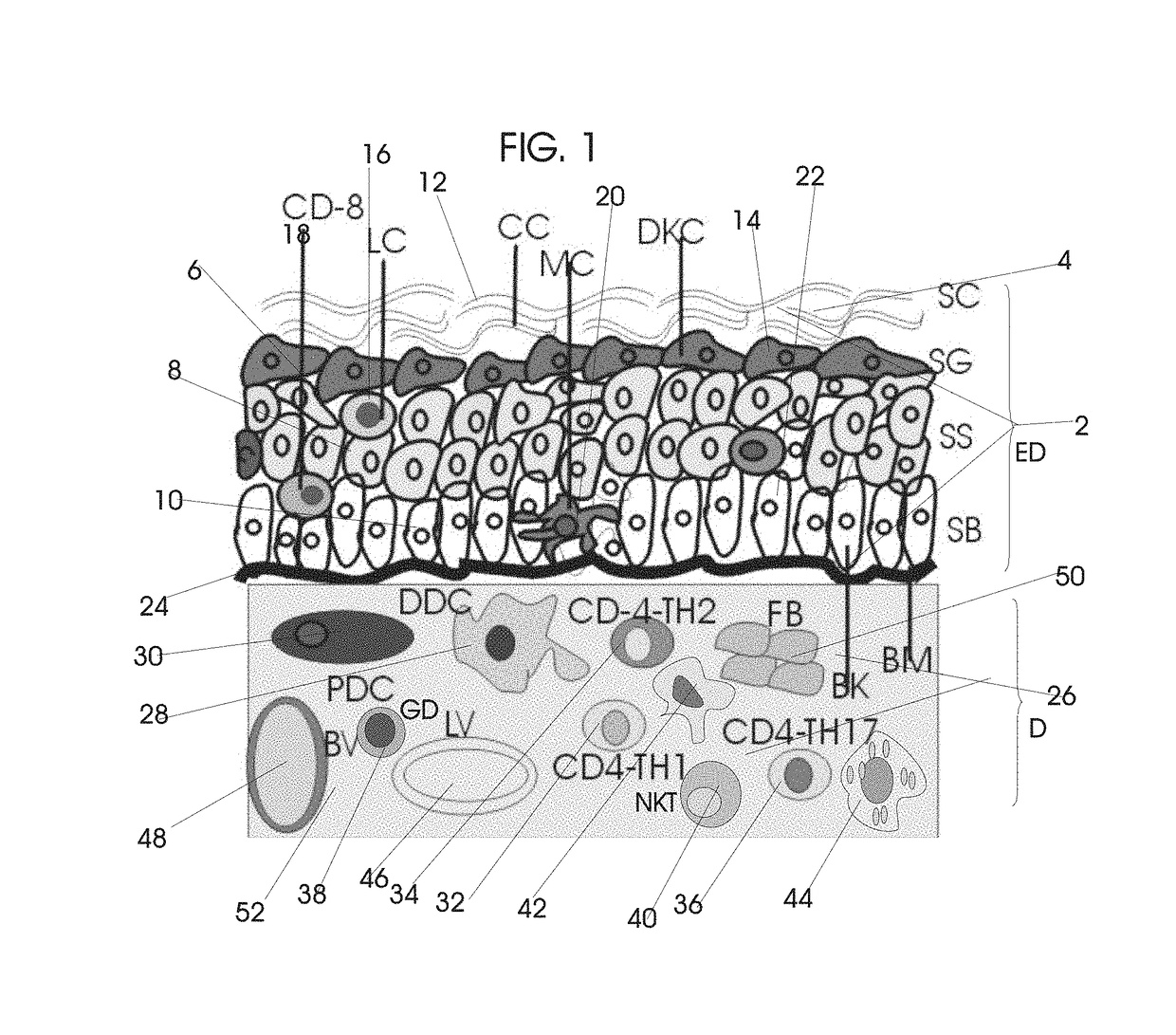
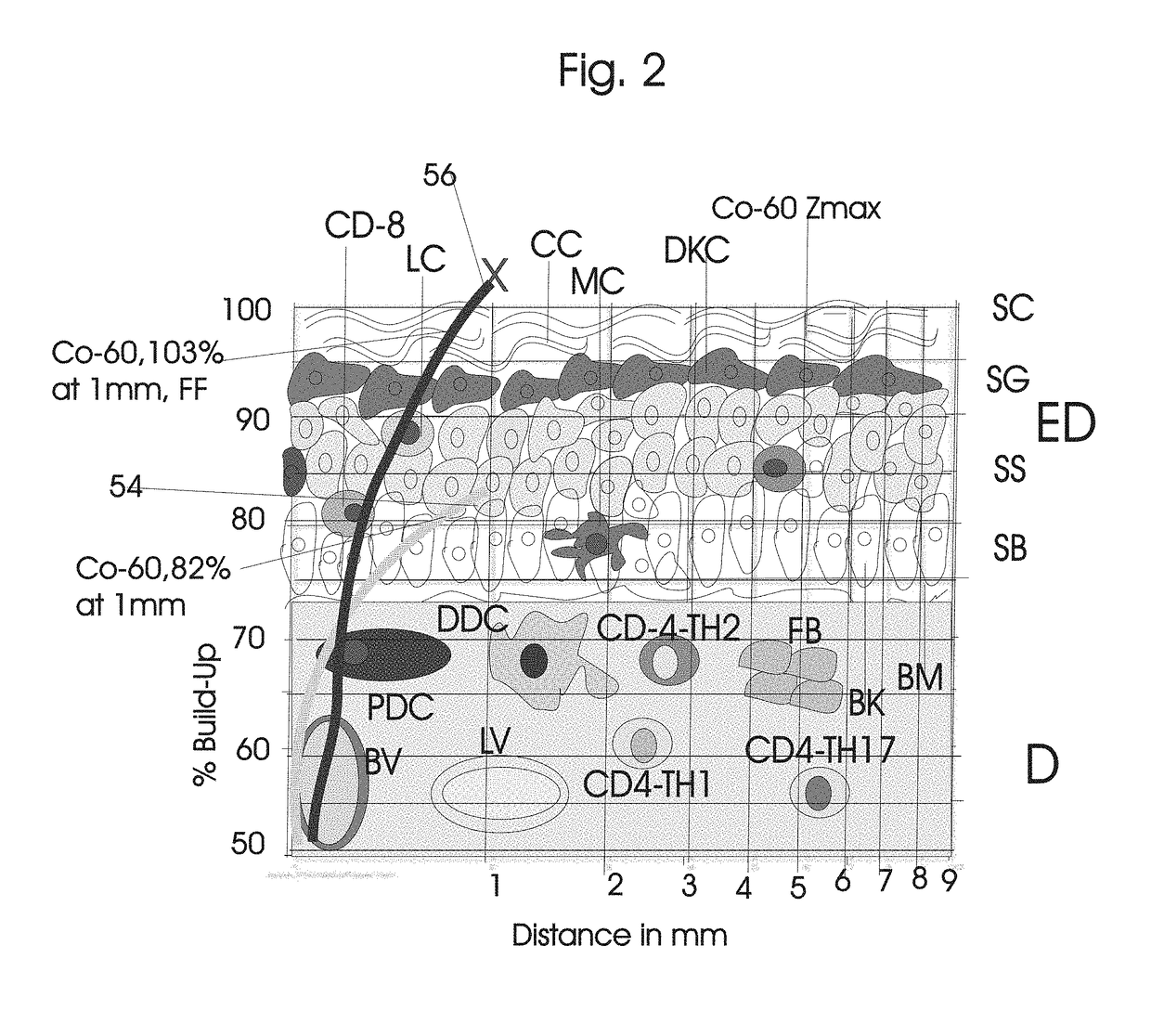
Normal Tissue Toxicity Reducing Microbeam-Broadbeam Radiotherapy, Skin's Radio-Response Immunotherapy and Mutated Molecular Apheresis Combined Cancer Treatments
InactiveUS20180154183A1Improve treatment outcomesIncreased toxicityOther blood circulation devicesHaemofiltrationAbnormal tissue growthGamma ray
Normal tissue complications limit curative broadbeam radiotherapy to tumors including lung cancer. Radiation retinitis causing blindness limits quality of life and long term survival for patients with ocular melanoma. This invention pertains to alternative, normal tissue sparing 100 to 1,000 Gy microbeam radiations with least normal tissue complications and concomitant radio-immunotherapy by innate immune response of epidermis and dermis to low dose radiation with 50 kV X-rays. Total body skin radiation with former airport passenger screening machines with 50 kV X-ray is disclosed. Microbeams are generated without contaminating scatter and neutron radiations from collinear gamma ray and electron beam produced by inverse Compton interaction with high energy laser and electron beam and from proton and carbon ion beams in tissue equivalent cylindrical collimators. Extracorporeal immunotherapy and chemotherapy and apheresis of mutated subcellular particles released into circulation in response to cancer-therapies are by clinical continuous flow ultracentrifugation combined chromatography.
