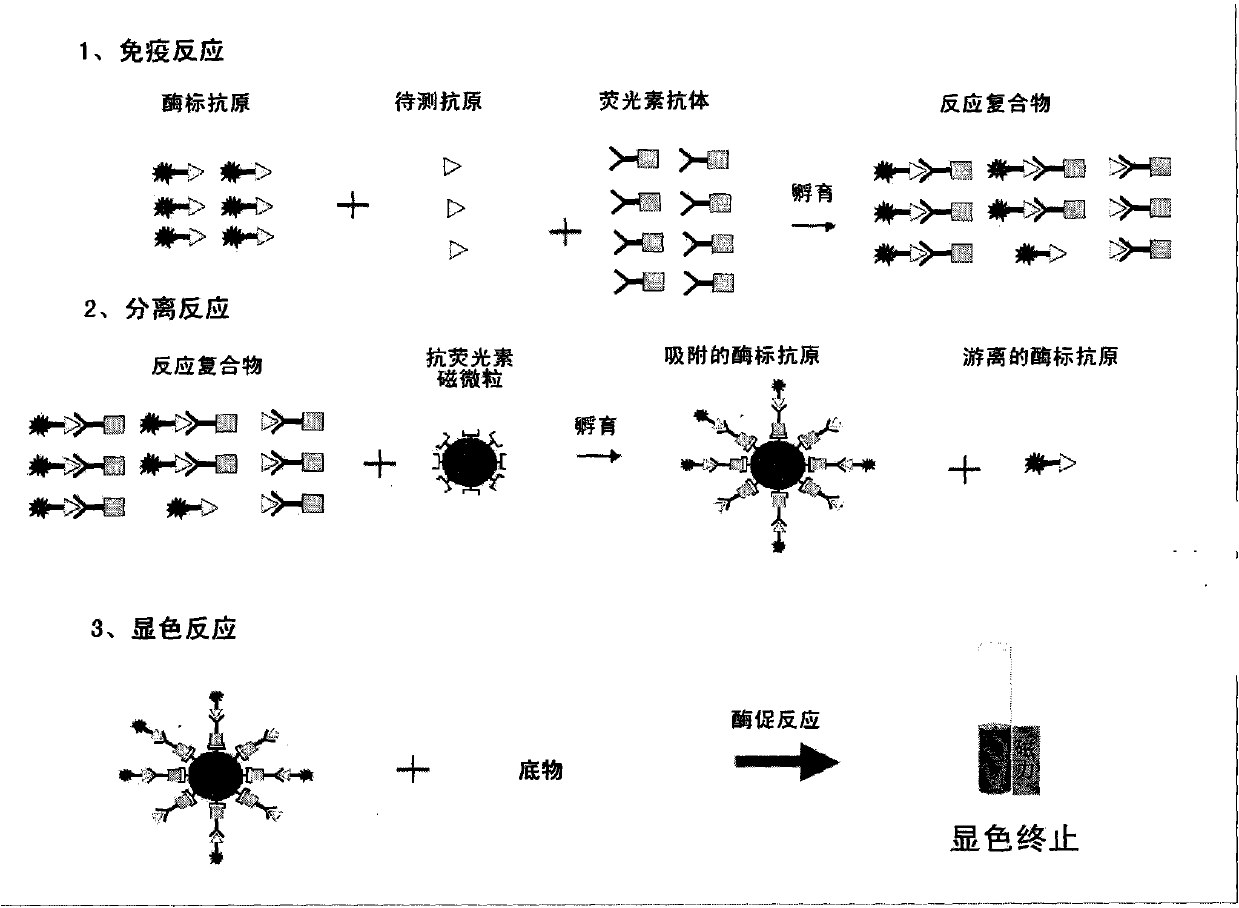
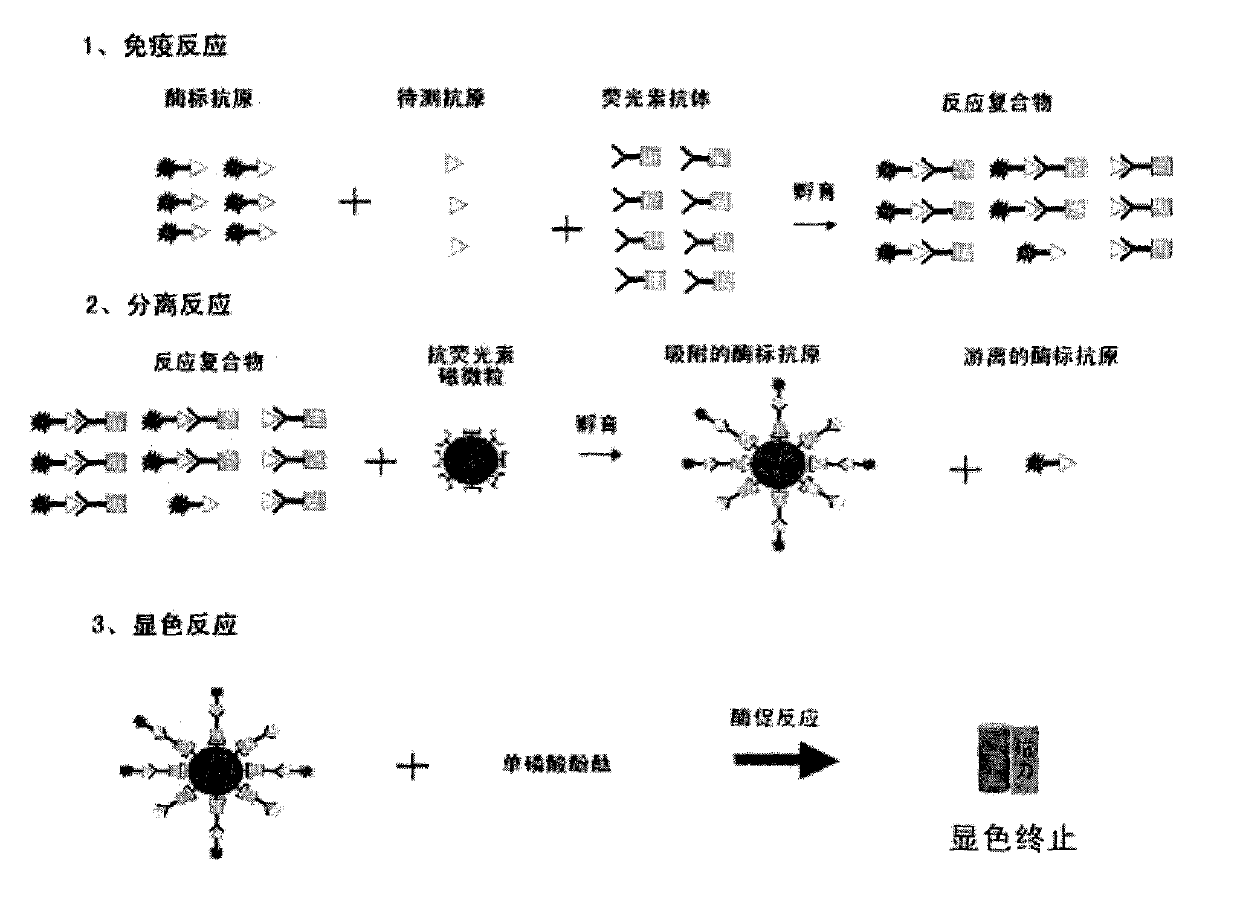
Clenbuterol hydrochloride magnetic particle separation enzyme-linked immunosorbent assay method
A technology of clenbuterol hydrochloride and magnetic particles, applied in the direction of color/spectral property measurement, measurement device, analysis material, etc., can solve the problems of weakened bronchodilation, increased concentration, endocrine disorder, etc., and achieves good repeatability, The effect of high sensitivity and simple processing method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Anti-FITC antigen coupled with surface amino (COOH-) magnetic particles to prepare magnetic separation reagent
[0028] Take 100 mg of magnetic particles containing carboxyl (COOH-) active groups on the surface and wash them three times with 0.1 M MES (2-[N-morpholino] ethanesulfonic acid), 10 ml of pH 4.5-5 solution. The magnetic particles were resuspended in 1ml of this solution, and 2mg of anti-FITC antibody was added, and mixed evenly. Add 100 μl of 10 mg / ml EDC solution, mix well and react at room temperature for 2 hours. After washing the magnetic beads 3 times with 10 ml of 0.01 M phosphate buffered saline (PBS) pH 7.4 containing 1% bovine serum albumin (BSA), the solution was used to prepare a 2.5 mg / ml magnetic separation reagent working solution.
Embodiment 2
[0029] Example 2: CLB-BSA linked alkaline phosphatase (ALP), preparation of enzyme-labeled antigen reagent
