Mosaic type DNA (Deoxyribonucleic Acid) vaccine pVAXI-Hsp 70/CD 80 for preventing and immunologically treating tuberculosis
A DNA vaccine and chimeric technology, applied in the field of tuberculosis vaccines and chimeric DNA molecules, can solve the problems of people who cannot improve the protection level of immunity, continued contact, and poor adult effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
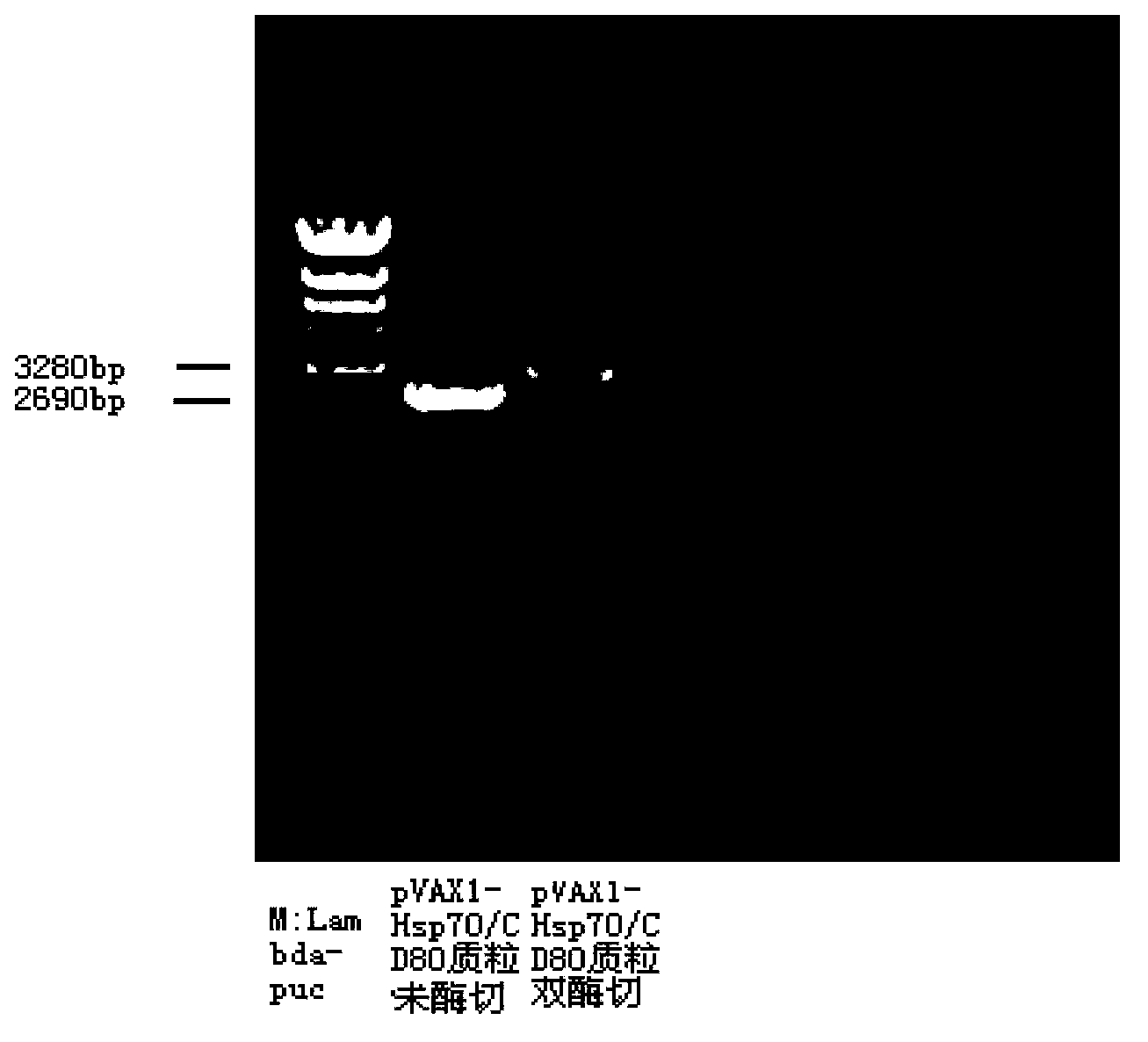
[0031] Example 1 Construction, identification and biological activity of the pVAXI-Hsp70 / CD80 vector of the present invention
[0032] (1) Materials and reagents:
[0033] 1.1 Materials and equipment
[0034] The pVAXI plasmid g was obtained from Invitrogen Company; the LIPOFECTAMINE2000 liposome transfection reagent was obtained from Invitrogen Company; the eukaryotic expression of pcDNA3.1-Hsp70 / CD80 was constructed and preserved in the previous stage of the laboratory, and it could also be constructed according to the records of the prior art (Chinese Patent ZL200410001785.0, publication number CN1562362); Competent cells E.coli DH5α were purchased from Invitrogen; human embryonic kidney cells 293T were purchased from the Cell Bank of the Chinese Academy of Sciences; 20 SPF-grade female healthy BALB / c mice were purchased from the Third Military Medical University ; Restriction enzymes HindIII, XbaI, T4 ligase were purchased from New England Biolabs (NEB Company); DNA fragm...