Owner:SAHADEVAN VELAYUDHAN
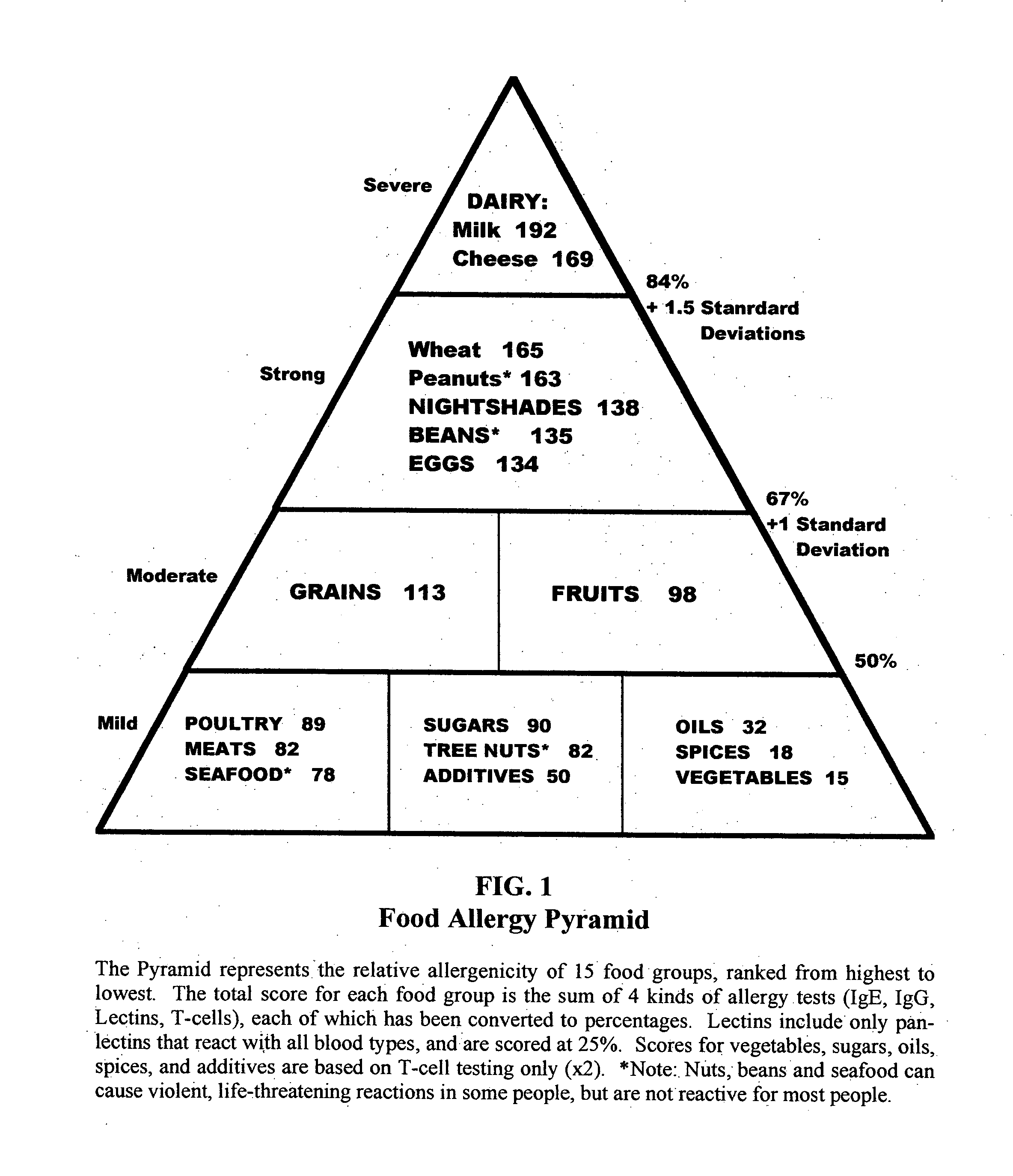
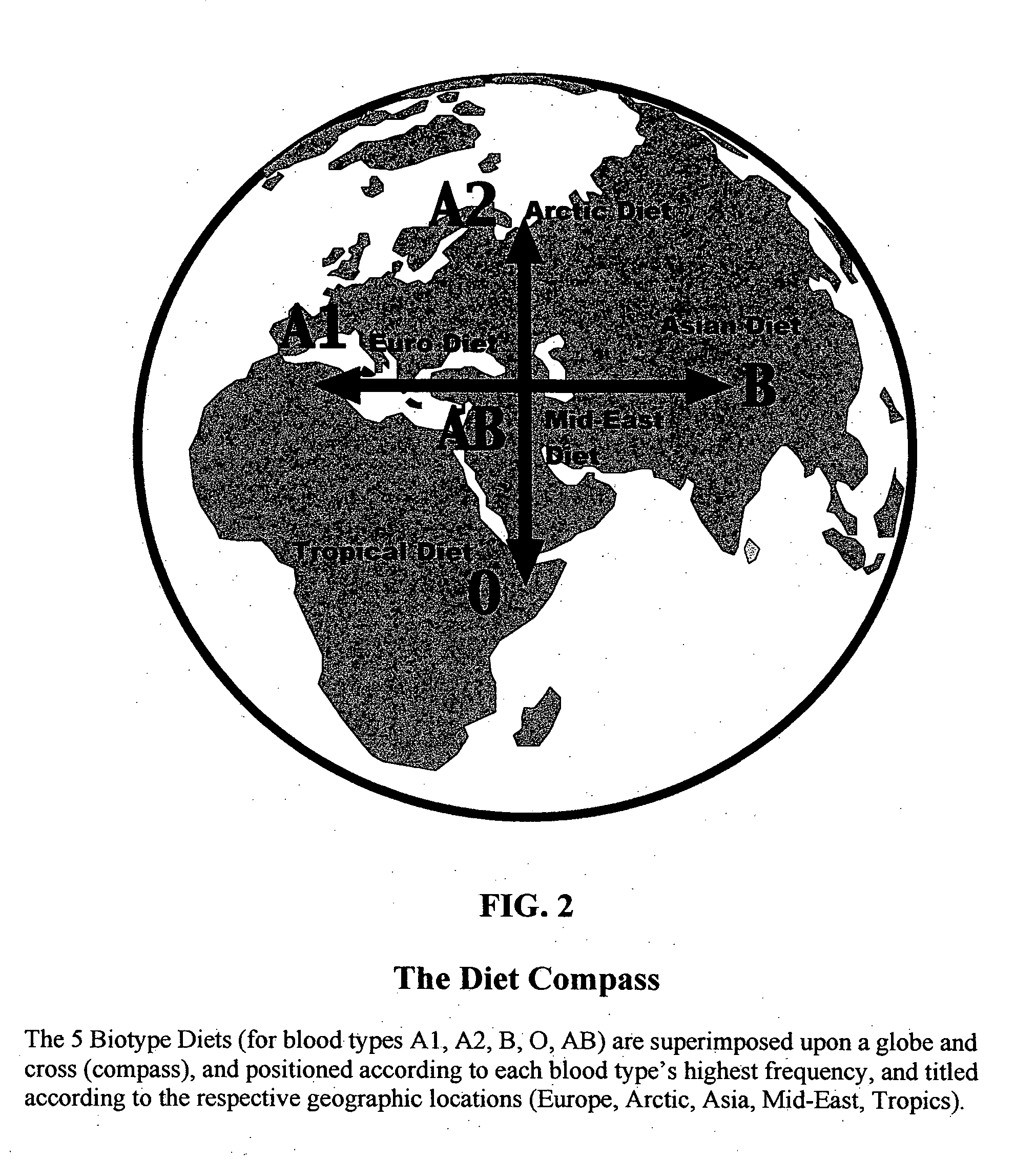
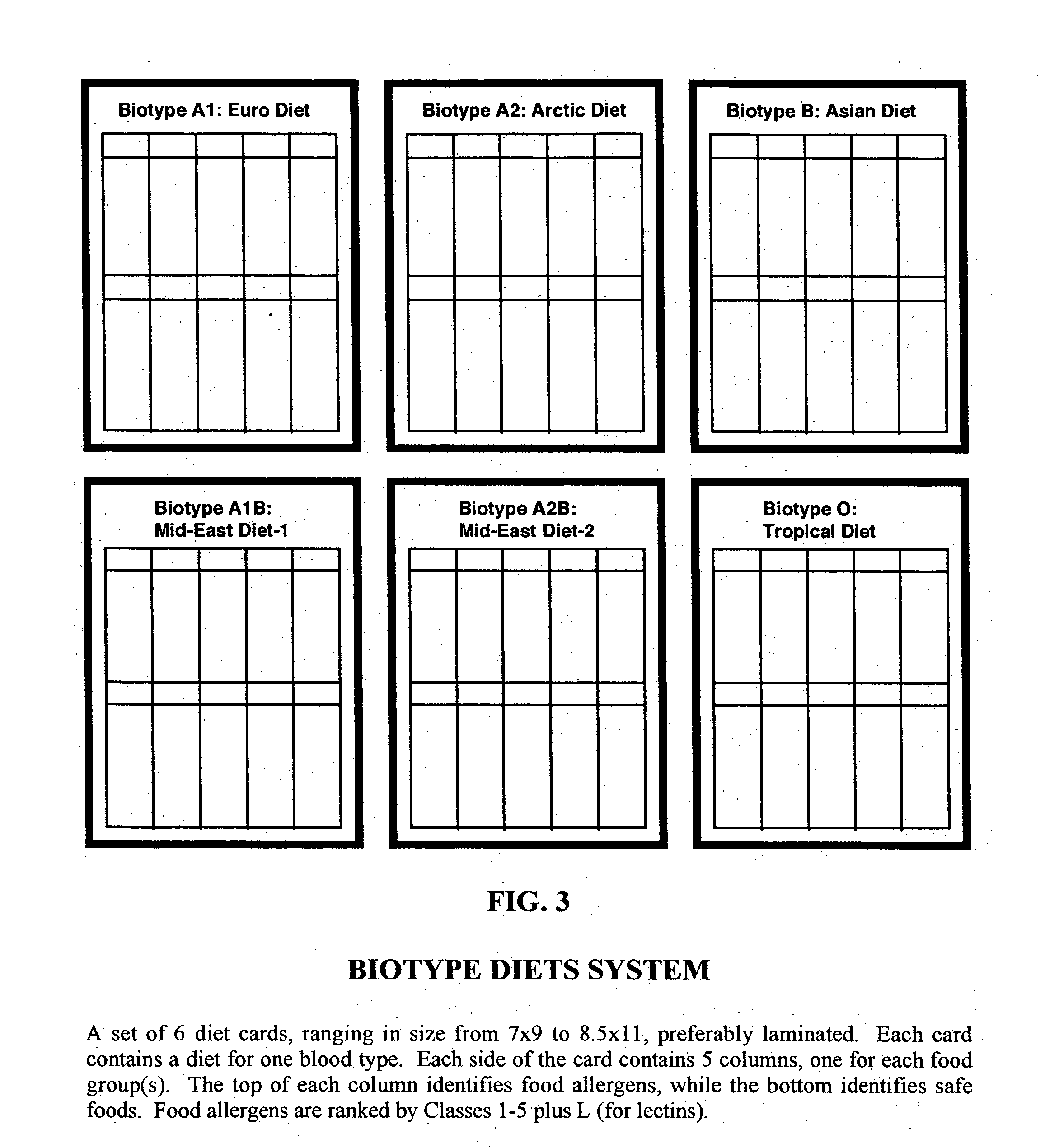
Biotype diets system: predicting food allergies by blood type
InactiveUS20060013773A1Ultrasonic/sonic/infrasonic diagnosticsAnalysis using chemical indicatorsGroup A - bloodAntigen
The invention is a diet-typing system for humans, including novel methods for diagnosis and treatment of food allergies and hypersensitivities. The diagnostic method correlates blood types (immunologically reactive antigens on RBC, skin and membranes) to four kinds of food allergies / hypersensitivities (IgE antibodies, IgG antibodies, T-cells, and Lectins). The results are used to identify and predict food allergies and hypersensitivities for six biological types (blood types A1, A2, B, O, A1B, and A2B), plus diet modifications for three subtypes (blood type Rh-negative, males and females). The treatment method uses the results to make food recommendations (to eat, limit, or avoid), based on the strength or classification of allergy scores, to mitigate the risk of food allergies and hypersensitivities in future persons. The diet-typing system presents the results on six diet cards, one for each blood type. The methods and resulting diets are unique, and differ substantially from prior inventions.
Owner:POWER LAURA W
A method for quantitative detection of biological molecules capable of being metabolized to generate H2O2 in serum by means of a ratiometric fluorescent probe
ActiveCN107478621AExcellent fluorescence performanceHighly sensitive assayFluorescence/phosphorescenceLinear relationshipHigh selectivity
The invention discloses a method for quantitative detection of biological molecules capable of being metabolized to generate H2O2 in serum by means of a ratiometric fluorescent probe. The method includes firstly preparing copper-nitrogen codoped carbon dots Cu-CDs; then reacting corresponding oxidase and the biological molecules capable of being metabolized to generate H2O2 to generate H2O2; catalyzing the H2O2 and a substrate that is o-phenylenediamine with horseradish peroxidase to generate an oxidation product DAP having yellow fluorescence; then adding the Cu-CDs into the DAP; allowing the DAP and the Cu-CDs to form ratiometric fluorescence, with the ratio I<572> / I<460> of fluorescence intensities of the DAP and the Cu-CDs being in a linear relationship with the concentration of a substance to be detected; and performing quantitative assay of the biological molecules in the serum according to the ratio of fluorescence intensities. The method is high in sensitivity and simple and convenient in detection, and has high selectivity and high affinity of immunoreactions.
