Immunostimulatory composition and use thereof
A technology of immune stimulation and composition, which is applied in the field of biopharmaceuticals, can solve the problems of huge difference in effect, and achieve the effect of excellent effect, high antibody level, and enhanced immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 prepares immunostimulatory composition of the present invention and hepatitis B vaccine
[0073] 1. HBsAg stock solution: the amino acid sequence of HBsAg protein is shown in SEQ ID NO:1.
[0074] The HBsAg protein is prepared by HBsAg gene recombinant yeast cells, and the yeast cell types include Hansenula, Saccharomyces and Pichia, preferably Hansenula. For the specific preparation steps, refer to the Chinese patent application CN108330145A, the HBsAg gene recombinant Hansenula cells are fermented and cultured, and the cells are harvested. Purified by bacteria-breaking treatment, silica gel adsorption, column chromatography and TFF.
[0075] 2. HBcAg stock solution: the amino acid sequence of HBcAg protein is shown in SEQ ID NO:2.
[0076] The HBcAg protein is prepared by HBcAg gene recombinant yeast cells, and the yeast cell types include Hansenula, Saccharomyces cerevisiae and Pichia pastoris, preferably Hansenula. For the specific preparation step...
Embodiment 2
[0084] Example 2 Screening experiment of CPG oligodeoxynucleotides
[0085] 1. Experimental animals: C57BL / 6(N) mice, male, 4 weeks old, 135 mice, Shanghai Lingchang Experimental Animal Technology Co., Ltd.
[0086] 2. Experimental grouping: see Table 2, each injection volume is 100 μL / rat. Among them, group A is the negative control, and the PBS solution is 100 μL per mouse.
[0087] Table 2 Grouping of experimental animals
[0088]
[0089] 3. Experimental steps: Take the spleen on the 7th day after the mouse is immunized, and prepare spleen lymphocytes according to the conventional method, as follows: take the spleen aseptically: cut the spleen with sterile forceps and scissors, put it in a 70 μm cell strainer, Place in a plate containing 2ml of pre-cooled 2% FBS (purchased from GIBCO)-PBS; grind the spleen with a grinding rod, and the spleen cells enter the plate through a sieve to obtain a cell suspension, and put the suspension into the plate with a Pasteur pipett...
Embodiment 3
[0092] The screening experiment of embodiment 3 immunostimulatory composition
[0093] 1. Experimental animals: C57BL / 6(N) mice, male, 4 weeks old, 81, Shanghai Lingchang Experimental Animal Technology Co., Ltd.,
[0094] 2. Experimental grouping: see Table 3, each injection volume is 100 μL / rat. Among them, group A is the negative control, and the PBS solution is 100 μL per mouse.
[0095] Table 3 Grouping of experimental animals
[0096]
[0097]
[0098] 3. Experimental steps: the same as in Example 2.
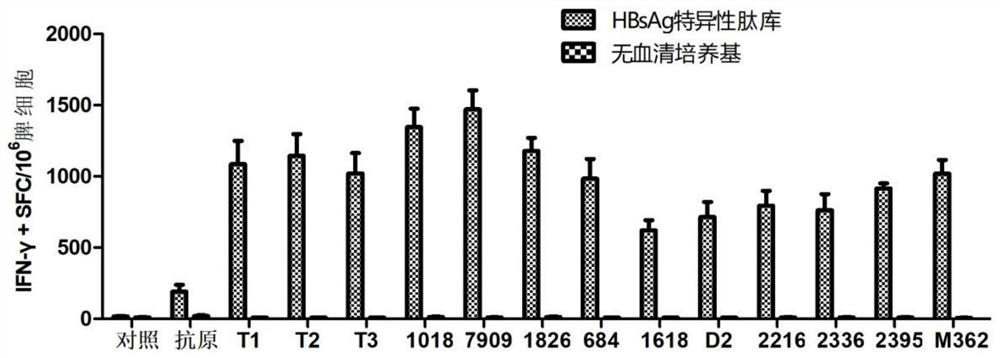
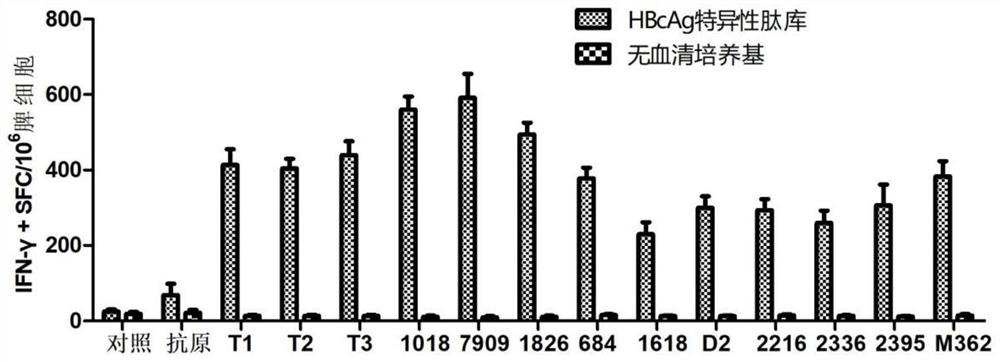
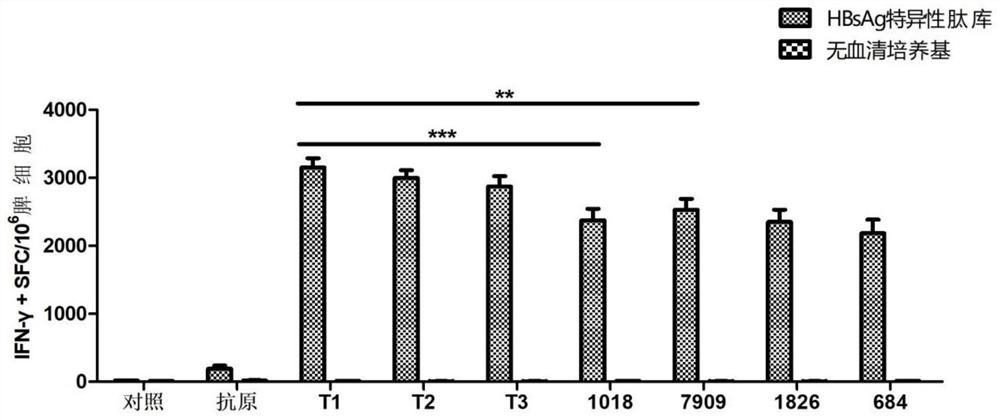
[0099] 4. Experimental results: ELISPOT spot results see image 3 and Figure 4 , the results showed that the combined application of CpG T1~T3 and QS21 had a highly efficient synergistic effect, and the level of HBsAg and HBcAg-specific IFN-γ induced was significantly higher than that of other CpG adjuvants such as CpG1018 and CpG7909, which had unexpected immune effects .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com