Embodiment 2
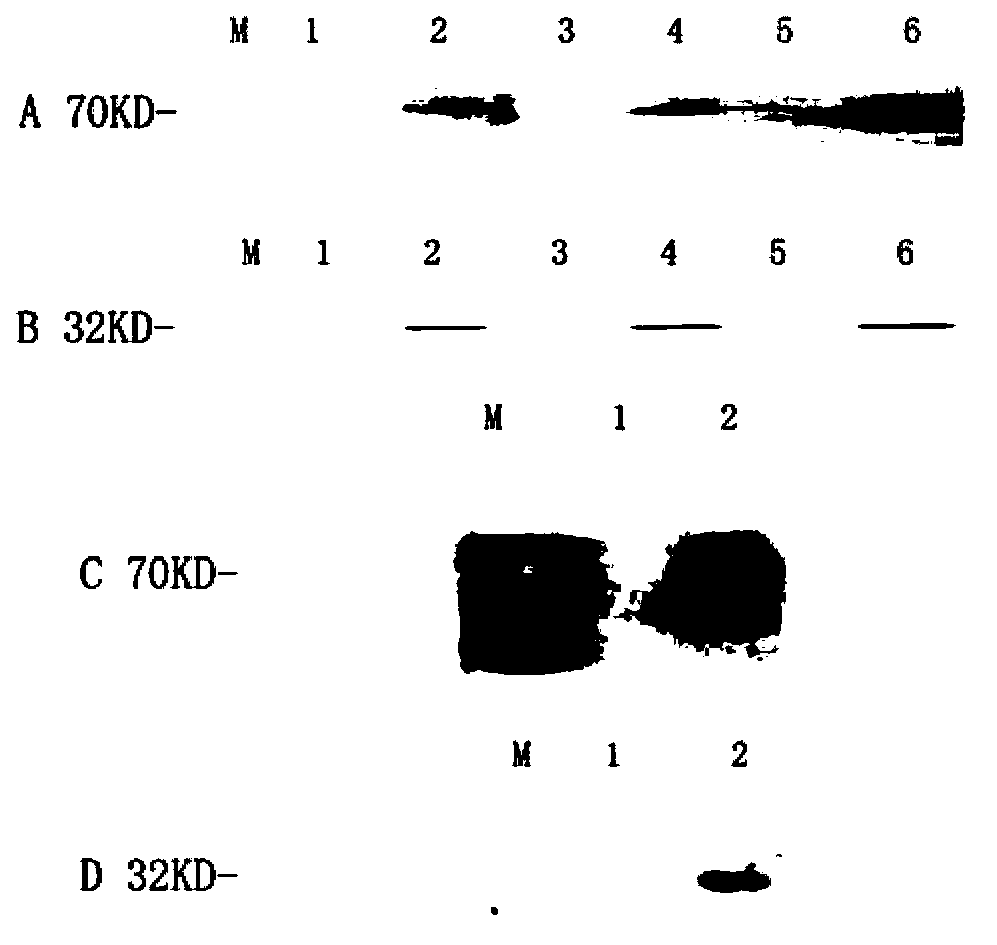
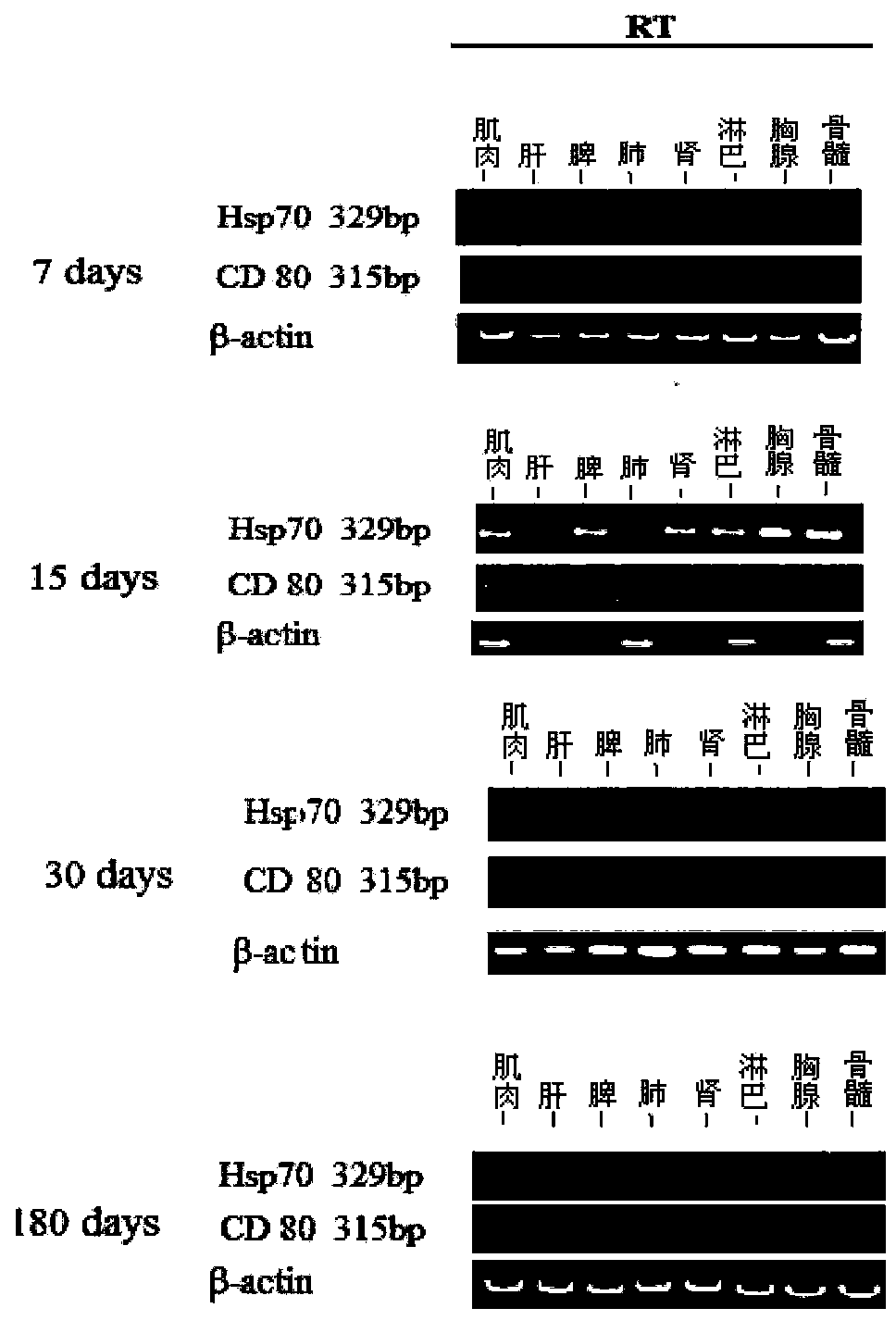
[0042] Example 2. Distribution and expression of PVAX1-Hsp70 / CD80 in mice
[0043] 1. Animal experiment Twenty inbred BALB / c mice were raised in an individually ventilated IVC system. The animals were divided into 5 groups according to the injection time (3 animals in each group): 7-day group, 15-day group, 30-day group, 180-day group and negative control group. At each time point, 125 μl of chimeric vaccine and 7.5 g / L bupivacaine suspension (4:1), containing 100 μg of plasmid, were injected into the quadriceps muscle of mice. The control group was injected with 125 μl of normal saline. At 7, 15, 30 and 180 days after the injection, eyeball blood as well as muscle, lymph node, bone marrow, spleen, liver, lung, kidney and thymus tissues were collected and frozen at -80°C for future use.
[0044] 1.2.4.2 RT-PCR detection of Hsp70 and CD80 expression in mice Extract RNA from muscle, lymph node, bone marrow, spleen, liver, lung, kidney, thymus tissue, according to Tiangen total...
Embodiment 3
[0065] Example 3 Study on the Immunoprotective Effect and Mechanism of pvAXI-Hsp70 / CD80 Chimeric DNA Vaccine on Tuberculosis
[0066] (1) Test method.
[0067] 1. Experimental materials:
[0068] Endotoxin-free plasmid purification system (Qiagen). IFN-γ, IL-4, (Miltenyi Biotec).
[0069] Preparation of DNA plasmids: Preparation of pVAXI-Hsp70 / CD80 and PcDNA with an endotoxin-free plasmid purification system 3.1 - Hsp70 / CD80 plasmid.
[0070] 2. Experiment group modeling and processing:
[0071] Animal grouping: random grouping according to the random number table, the experimental animals were 6-8 week old, clean grade healthy mice C57BL / 6N, weighing about 18±2g, half male and half male, purchased from the Experimental Animal Center of Chongqing Medical University. Divided into blank control group (referred to as control group), BCG vaccine group (BCG group, referred to as BCG group), tuberculosis experimental model group (referred to as tuberculosis group), pcDNA3.1-Hsp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com