Owner:NANJING MEDICAL UNIV
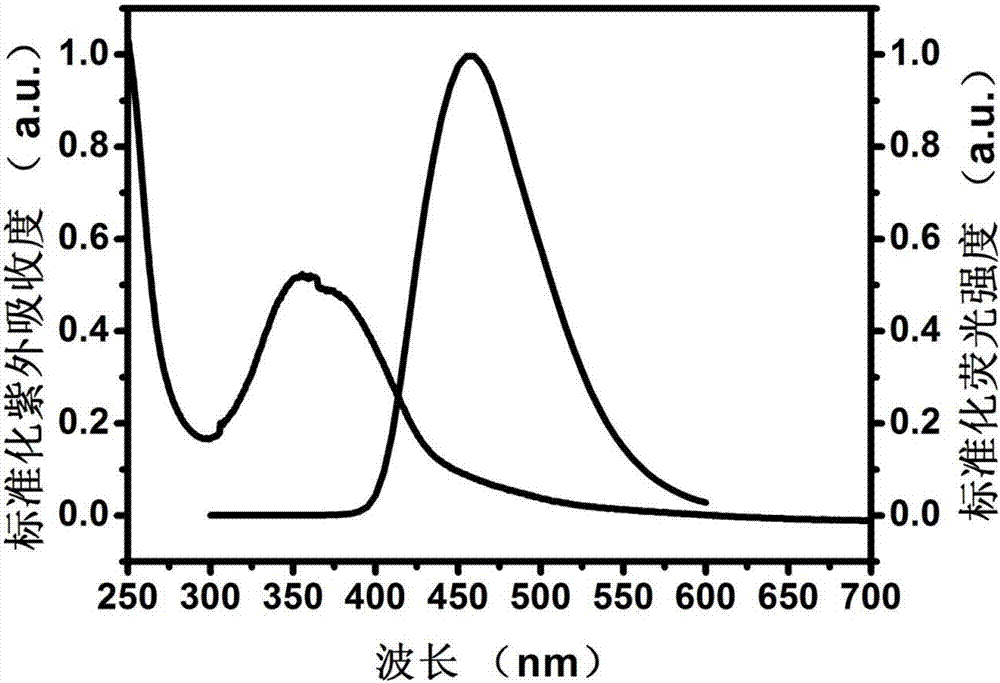
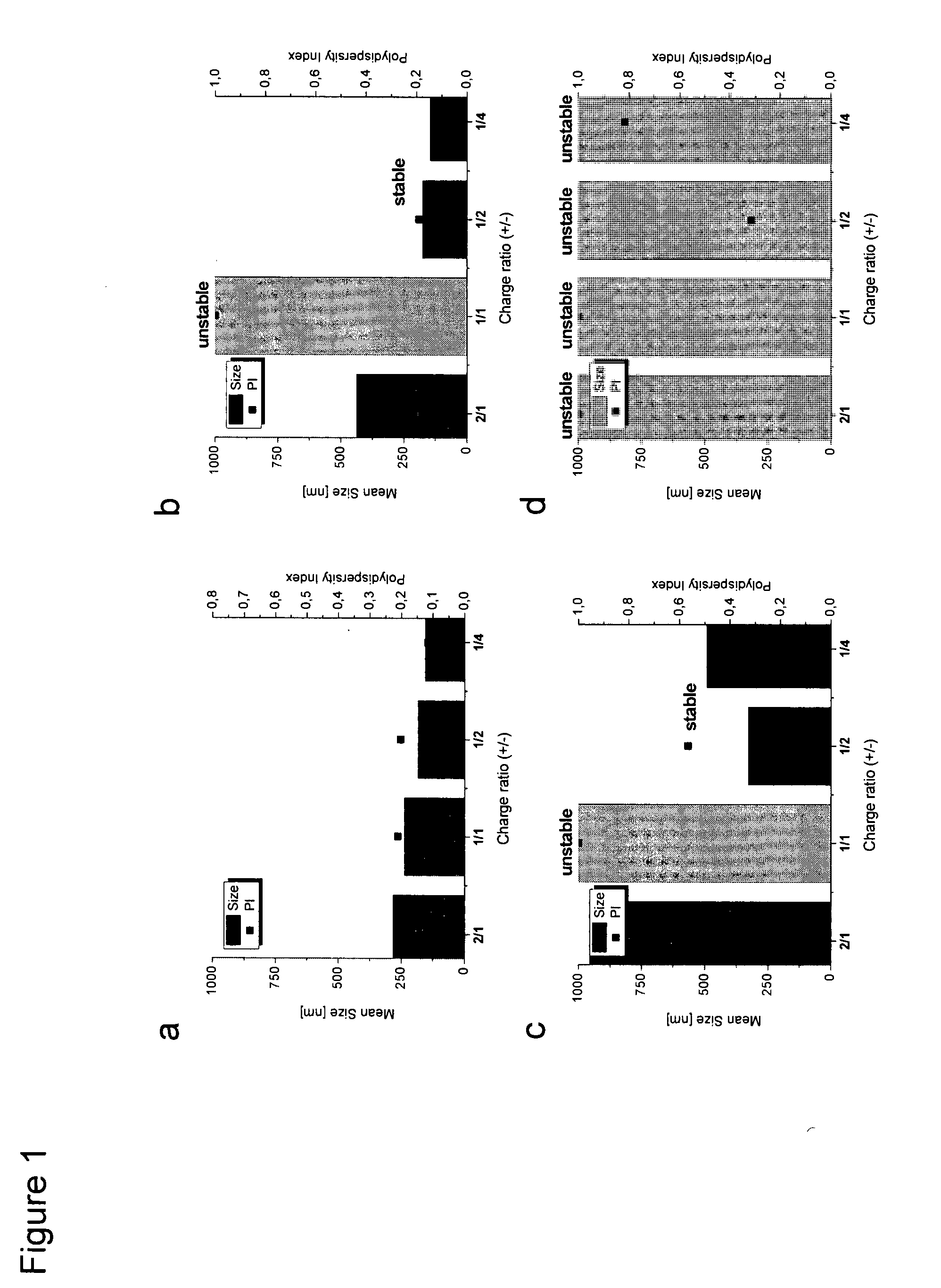
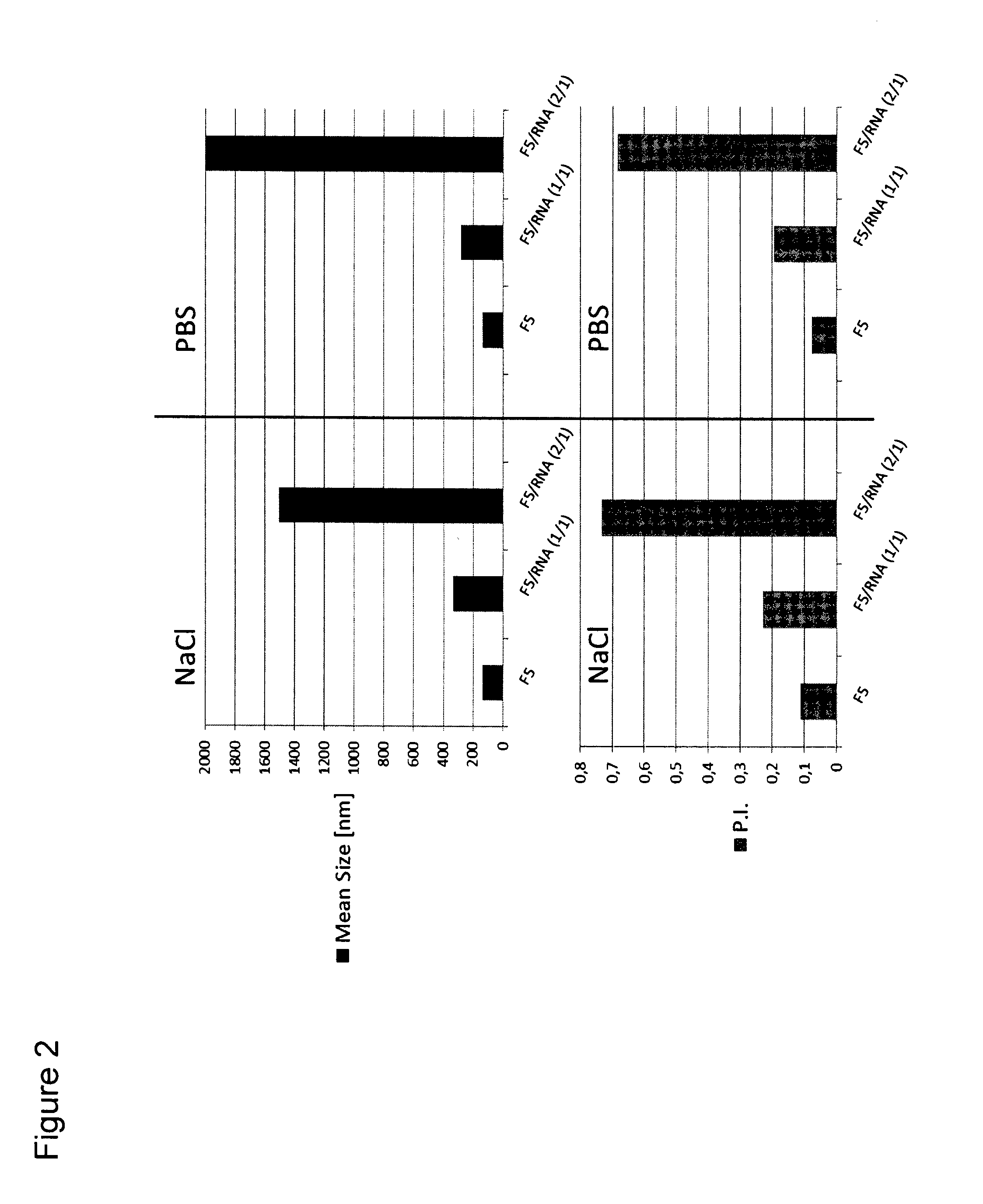
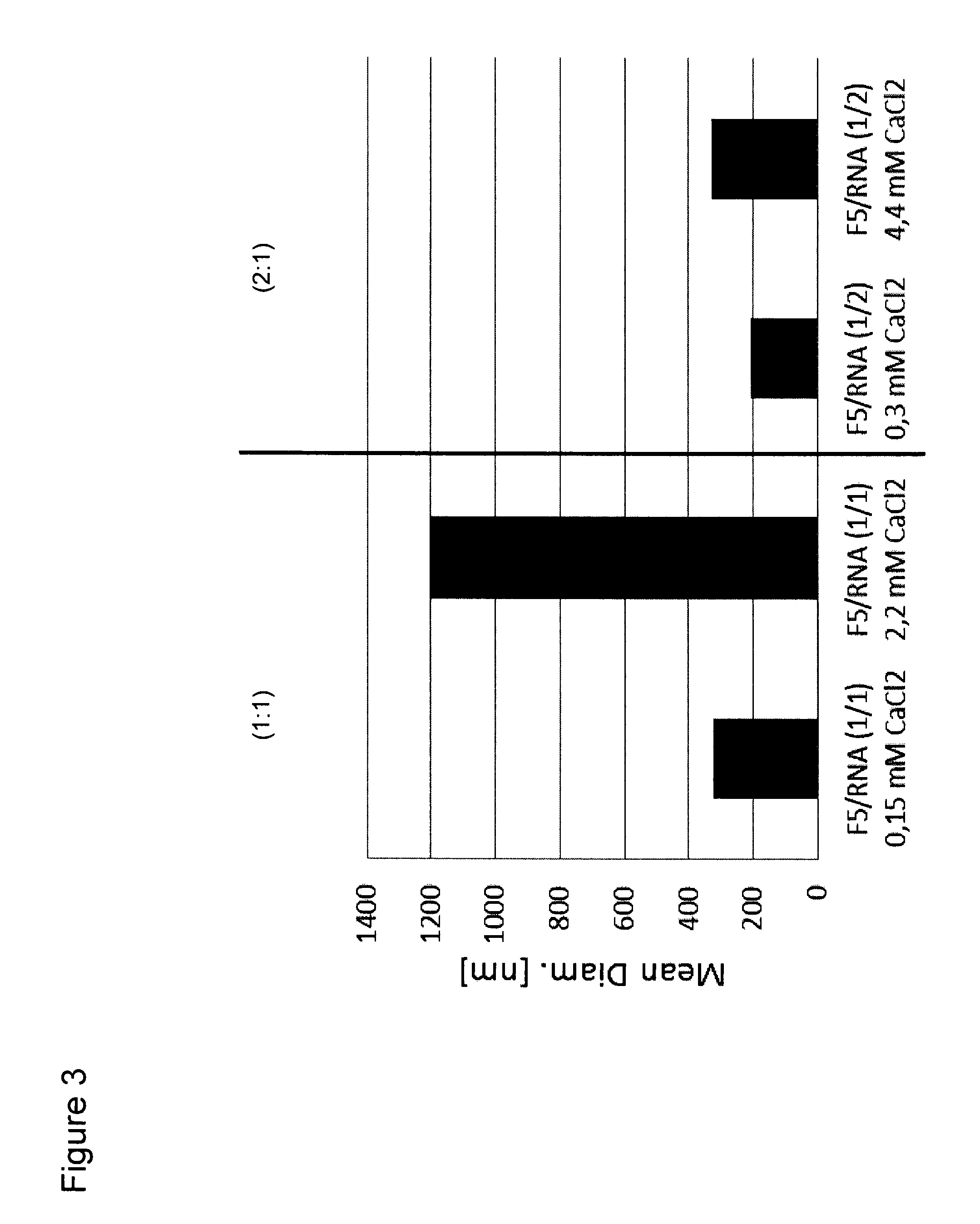
RNA Formulation for Immunotherapy
ActiveUS20150086612A1Minimize adverse effectsPowder deliveryGenetic material ingredientsAntigenWhole body
The present invention is in the field of immunotherapy, in particular tumor immunotherapy. The present invention provides pharmaceutical formulations for delivering RNA to antigen presenting cells such as dendrite cells (DCs) in the spleen after systemic administration. In particular, the formulations described herein enable to induce an immune response after systemic administration of antigen-coding RNA.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
AAV virions with decreased immunoreactivity and uses therefor
ActiveUS20080008690A1Decreased immunoreactivityEfficient transductionVirusesHydrolasesVirosomeSerotype
Owner:GENZYME CORP
Ionic liquid-graphene nanocomposite, preparation method and electrochemical immunodetection method thereof
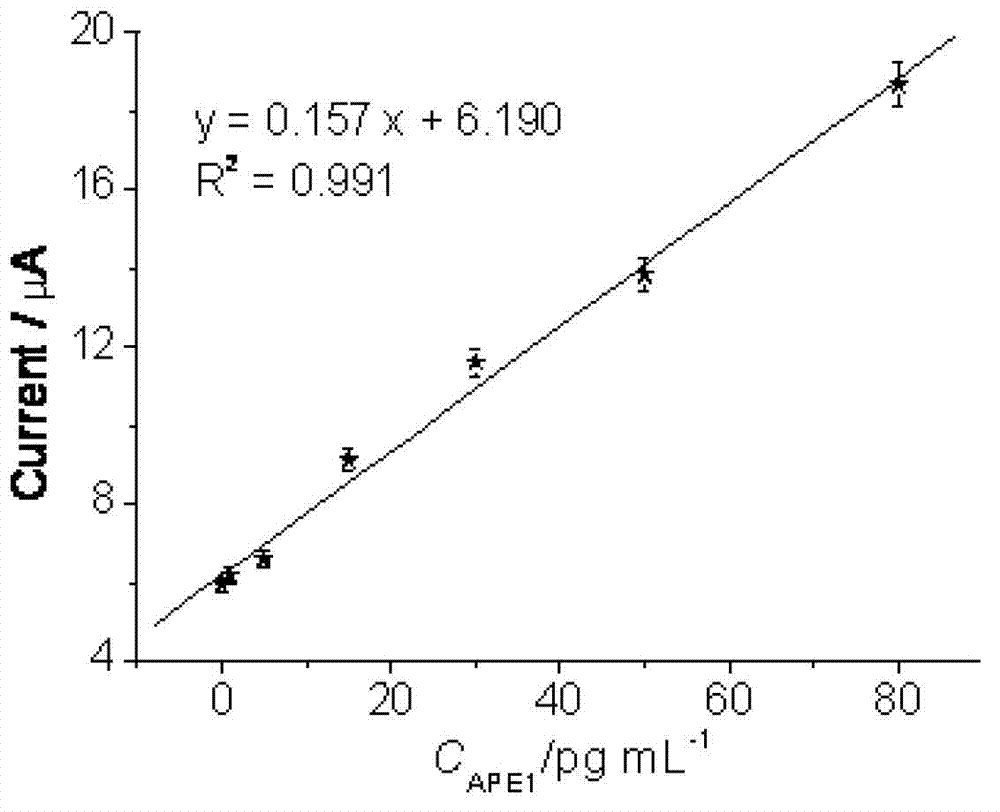
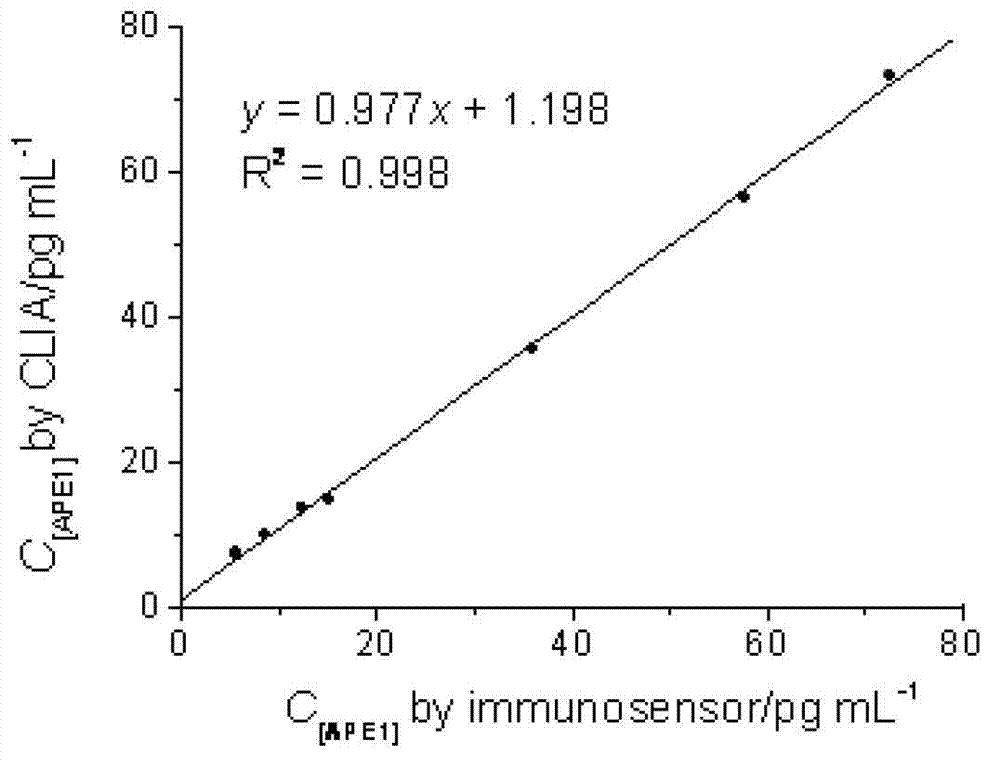
The invention relates to the field of electrochemical immunodetection, and especially relates to an ionic liquid-graphene nanocomposite, a preparation method and an electrochemical immunodetection method thereof. According to the immunodetection method, through using a double-antibody sandwich method, an apurinic / apyrimidinic endonuclease / redox factor antibody (anti-APE1) fixedly carried on the surface of an electrode carries out immunoreaction with an apurinic / apyrimidinic endonuclease / redox factor (APE1) in a sample solution, and is then combined with a room-temperature ionic liquid-graphene nanocomposite and an anti-APE1 co-coupling object marked by alkaline phosphatase (ALP) and ferrocene (Fc). Based on the electrochemical activities of the ALP-Fc-anti-APE and the room-temperature ionic liquid-graphene nanocomposite, a CV (cyclic vohammetry) catalytic current value is measured, and then the concentration of the APE1 in a detection sample is detected. The linear response range of the electrochemical immunodetection method provided by the invention is 0.1-80 pg / mL, and the lower detection limit is 0.04 pg / mL, therefore, the electrochemical immunodetection method is good in specificity and high in sensitivity.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Nano antibody of anti-deoxynivalenol antibody
InactiveCN104592389AHave immune response propertiesGood effectImmunoglobulinsGenetic engineeringEpitopeEndurance capacity
The invention belongs to the technical field of biology and specifically relates to a preparation method and an application of a nano antibody capable of specially binding with an anti-deoxynivalenol antibody. The amino acid sequence of the nano antibody is SEQ ID No. 1. The invention also relates to a nucleotide for encoding amino acid. The nano antibody is capable of taking the place of the expensive high-toxicity DON standard substances, and can be applied to the immunological detection of the DON as a competitive antigen or a