Methacrylic ester polymer, compounds thereof as well as preparation methods and application of all
A technology of methacrylate and compound, which is applied in the field of preparation method and its application as a gene carrier, can solve problems such as difficulty in determining possible toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example 1: Synthesis of ethyl xanthate
[0040] Add 100 mL of chloroform, 0.1 mol of bromoethane, and 0.11 mol of ethyl xanthate sodium into a 500 mL round-bottomed flask, stir at room temperature for 72 hours, separate by filtration under reduced pressure, wash with chloroform, and saturated sodium bicarbonate. Dry over magnesium sulfate and distill off the solvent to obtain a yellow oily liquid, which is separated by silica gel column chromatography, and the eluent is a mixed solvent of hexane and ethyl acetate (volume ratio 90:10) to obtain ethyl xanthate.
[0041] 1 H NMR (CDCl 3 ): 1.11, 3.57, 2.91, 1.31
[0042] 13 C NMR (CDCl 3 ): 13.5, 60.5, 72.0, 24.5, 15.5
[0043] synthetic route:
example 2
[0044] Example 2: Synthesis of xanthate-based poly-2-dimethylaminoethyl methacrylate (OPDMAEMA)
[0045] Add 1 g of 2-dimethylaminoethyl methacrylate (DMAEMA), 4 mg of azobisisobutyronitrile (AIBN), 80 mg of ethyl xanthate, and 2 mL of tetrahydrofuran into a 5 mL round bottom flask, [ethyl yellow Ethyl orthoate]: [AIBN]=20. Freeze with liquid nitrogen to remove oxygen, then seal under reduced pressure and remove oxygen, and react in a water bath at 70°C for 48 hours. After cooling to room temperature, excess hexane was added to precipitate and isolate the polymer-xanthate-based poly(2-dimethylaminoethyl)methacrylate (OPDMAEMA). for polymer 1 H NMR characterization, D 4 -Methanol and D-chloroform as solvents.
[0046] 1 H NMR (CDCl 3 ): 1.11, 3.57, 1.69, 1.33, 0.96, 2.27, 4.18, 2.64
[0047] 13 C NMR (CDCl 3 ): 13.5, 60.5, 172.0, 50.6, 39.7, 16.4, 14, 22.1, 41.2, 58.2, 66.1, 174.5
example 3
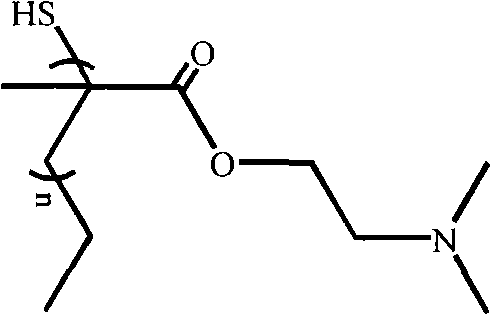
[0048] Example 3: Preparation of mercapto-terminated poly-2-dimethylaminoethyl methacrylate (MPDMAEMA) by OPDMAEMA
[0049] Dissolve OPDMAEMA in tetrahydrofuran solution, add a few drops of ammonium persulfate solution, fill with nitrogen for 30 minutes, add n-butylamine, and stir for 5 hours under nitrogen protection. The reaction mixture was added into a 10-fold excess of hexane, precipitated and filtered to obtain mercapto-terminated poly 2-dimethylaminoethyl methacrylate (MPDMAEMA).
[0050] 1 H NMR (CDCl 3 ): 1.5, 1.9, 1.33, 0.96, 1.69, 4.18, 2.64, 2.27
[0051] 13 C NMR (CDCl 3 ): 44.9, 42.4, 16.4, 13.6, 24.3, 174.5, 65.7, 58.2, 41.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com