[0030] Take 5 mg of CLB-BSA antigen, concentrate to 5 mg / ml, add 10 μl of 13.76 mg / ml activator 2-Iminothiolane HCl (2IT) solution, place at room temperature for 30 minutes, add glycine to terminate the activation reaction, and place at room temperature for 5 minutes. Use Sephadex G25 column to desalt and collect protein elution peaks.
[0031] Take 5 mg of ALP solution, add 50 μl of 6.69 mg / ml Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) solution, leave it at room temperature for 15 minutes, add glycine to terminate the activation reaction, and leave it at room temperature for 5 minutes. Use Sephadex G25 column to desalt and collect protein elution peaks.
[0032] The activated CLB-BSA was mixed with the activated ALP, left at room temperature for 10 hours, then separated and purified using Supperdex200 gel chromatography column to remove unlinke...
Embodiment 3
[0035] Example 3: Anti-CLB antibody linked to FITC to prepare anti-reagent
[0036] Take 5mg of anti-CLB antibody solution and dialyze against 20mM pH9.0 carbonate buffer for more than 12h, and the concentration after dialysis is required to be greater than 1mg / ml. Add 500 μl of 0.5 mg / ml FITC solution (prepared with 20 mM pH9.0 carbonic acid buffer), mix well and react at room temperature for more than 12 hours. Use Sephadex G25 column to remove unbound FITC, and collect the protein elution peak.
[0037] The preparation method of the anti-reagent diluent is the same as that of the enzyme-labeled antigen reagent diluent. The above-mentioned CLB antibody FITC conjugate is diluted to 1-5 μg / ml with the diluent to prepare the anti-reagent working solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com