solid-phase envelope antigen; the nano antibody has immunoreaction characteristic similar to that of natural DON molecule, and is excellent in effect. Compared with the traditional antigen mimic epitopes based on polypeptides and the traditional anti-idiotype antibodies based on IgG, the nano antibody has the characteristics of more stable structure, acid-alkali resistance, high temperature resistance, high detection sensitivity and the like, and therefore, the immunodetection stability of the nano antibody is greatly improved, and meanwhile, the endurance capacity of the nano antibody to the environment is also improved.
Owner:NANCHANG UNIV
Cancer immunotherapy
InactiveUS20060127408A1Eliminate the effects ofIncreasing breadth and hopefully potency of immune responseGenetic material ingredientsTissue cultureAntigenPolycomb-group proteins
The invention relates to the use of polycomb group proteins as a tumour-associated antigens. Polycomb group proteins are highly involved in body architecture development, haematopoiesis and cell cycle control. Aberrant expression of polycomb proteins has been linked with haematological malignancies, mainly lymphomas. This invention relates to the use of these polycomb group proteins as antigens for cancer immunotherapy. Immunological responses can be raised against these proteins and such responses are active against polycomb protein over-expressing tumour cells.
Owner:THE UNIV OF BIRMINGHAM
Electrochemical immunosensor for detecting toxoplasma gondii IgM antibody and preparation method thereof
InactiveCN102914648AImprove conductivityEasy to passMaterial electrochemical variablesMedical diagnosisElectronics
The invention belongs to the technical field of analytical chemistry and chemical sensors and discloses an electrochemical immunosensor for detecting a toxoplasma gondii IgM (Immunoglobulin m) antibody (Tg-IgM) of a gravida and a preparation method of the electrochemical immunosensor. The immunosensor is prepared by sequentially modifying graphene, polythionine, gold nanoparticles and capture antigen to the surface of a glassy carbon electrode. An enzyme-functionalized nano-composite detection probe with an electrical signal amplifying function is prepared by assembling enzyme and a second antibody with high proportions on an Au-Fe3O4 surface. According to the sandwich immunoassay principle, the concentration of Tg-IgM is determined by using an electrochemical signal generated by catalysis of enzyme to a substrate. According to the electrochemical immunosensor, the specificity of immunoreaction is combined with the sensitivity of electrochemical detection; the transmission of electronics is promoted by using the graphene, the polythionine, the gold nanoparticles, Au-Fe3O4 and other material; and the sensitivity of the detection is improved. The electrochemical immunosensor has the advantages of simplicity and convenience for operation, favorable regeneration performance and detection cost reduction. The electrochemical immunosensor prepared on the basis can be also used for detecting other immunological markers and has favorable application prospect in medical diagnosis.
