Cation polymer capable of removing positive charges through oxidative response, and preparation method and application thereof
A cationic polymer and positive charge technology, which is applied in the direction of medical preparations, drug combinations, and pharmaceutical formulations of non-active ingredients, can solve the problems of reporting, no preparation methods, and applications, and achieve easy synthesis, low cytotoxicity, and structure simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of polymer (1)
[0061] Polymer (1) can be synthesized by two methods;
[0062] Synthesis method 1:
[0063] Monomer N,N-diethylaminoethyl acrylate (2-(N,N-diethylaminoethyl)acrylate, DEAEA) was purified by vacuum distillation before use, weighed 5g of DEAEA, AIBN (azobisisobutyronitrile) 0.05g was mixed with a molar ratio of 100:1, argon was bubbled for 30 minutes to remove oxygen, heated to 65°C under anaerobic conditions and stirred for bulk polymerization, reacted for 24 hours, and then separated and purified, and the polymer was dissolved in Dichloromethane was precipitated with ice n-hexane, repeated three times, and dried to obtain 4.5 g of polymer PDEAEA as light yellow viscous liquid with a yield of 90%.
[0064]
[0065] The PDEAEA obtained above is carried out molecular weight determination, and assay method is gel permeation chromatography, polyethylene glycol (PEG) standard substance and the PDEAEA prepared by embodiment are samples, dissolve...
Embodiment 2
[0092] Oxidative response of polymer (1)
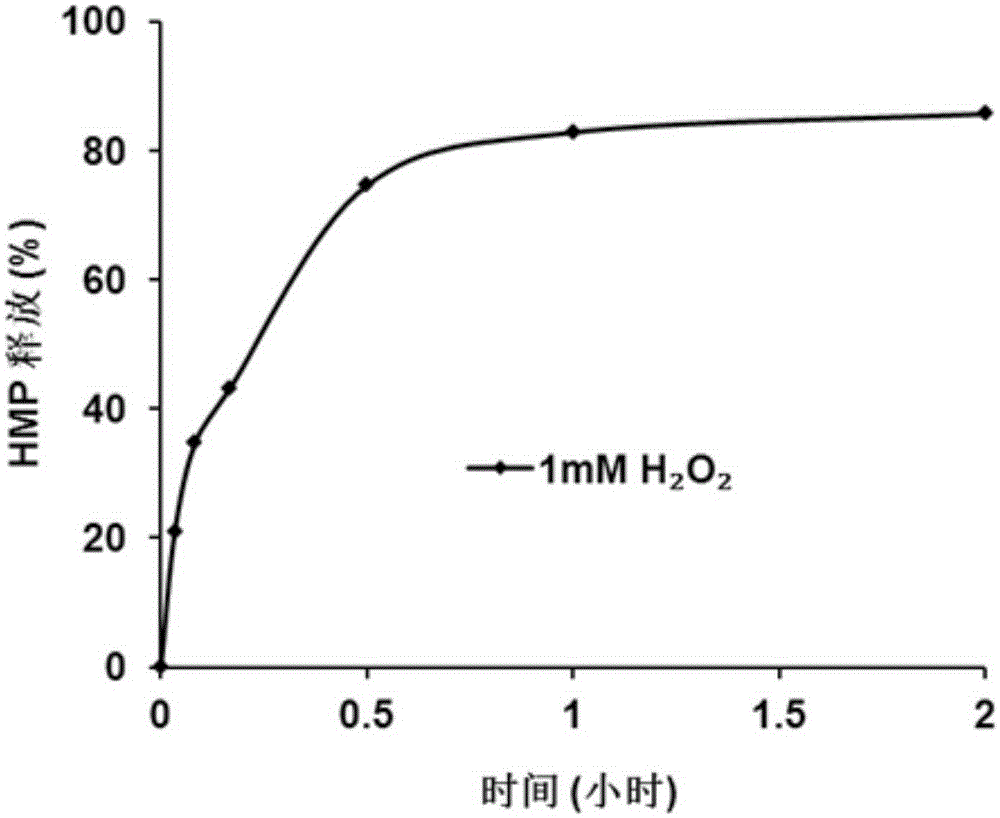
[0093] A certain amount of polymer (1) was weighed and dissolved in ultrapure water at a concentration of 0.3 mg / mL, and aqueous hydrogen peroxide solution was added, and the final concentration of hydrogen peroxide was 1 mM (mM stands for millimole per liter). The quinone generated by polymer (1) under oxidative conditions becomes p-hydroxybenzyl alcohol (HMP) very quickly in water, with 10% methanol aqueous solution as mobile phase, flow rate is 1mL / min, with high performance liquid chromatography (HPLC) When analyzing HMP, the peak time is 3.5 minutes, and the release curve of HMP can be obtained by monitoring the amount of HMP released at different times. This experiment proves that under the condition of 1mM hydrogen peroxide, the polymer polymer (1) is oxidized rapidly within 30 minutes, and is almost completely oxidized within 2 hours. For the test results, see figure 1 .
