Tissue decellularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example ii
Experimental details
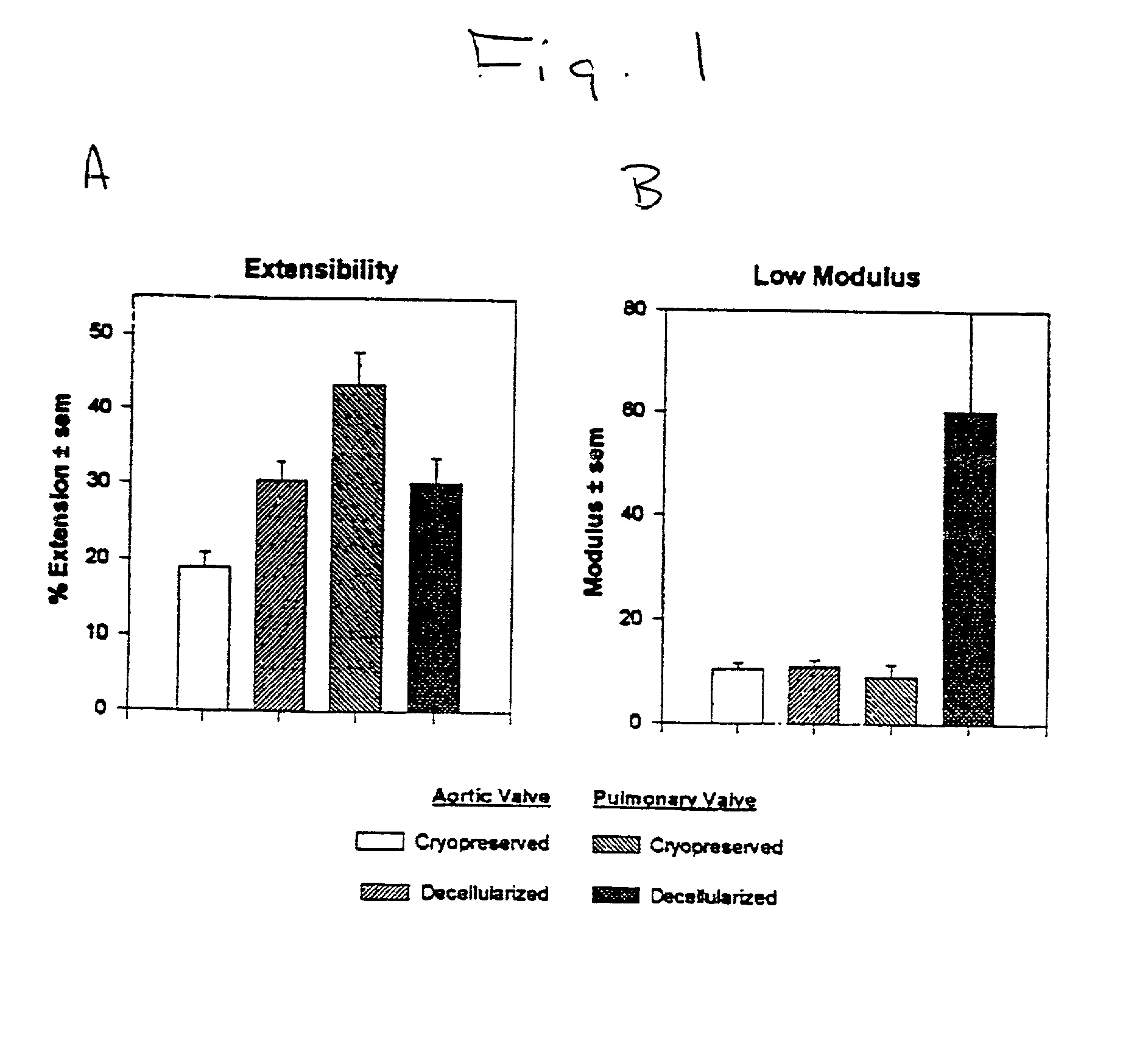
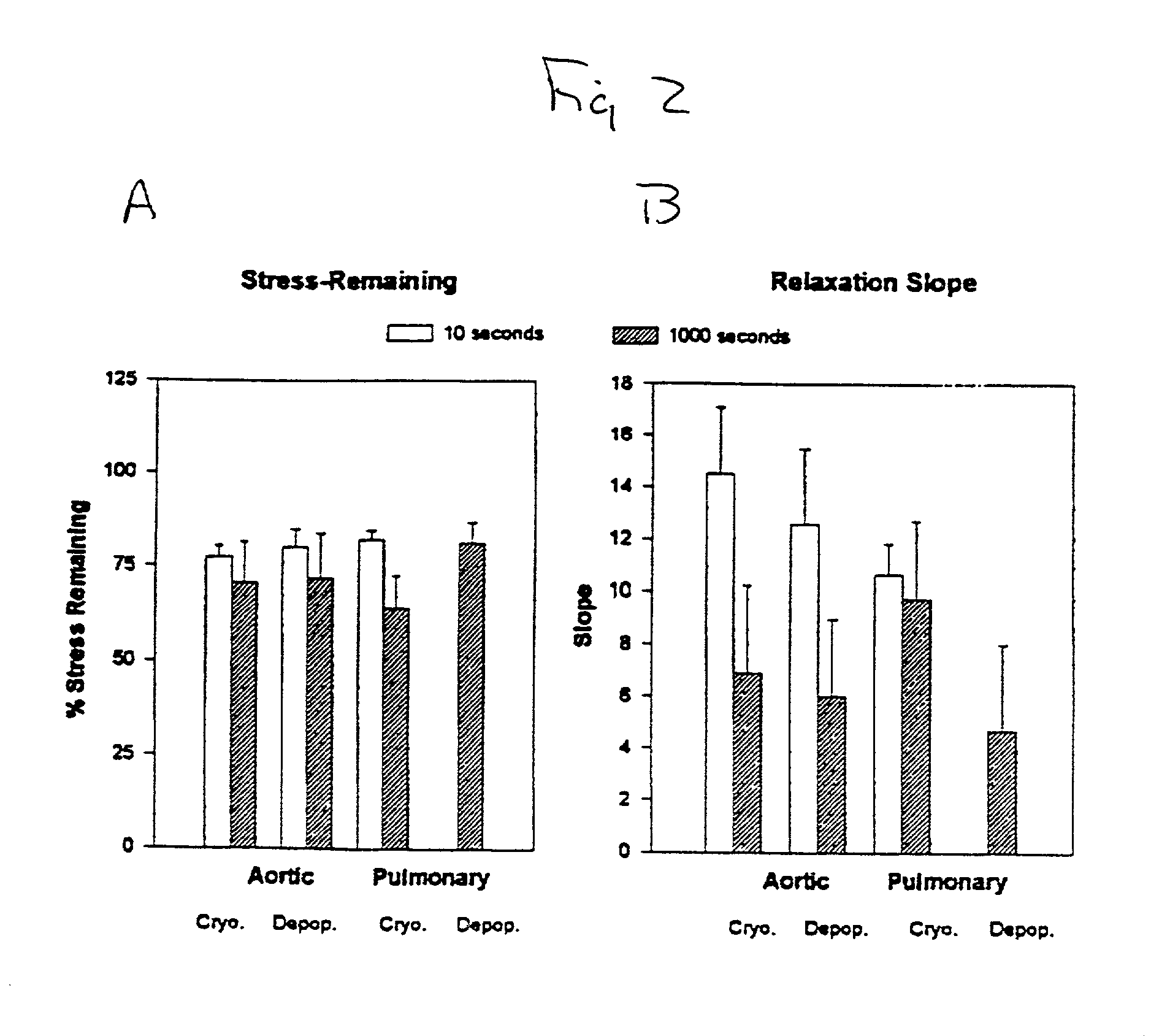
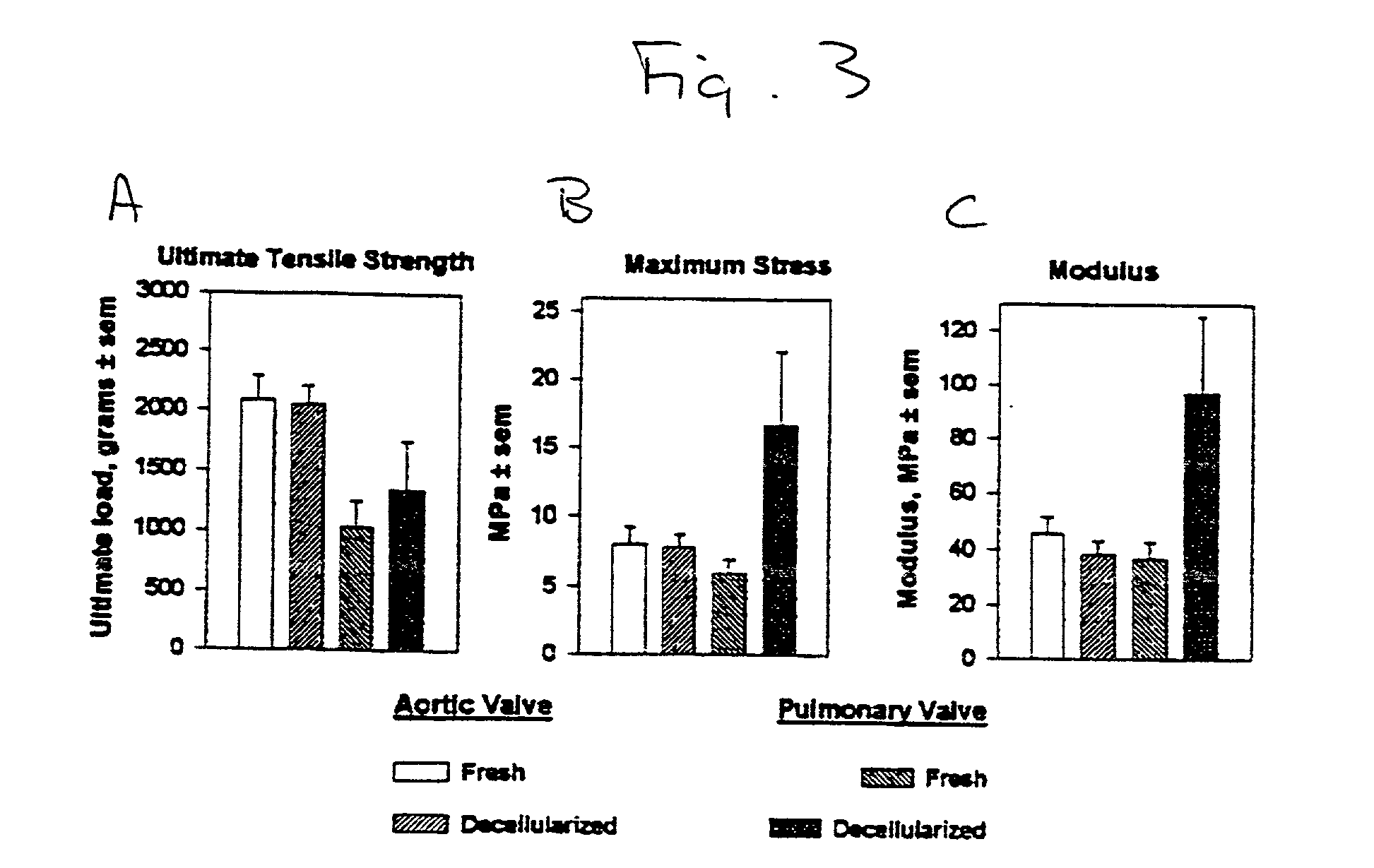
39. Porcine heart valves. Porcine hearts were obtained from market weight pigs (>120 kg). After rinsing in sterile phosphate buffered saline, the hearts were field dissected (apex removed) and shipped at 4.degree.C. in sterile PBS. All hearts arrived within 24 hr of animal slaughter. Aortic and pulmonary valves were dissected as roots. These tissues were subjected to a bioburden reduction step of incubation in a mixture of antibiotics and antimycotics for 48 hr at 48.degree.C. The disinfected tissues were either cryopreserved (10% (v / v) DMSO and 10% (v / v) fetal bovine serum, -1.degree. C. / min) or were decellularized by a procedure involving treatment with hypotonic medium followed by digestion with a mixture of deoxyribonuclease I and ribonuclease A. After 12 days, the decellularized valves were either cryopreserved as above or chemically fixed in 0.35% (w / v) glutaraldehyde at 2 mmHg in phosphate buffered saline (pH 7.4) for a total of 7 days; the low pressure fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com