Owner:CHONGQING MEDICAL UNIVERSITY
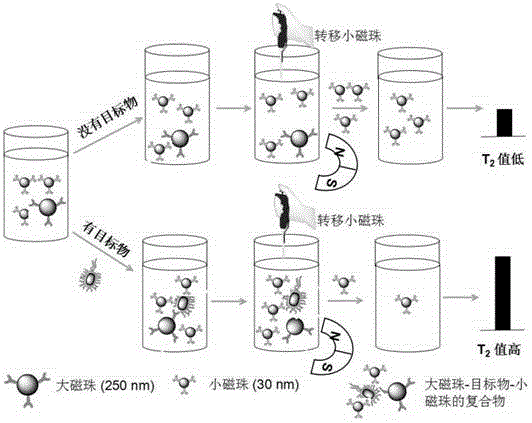
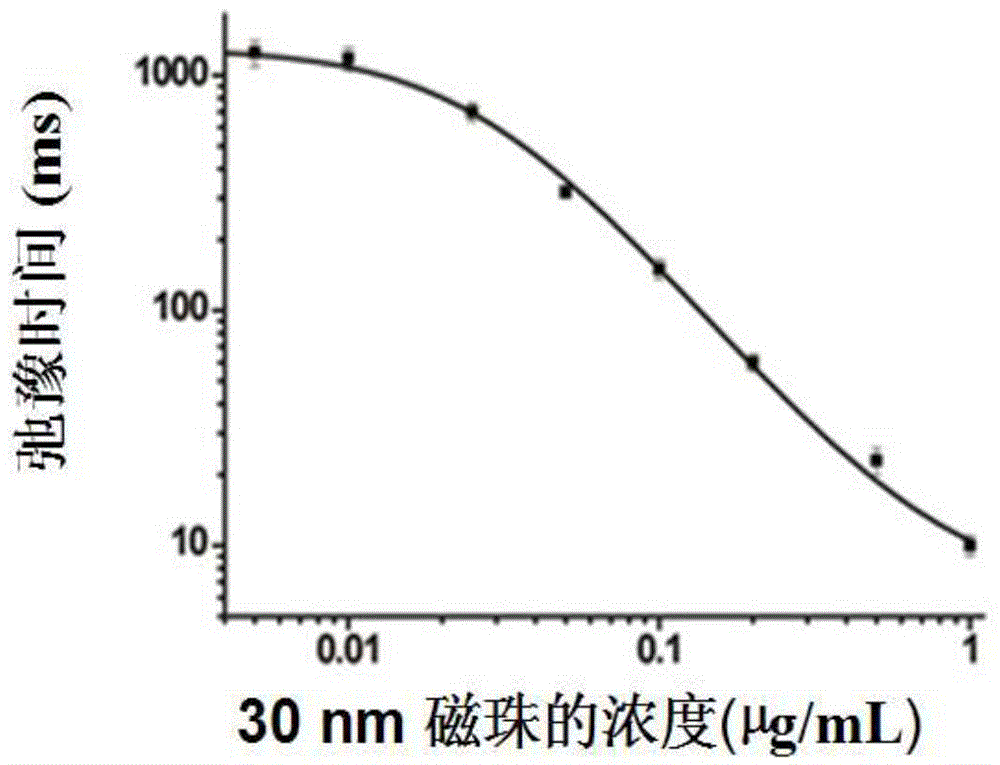
Relaxation time immunosensing analysis method based on magnetic separation
ActiveCN104614513AStable detectionAccurate detectionMaterial analysisMagnetic beadBiomarker (petroleum)
The invention relates to a relaxation time immunosensing analysis method based on magnetic separation. The method comprises the following steps: selecting two magnetic beads (one can be quickly separated and the other cannot be separated) different in saturated magnetization intensity and remarkably different in separation speed in the same magnetic field, so that the magnetic beads can be separated in the magnetic field; coupling the magnetic beads to an antibody used for identifying different sites of the same target object respectively to prepare immunomagnetic beads; producing an immunoreaction of the immunomagnetic beads and a to-be-detected sample; performing magnetic separation on a mixed system, measuring transverse relaxation time for supernatant liquor subjected to magnetic separation, and determining the concentration of biomacromolecules in the to-be-detected sample according to change of the transverse relaxation time. The immunomagnetic beads different in saturated magnetization intensity are different in separation speed in the same magnetic field, the magnetic separation is combined with magnetic relaxation time analysis, the reaction time only needs 30 minutes, and the method can be used for quickly detecting bacteria, viruses and proteins and has a very good application prospect in an aspect of biomarker detection.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
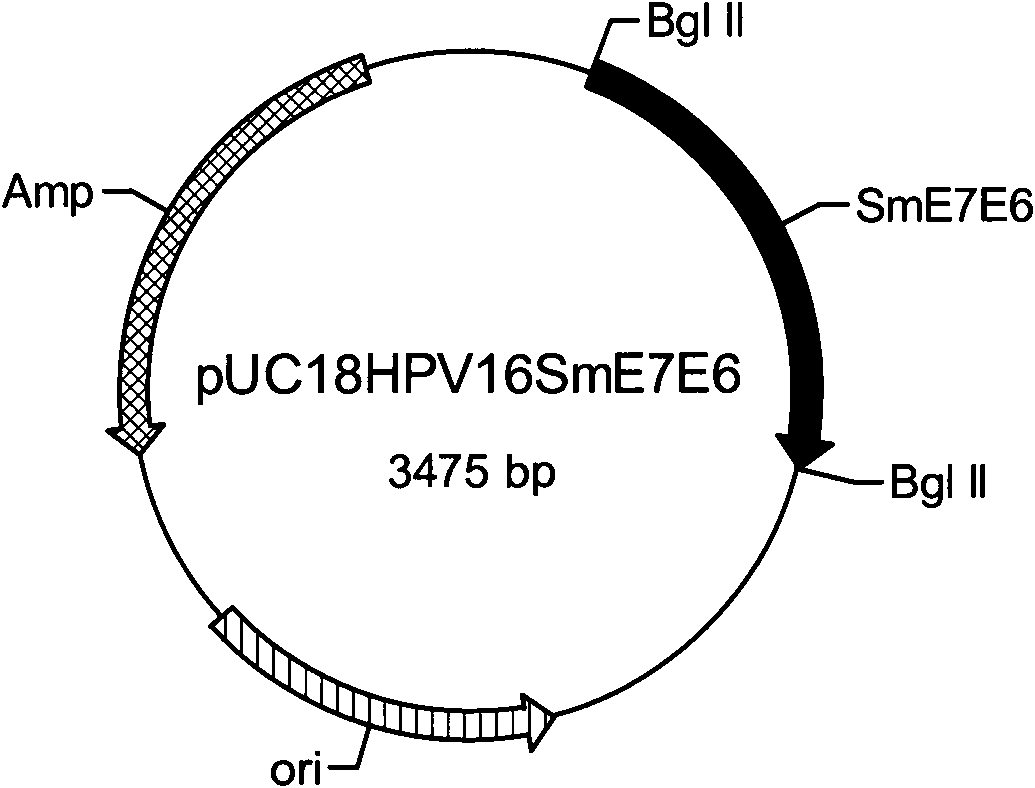
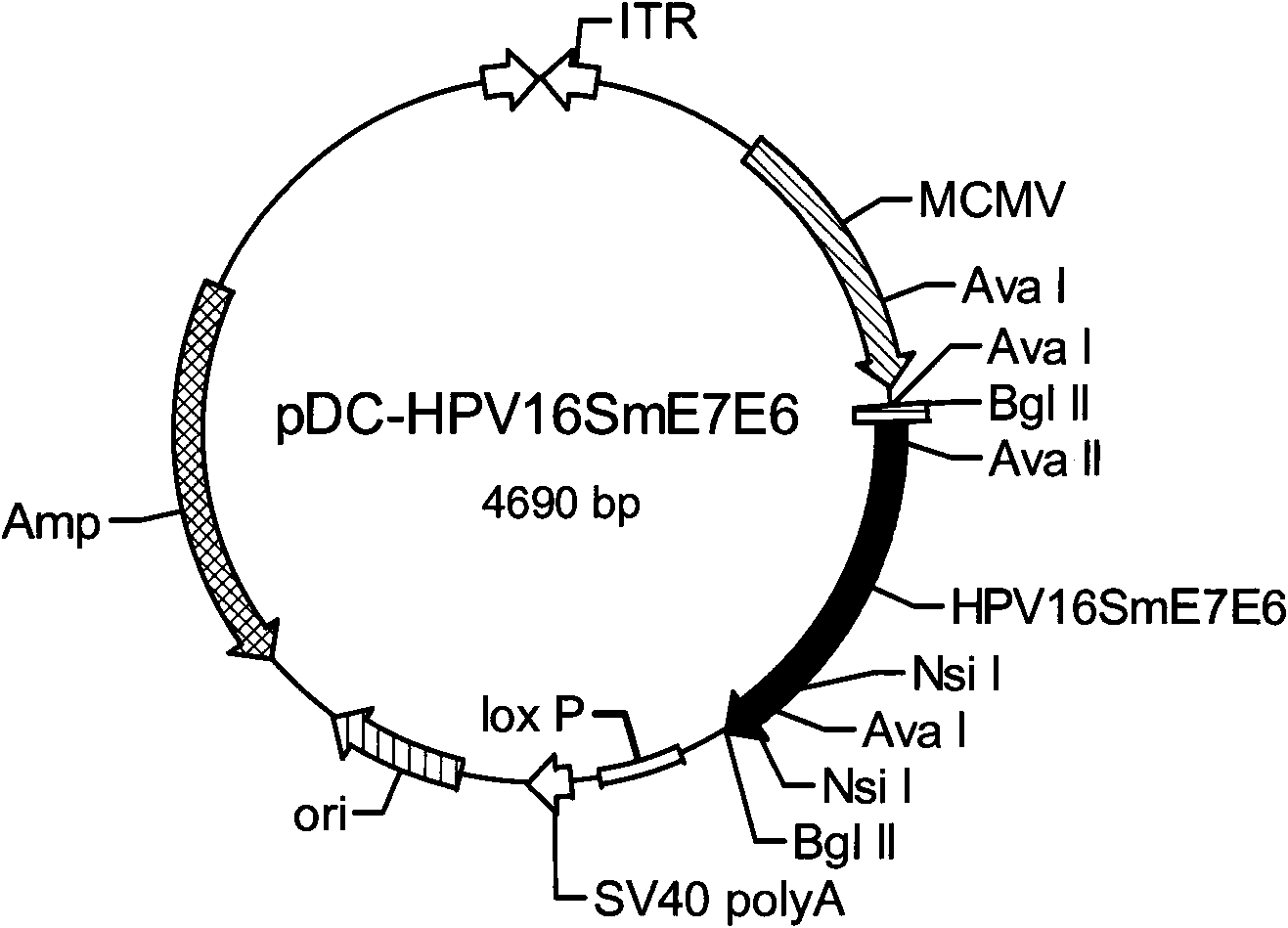
Gene, expression vector, expression method, expression cell and application of human papilloma virus (HPV) 16 E7E6 fusion protein
ActiveCN102002105AStrong immunogenicityPreserve antigenicityViral antigen ingredientsGenetic material ingredientsT cell immunityFhit gene
The invention provides a gene, an expression vector, an expression method, an expression cell and an application of a human papilloma virus (HPV) 16 E7E6 fusion protein. In the invention, recombinant adenovirus is adopted to fuse E6 and E7 protein genes which have no converting activity after modification, and the mammalian cell codon optimization and transformation is performed based on the amino acid sequences of the fusion protein to lead the codon to be expressed in the mammalian cells in a high level. In the invention, an E7E6 fusion protein mammalian cell codon optimization gene is designed successfully, the recombinant adenovirus capable of expressing the HPV16 E7E6 fusion proteins in a high level is successfully constructed, has strong immunogenic performance, can induce immunoreaction of specific T cells, and completely protects immune mice from attacking of anti-tumor cells. The invention can be used for searching and developing therapeutic vaccines of cervical cancer.
Owner:中国疾病预防控制中心病毒病预防控制所
Methods and devices to enhance sensitivity and evaluate sample adequacy and reagent reactivity in rapid lateral flow immunoassays
ActiveUS20110143365A1Reduce chanceBioreactor/fermenter combinationsBiological substance pretreatmentsIntravenous gammaglobulinLateral flow immunoassay
Methods and devices for rapid lateral flow immunoassays to detect specific antibodies within a liquid sample while also validating the adequacy of the liquid sample for the presence of immunoglobulin and the integrity and immunoreactivity of the test reagents that detect the antibodies of interest, without requiring instrumentation. The methods and devices provide for delivery of a diluted liquid sample to a single location that simultaneously directs the liquid flow along two or more separate flow paths, one that serves as a positive control to confirm that all critical reagents of the test are immunoreactive, and that the sample being tested is adequate, and the other to detect specific antibodies if present.
Owner:BUCHANAN
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Method for obtaining tumor specific T cell receptor
InactiveCN109485721AHigh killing efficiencyImmunoglobulin superfamilyGenetically modified cellsT-Cell Receptor GeneWilms' tumor
The invention discloses a method for obtaining a tumor specific T cell receptor. The method comprises the following steps: firstly, obtaining a T cell receptor gene sequence in an antigen peptide specific T cell which comes from an immunoreactive positive and / or tumor symptom relieved tumor patient after antigen peptide treatment; then, preparing an antigen specific T cell receptor according to the antigen specific T cell receptor gene sequence; introducing the antigen peptide specific T cell receptor gene into the T cell, and expressing the antigen peptide specific T cell receptor gene to obtain the specific TCR-T cell. The specific TCR-T cell can be used for successfully killing gene mutation corresponding to antigen peptide and HLA typed tumor cell, and has high killing efficiency and agood application prospect.