Embodiment 3
[0095] Charge reversal of polymer (1) under oxidative conditions
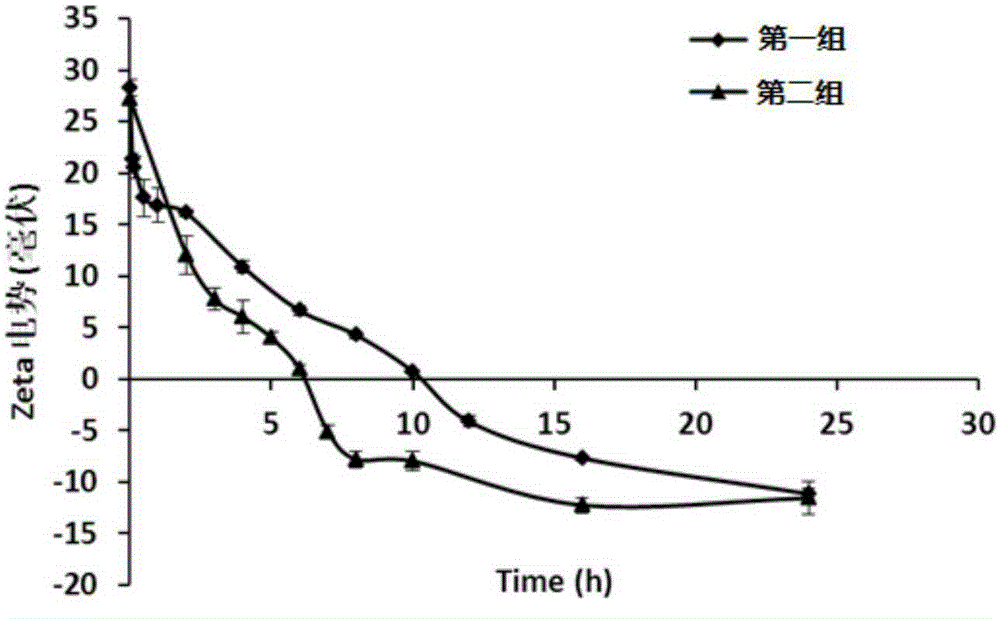
[0096] The first group: Weigh a certain amount of polymer Polymer (1) is dissolved in HEPES buffer solution (pH value is 7.4, 10mM), the concentration is 3mg / mL, and the final concentration of adding hydrogen peroxide solution is 80mM. Always maintain the pH value of the solution at 7.4, incubate at 37°C, and measure the Zeta potential of the solution at different times.
[0097] The second group: take a certain amount of polymer (1) and dissolve it in HEPES buffer solution (pH value is 7.4, 10mM), the concentration is 3mg / mL, add hydrogen peroxide aqueous solution and the final concentration is 80mM, the solution pH value is adjusted to 5.0, after incubating at 37°C for 2 hours, adjust the pH value of the solution to 7.4, maintain the pH value of the solution at 7.4, incubate at 37°C, and measure the Zeta potential of the solution at different times.
[0098] Test results such as figure 2 As shown, the expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com