Owner:杜学明
Clenbuterol hydrochloride magnetic particle separation enzyme-linked immunosorbent assay method
InactiveCN101907627ASimple processing methodHigh detection sensitivityColor/spectral properties measurementsElisa methodFluorescein isothiocyanate
The invention provides a clenbuterol (CLB) magnetic particle separation enzyme-linked immunosorbent assay (ELISA) method, belonging to the technical field of food safety immunodetection. The method comprises the following steps: the immunodetection principle of the competition method is adopted to connect CLB with bio-enzyme and prepare an enzyme labeled antigen reagent, antibody against fluorescein isothiocyanate (FITC) is absorbed on the surface of magnetic particles to prepare a magnetic separation reagent, FITC is connected with the antibody against CLB to prepare an antibody reagent; CLB in a sample and enzyme-labeled CLB compete to combine with a small quantity of FITC-labeled antibody against CLB and form an antigen-antibody composite; after the magnetic separation reagent is added, the antibody against FITC connected with the surface of magnetic particles captures the composite on the surface of magnetic particles; and washing, and finally adding substrate to detect. The invention has the following advantages: (1) magnetic particles replace the traditional ELISA plate to be used as solid carrier and ensure that the immunoreaction is performed more fully and fast under the near-liquidus condition; compared with the traditional ELISA method, the method is characterized by high specificity and good repeatability; and (2) the one-step competition method principle is adopted, thus the detection time is short.
Owner:北京倍爱康生物技术有限公司
Electrochemical immunoassay method based on Au-PB-SiO2 composite nano-particles
The invention discloses an electrochemical immunoassay method based on Au-PB-SiO2 composite nano-particles. The detection method adopts a double antibody sandwich method, allows neuron-specific enolase antibodies solid-supported on an electrode surface to perform an immunoreaction with neuron-specific enolase in a sample solution, and to combine with a co-coupled substance of Au-PB-SiO2 composite nano-particles and neuron-specific enolase antibodies. Based on the electrochemical activity of Au-PB-SiO2 composite nano-particles, the detected peak current value of a cyclic voltammetry reduction peak is positively correlated with the concentration of the neuron-specific enolase, and thus the concentration of the neuron-specific enolase in the sample to be detected can be detected. The electrochemical immunoassay method provided by the invention has a corresponding linear range of 0.25-500 ng / ml, and a lower detection limit of 0.08 ng / ml, has good specificity and high sensitivity, and has important significance for the diagnosis of small cell lung cancer (SCLC).
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Enzyme linked immunosorbent assay kit for detecting progesterone and detection method thereof
InactiveCN104655846ASensitive detectionAccurate detectionColor/spectral properties measurementsElisa kitAntiendomysial antibodies
The invention provides an enzyme linked immunosorbent assay (ELISA) kit for detecting progesterone and a detection method thereof. The kit is sensitive, accurate, quick in detection, is simple in operation, is strong in specificity and is suitable for detection of samples in large amount. The kit includes: an ELISA plate coated by a progesterone antigen, a progesterone standard sample, a progesterone antibody operating fluid, a progesterone enzyme label second antibody operating fluid, a substrate liquid A, a substrate liquid B, a stop buffer liquid, a concentrated diluent and a concentrated washing liquid. The principle of the ELISA kit for detecting progesterone is solid-phase indirect competitive ELISA. In the detection method, an extracted sample, the progesterone enzyme label second antibody operating fluid and the progesterone antibody operating fluid are added to corresponding microholes in the ELISA plate. After incubation for a certain time, the ELISA plate is washed and is added with the substrate liquid A and the substrate liquid B, and then a color developing agent develops a blue color under effect of enzymes. After the stop buffer liquid added, the color is turned into yellow from blue. An inversely proportional relationship is formed between the depth of the developed color and the content of the progesterone in the standard sample or the sample. The method can be directly used for detecting residual amount of the progesterone in chicken tissue.
Owner:ZHENJIANG XIANCHUANG BIOTECH CO LTD
Humanized avian influenza virus H7N9 resisting neutralizing antibody M5 as well as preparation method and application thereof
ActiveCN104031146AImmunoglobulins against virusesAntiviralsAcute respiratory tract infectionNeutralizing antibody
The invention provides a humanized avian influenza virus H7N9 resisting neutralizing antibody M5 which is obtained through screening by using a phage surface display technology. Amino acid sequences of light-chain and heavy-chain variable regions of the antibody are respectively represented by SEQ ID No. 1 and SEQ ID No. 2. The antibody can specifically identify a particulate antigen of a virus H7N9, can be subjected to remarkable enzyme-linked immunoreaction with the virus H7N9 and has the neutralizing active function of resisting virus H7N9 infection; in addition, the antibody provided by the invention can be prepared into specific antibody drugs for preventing and treating the avian influenza virus H7N9 and sequentially is clinically used for preventing and treating acute respiratory tract infections caused by the virus H7N9.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Preparation method of label-free photoelectrochemical sensor for zearalenone
InactiveCN107748190AFast transmissionHigh sensitivityMaterial electrochemical variablesAntibodyPlasma effect
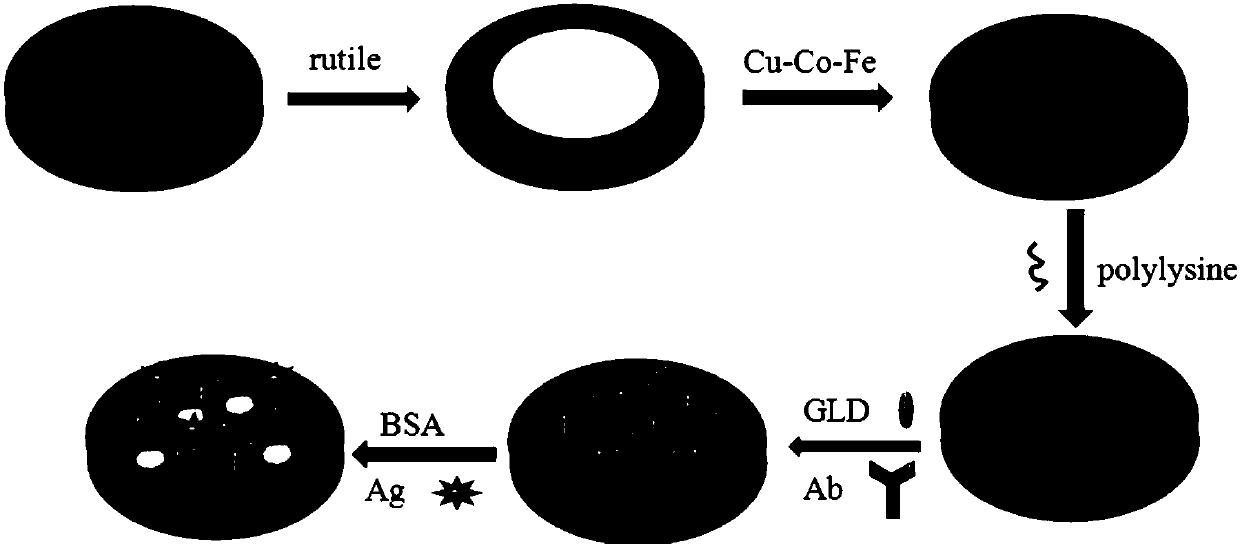
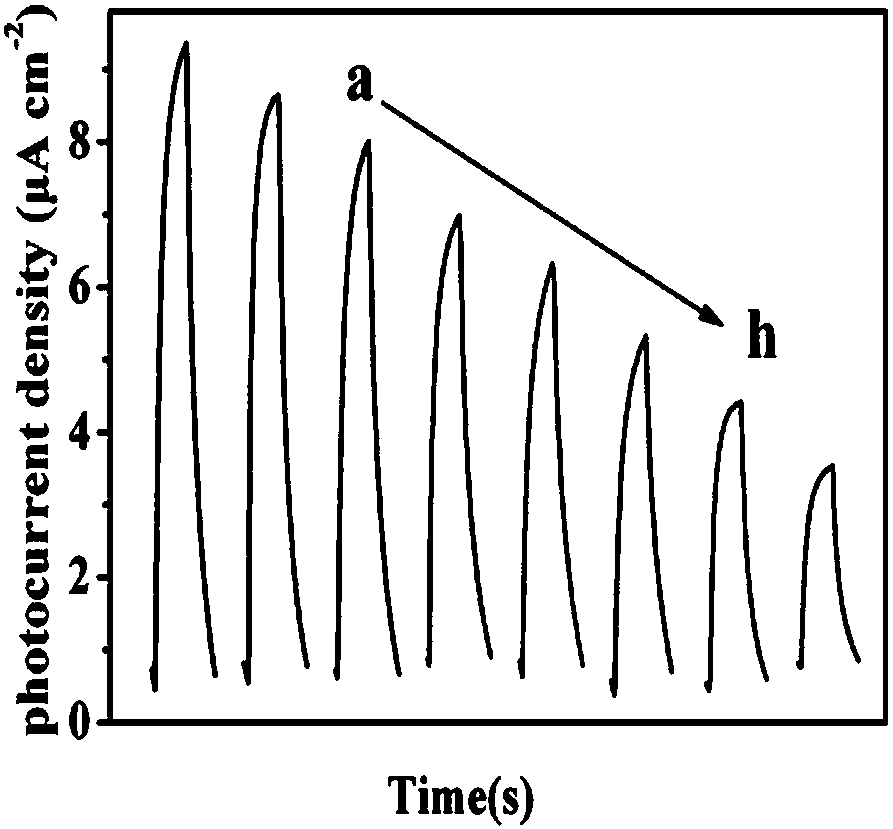
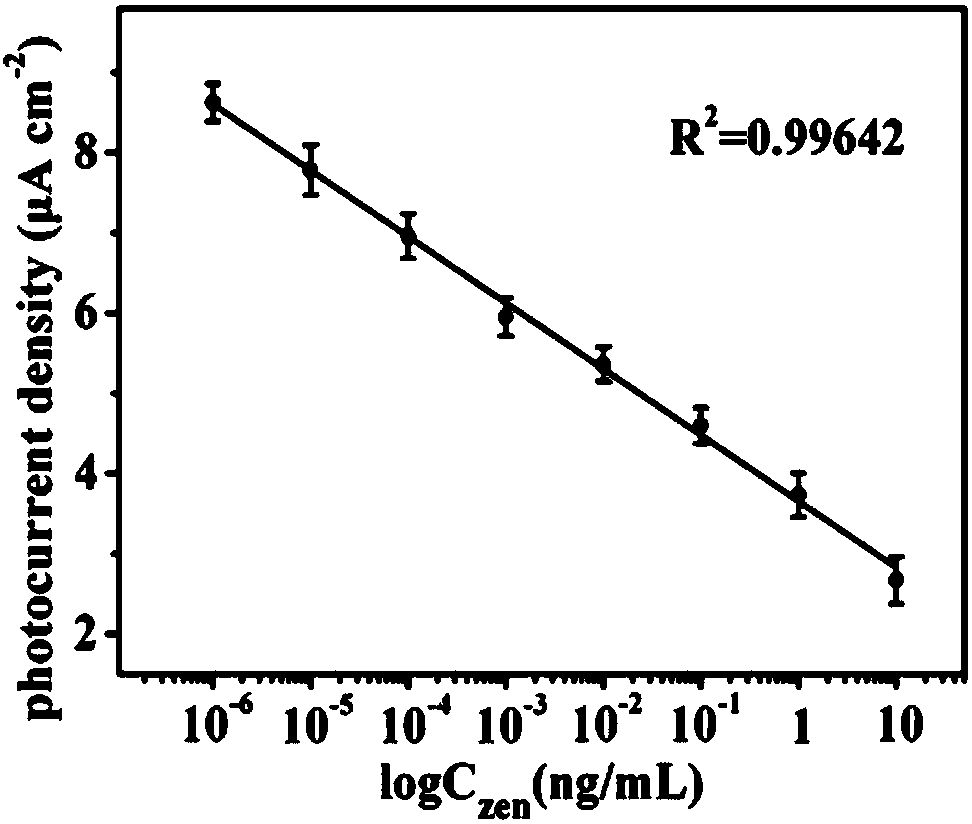
The invention discloses a preparation method of a label-free photoelectrochemical sensor for zearalenone. According to a construction method of a sensing interface, a nano copper-cobalt-iron material,polylysine and nano-rutile type TiO2 mesoscopic crystals are taken as construction elements, and a zearalenone antibody (Ab) is further immobilized; due to the surface plasma effect of the nano copper-cobalt-iron material and the excellent conductivity of the polylysine, the sensing interface can accelerate the photo-induced electron transfer rate of the nano-rutile type TiO2 mesoscopic crystalsand improve photocurrent signals of the nano-rutile type TiO2 mesoscopic crystals; furthermore, the polylysine is rich in amino, thus being conducive to the load of the antibody; when the zearalenoneand the immobilized zearalenone antibody generate an immunoreaction, the photoelectric signals at the sensing interface are significantly weakened due to a steric hindrance effect. A label-free photoelectric sensing method established based on the phenomenon can realize high sensitive detection of the zearalenone having a concentration within a range of 1*10<-6>-10ng / mL.
Owner:FUJIAN NORMAL UNIV
Immunoprotective primary mesenchymal stem cells and methods
ActiveUS20140271580A1Prevent toxicityPrevent infectionAntibacterial agentsBiocidePathogenSingle-Chain Antibodies
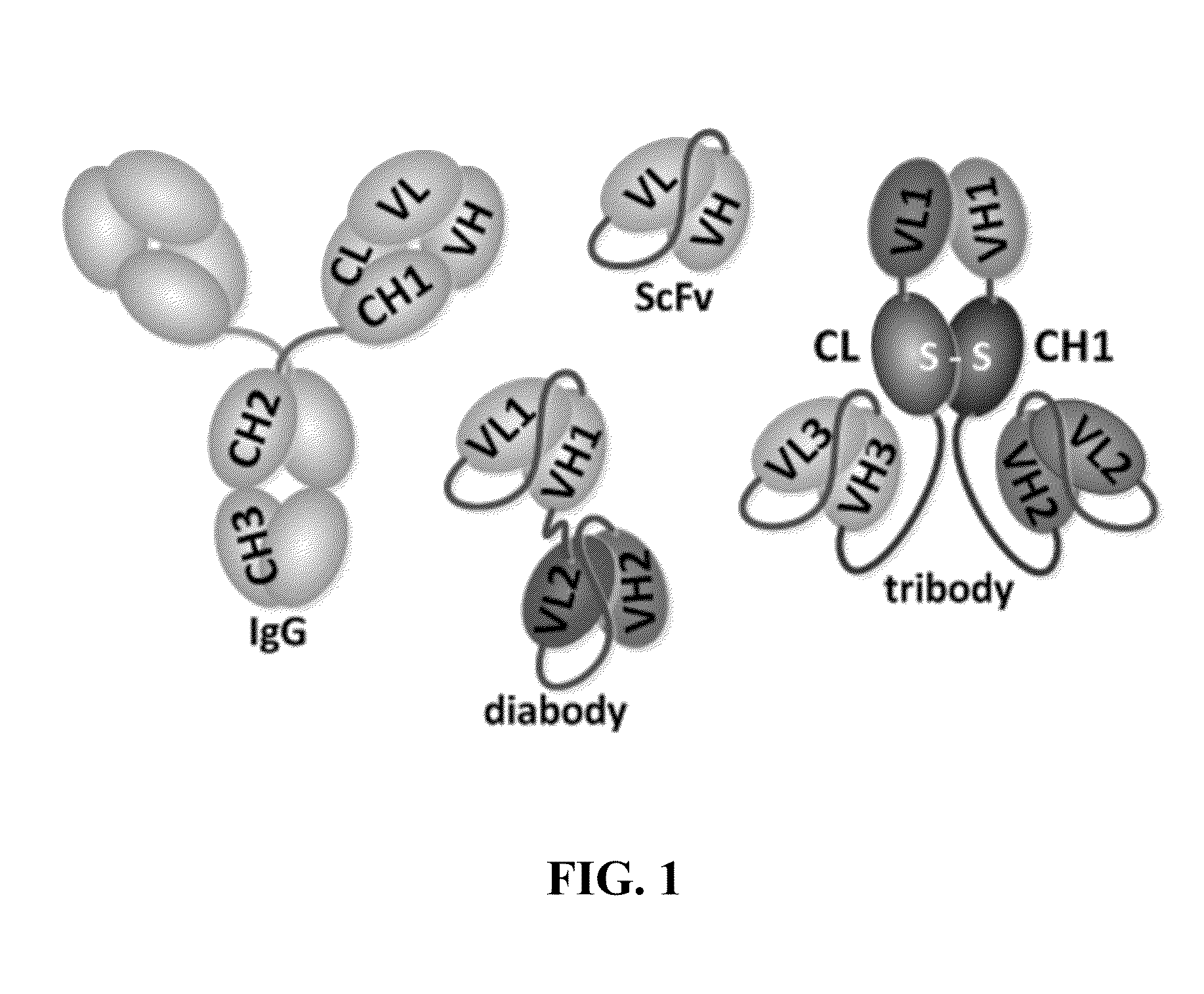
Immunoprotective primary mesenchymal stems cells (IP-MSC) which episomally express multiple immunoreactive polypeptides that specifically target a pathogen (e.g., an infectious species of virus, bacterium, or parasite) or toxin are described herein. The IP-MSC express two or more (e.g., 2 to about 100) immunoreactive polypeptides (e.g., full antibodies, single-chain antibodies (ScFV), Fab or F(ab)2 antibody fragments, diabodies, tribodies, and the like), and optionally one or more other immunomodulating polypeptides, e.g., a cytokine such as an interleukin (e.g., IL-2, IL-4, IL-6, IL-7, IL-9, and IL-12), an interferon (e.g., IFNα, IFNβ, or IFNω), and the like, which can enhance the effectiveness of the immunoreactive polypeptides.
Owner:TULANE EDUCATIONAL FUND +1
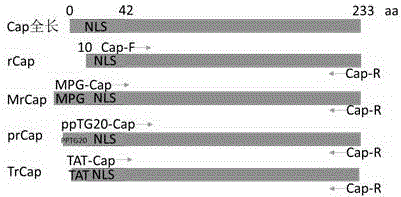
Gene encoding recombinant porcine circovirus type 2 Cap protein and application thereof
The present invention provides a gene encoding a recombinant porcine circovirus type 2 Cap protein and application thereof. The present invention aims to obtain a soluble recombinant PCV2 Cap protein, and adopts a PCR method for fusion of MPG and NLS partially deleted Cap protein N-terminal gene to construct a new gene; and then the new gene is cloned into an E. coli expression vector pET28a to obtain a recombinant plasmid; the recombinant plasmid is transformed into E. coli BL21 to obtain recombinant engineering bacteria; the recombinant engineering bacteria is subjected to induced expression by IPTG to obtain the soluble recombinant PCV2 Cap protein. SDS-PAGE and Western-blot identification shows that most of the recombinant protein MrCap is present in the bacterial lysis supernatant and is soluble; and the recombinant protein MrCap has high immunogenicity and immunoreactivity, and lays foundation for the development of a PCV2 antibody detection kit and subunit vaccines.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Preparation of ELISA kit for detecting deoxynivalenol
The invention relates to preparation of an ELISA kit for detecting deoxynivalenol. The kit with sensitive detection, accuracy, fastness, simple operation and strong specificity is suitable for determination of a large number of samples. The kit includes an ELISA plate coated with deoxynivalenol antigens, a deoxynivalenol standard sample, a deoxynivalenol antibody working solution, an enzyme-labeled?second antibody working solution, a substrate solution A, a substrate solution B, a stop solution, a concentrating sample weak solution and a washing concentrate. The principle of the deoxynivalenol detection kit is solid phase indirect competitive enzyme-linked immunosorbent assay. An extracted sample, the enzyme-labeled?standard second antibody working solution and the antibody working solution are added to corresponding enzyme-labeled holes; after incubation for a period of time, the substrate solution A and the substrate solution B are added into a washing plate; under the action of the enzyme, blue color occurs in the holes; and the stop solution adding is added in, and the color changes from blue to yellow. The degree of color is in inverse proportion relationship with the content of deoxynivalenol in the standard substance or sample. The method can be directly used for detecting deoxynivalenol in cereals and cereal products.
Owner:JIANGSU WISE SCI & TECH DEV
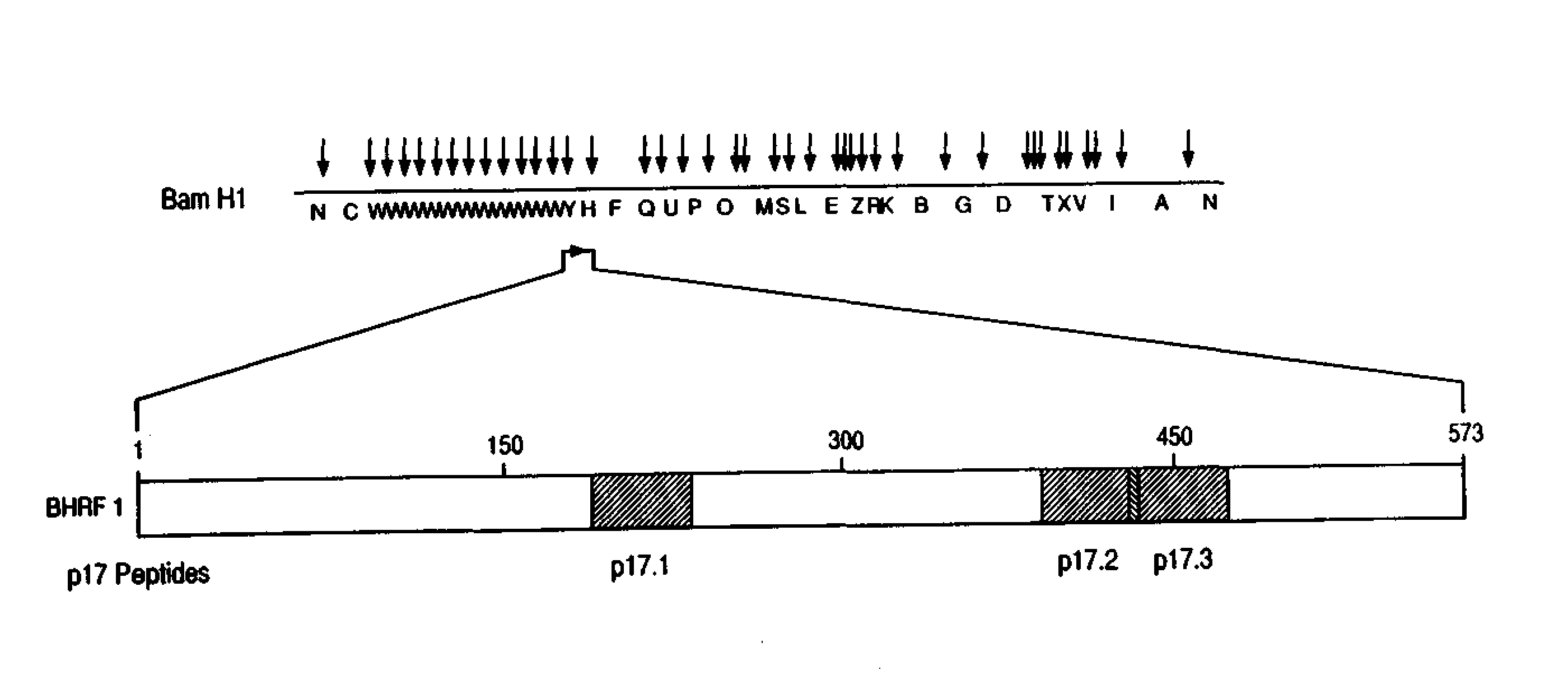
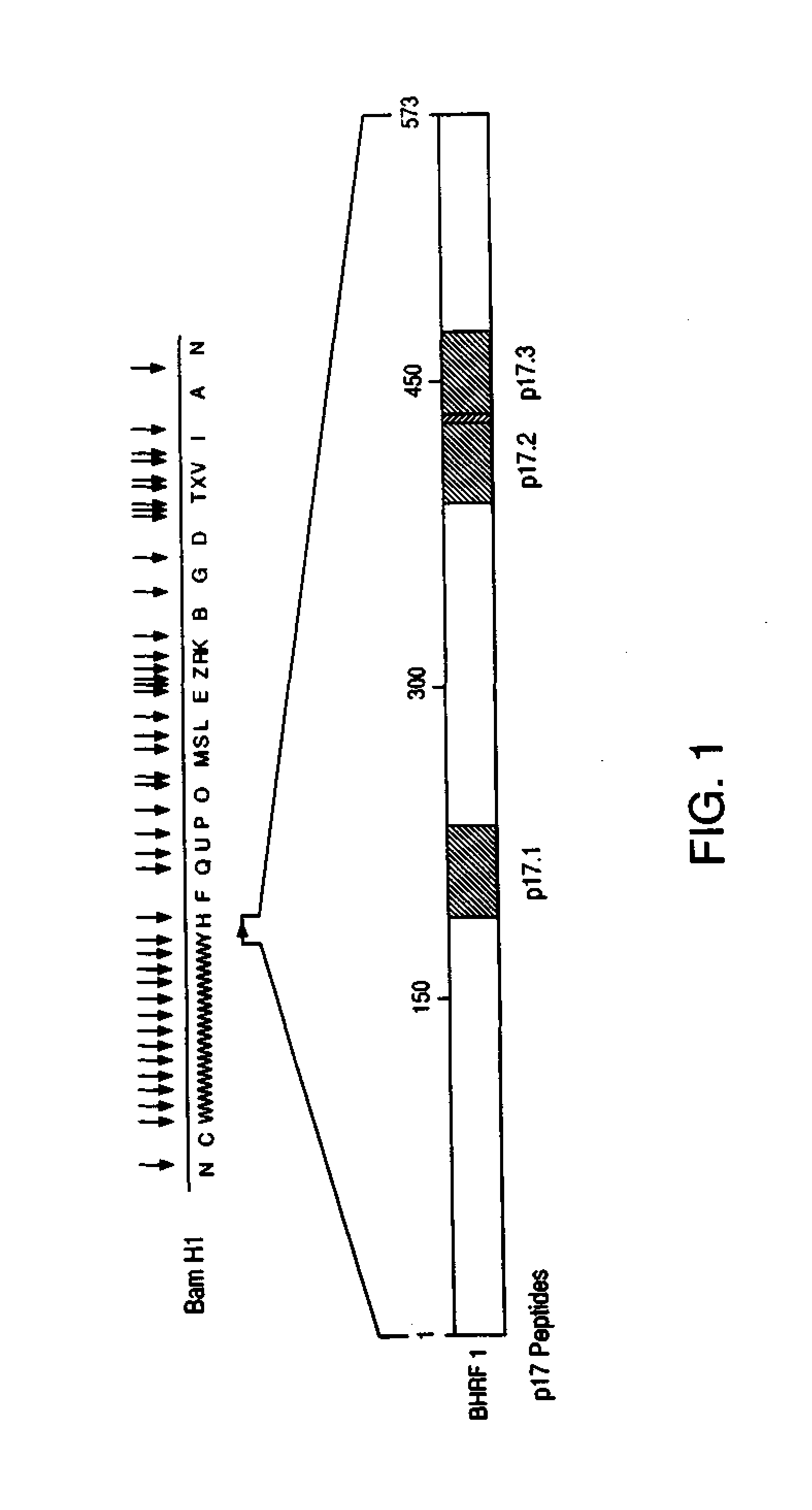
Immunoreactive peptides from epstein-barr virus
Owner:ORTHO-CLINICAL DIAGNOSTICS
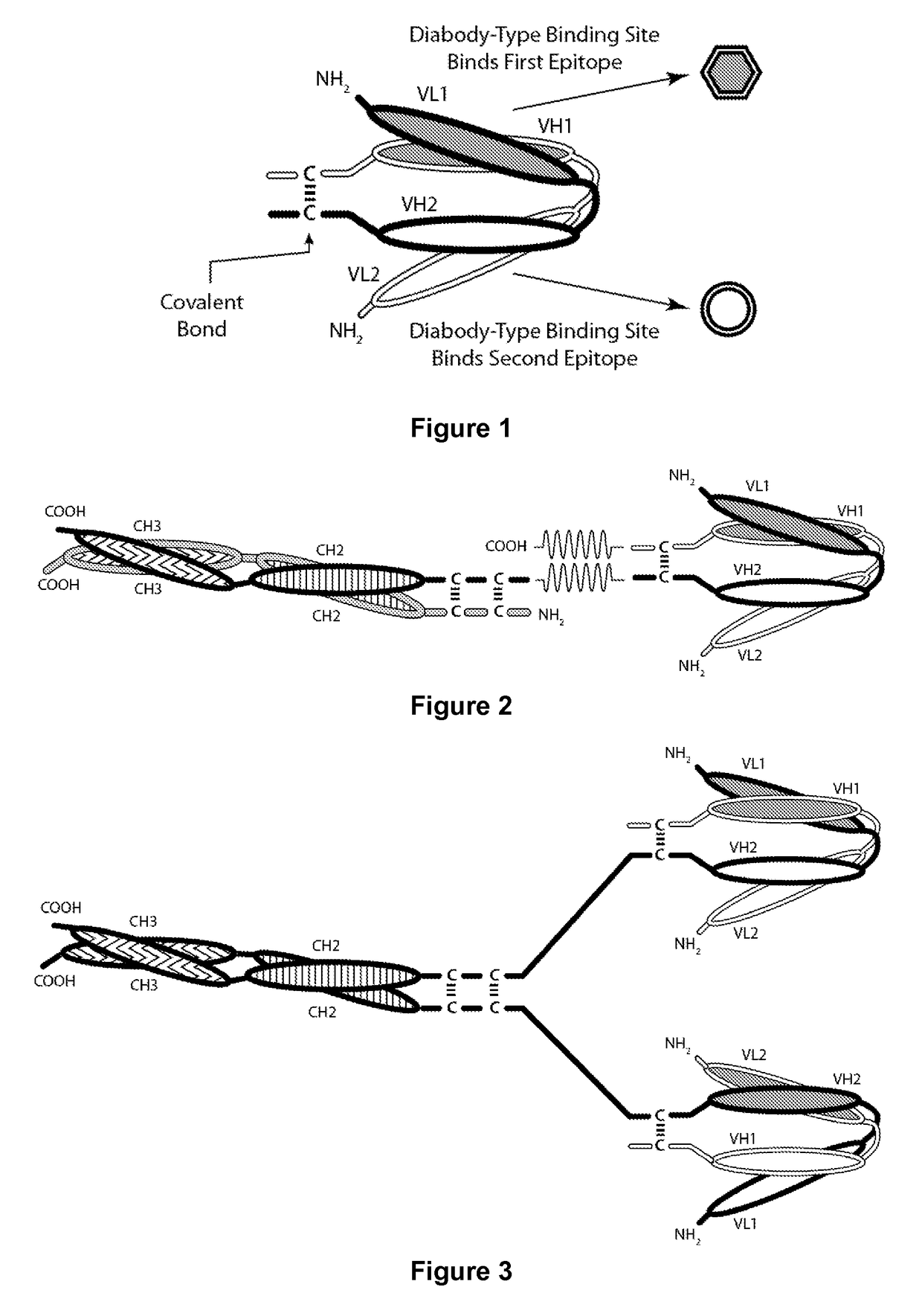
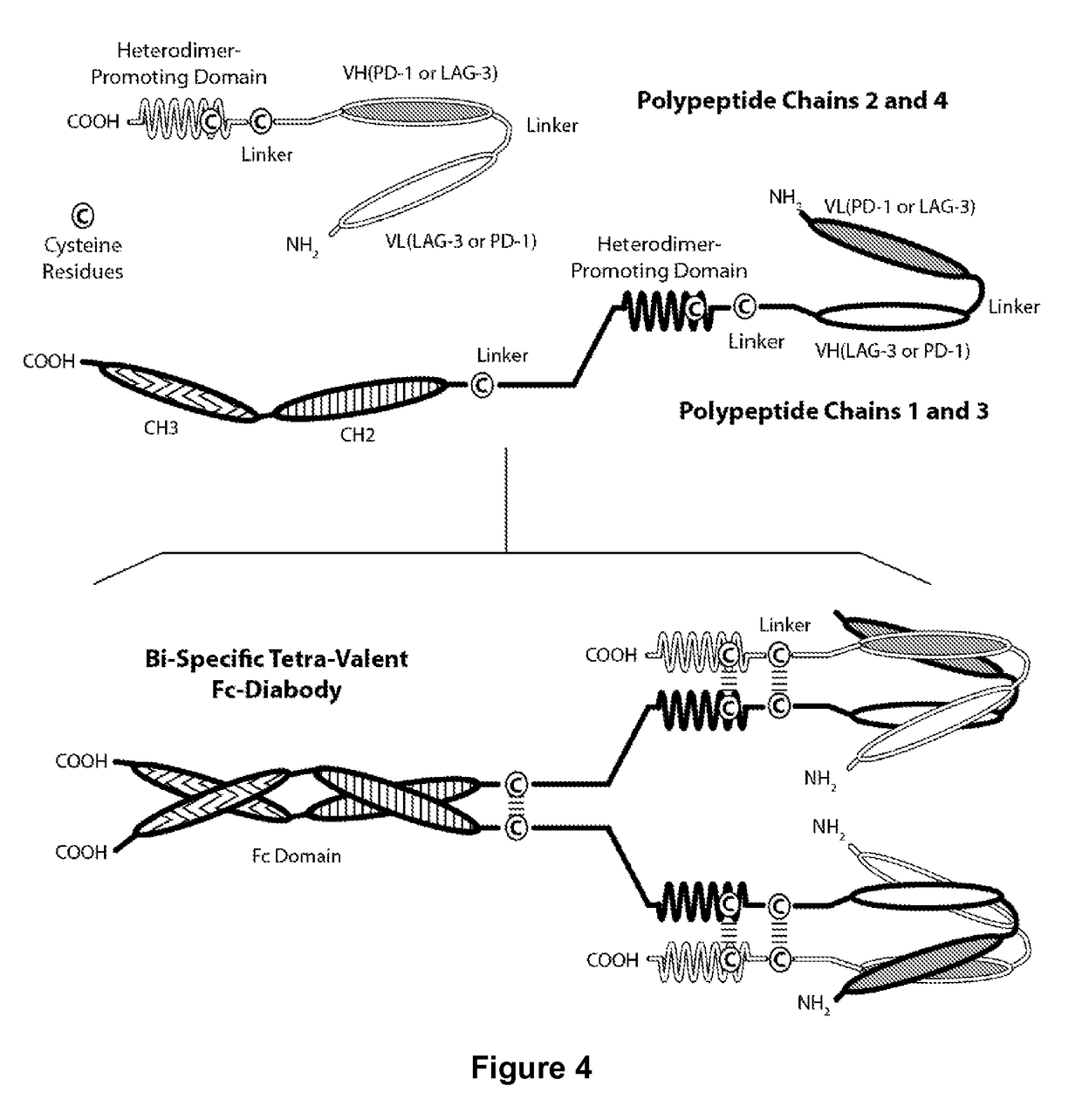
Covalently bonded diabodies having immunoreactivity with PD-1 and LAG-3, and methods of use thereof
The present invention is directed to bi-specific diabodies that comprise two or more polypeptide chains and which possess at least one Epitope-Binding Site that is immunospecific for an epitope of PD-1 and at least one Epitope-Binding Site that is immunospecific for an epitope of LAG-3 (i.e., a “PD-I×LAG-3 bi-specific diabody”). More preferably, the present invention is directed to bi-specific diabodies that comprise four polypeptide chains and which possess two Epitope-Binding Sites that are immunospecific for one (or two) epitope(s) of PD-1 and two Epitope-Binding Site that are immunospecific for one (or two) epitope(s) of LAG-3 (i.e., a “PD-1×LAG-3 bi-specific, tetra-valent diabody”).
Owner:MACROGENICS INC
Portable electrochemical quantitative immunochromatography filtering paper as well as test paper and application thereof
The invention discloses portable electrochemical quantitative immunochromatography filtering paper as well as test paper and application of the electrochemical quantitative immunochromatography filtering paper. The immunochromatography filtering paper is formed by arranging a hydrophilic channel on hydrophilic printing filtering paper; a sample feeding region is arranged at one end of the channel and a water absorption region is arranged at the other end of the channel; an electrochemical detection region is arranged between the sample feeding region and the water absorption region; water absorption paper is arranged in the water absorption region; the electrochemical detection region is coated with an antigen or antibody for detecting a target substance; the portable electrochemical quantitative immunochromatography filtering paper is manufactured by immunochromatography filtering paper, a conductive film and a bottom plate; the test paper can be used for quantitatively analyzing immunoreaction and eliminating the non-specific interferences, and has the advantages of accurate result, accuracy in measurement and the like.
Owner:SOUTHWEST UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com