Chimeric type DNA vaccine HSP70/CD80 for asthma prevention and immunotherapy
A DNA vaccine and immunotherapy technology, applied in the field of biomedicine, can solve the problems of insufficient safety and unsatisfactory efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Construction and identification of the pVAXI-Hsp70 / CD80 vector of the present invention
[0035] (1) Materials and reagents:
[0036] pVAXI plasmid (invitrogen company), LIPOFECTAMINE 2000 liposome transfection reagent (invitrogen company), endonuclease Hind III, Xba I and T4 ligase (NEB company). PcDNA 3.1 - The Hsp70 / CD80 plasmid was constructed according to the description in the background art. Hsp70 monoclonal antibody (Lifespan Biosciences Company), CD80 monoclonal antibody (abcam Company), Western blot reagent (Shanghai Beyontian Biological Company).
[0037] (2) Construction process
[0038] PcDNA3--Hsp70 / CD80 plasmid and vector pVAXI were double-digested with endonucleases Hind III and Xba I, and the target gene Hsp70 / CD80 was connected to pVAXI with T4 ligase to construct pVAXI-Hsp70 / CD80. The constructed plasmid was sent to Shanghai Sangon Biotechnology Co., Ltd. for sequencing verification.
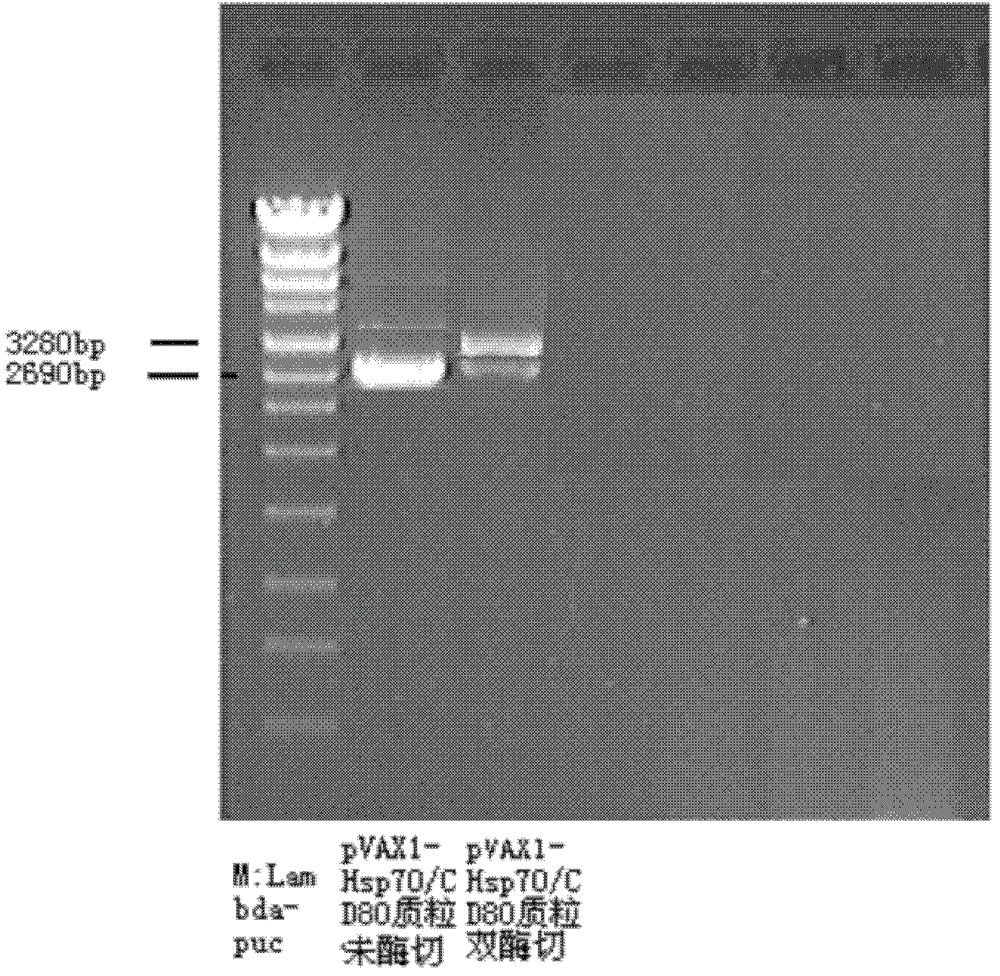
[0039] (3) Identification of pVAXI-Hsp70 / CD80:
[...
Embodiment 2
[0041] Example 2 The present invention pVAXI-Hsp70 / CD80 expression and biodistribution detection
[0042] (1) Verification of eukaryotic expression
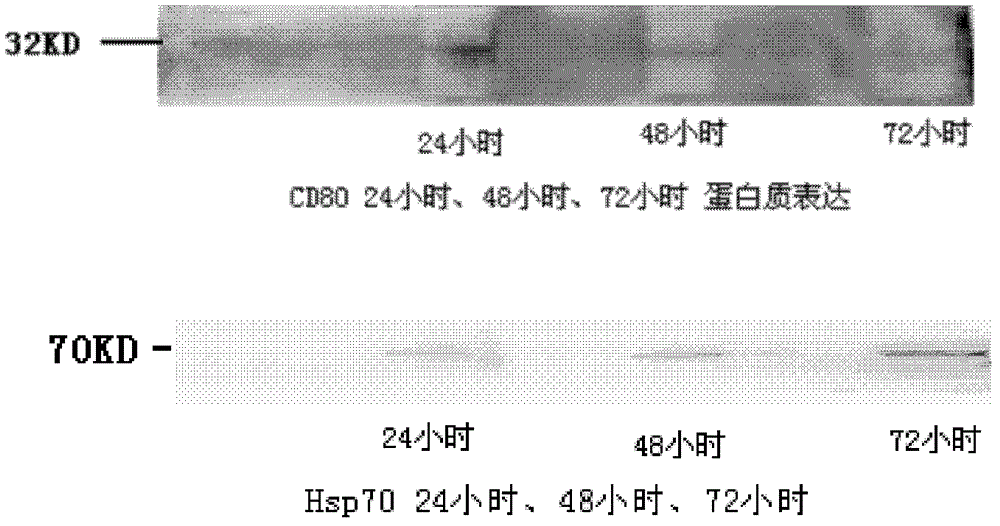
[0043] 4 μg (2 μl) of the pVAXI-Hsp70 / CD80 plasmid was encapsulated with 10 μl of LIPOFECTAMINE 2000 liposome transfection reagent and transfected into 293T cells. The cells were harvested at 24 hours, 48 hours, and 72 hours, and the lysate was added to release the protein. The supernatant was mixed with 5x loading buffer and boiled to denature the protein. SDS-page electrophoresis, membrane transfer. Add Hsp70 and CD80 primary antibodies and incubate overnight at 4°C, and incubate with secondary antibodies for one hour after washing the membrane. After washing the membrane, there were single bands at 70kD (Hsp70) and 32KD (CD80) by chemiluminescence (results in figure 2 ), indicating that the vector can simultaneously express Hsp70 and CD80 proteins.
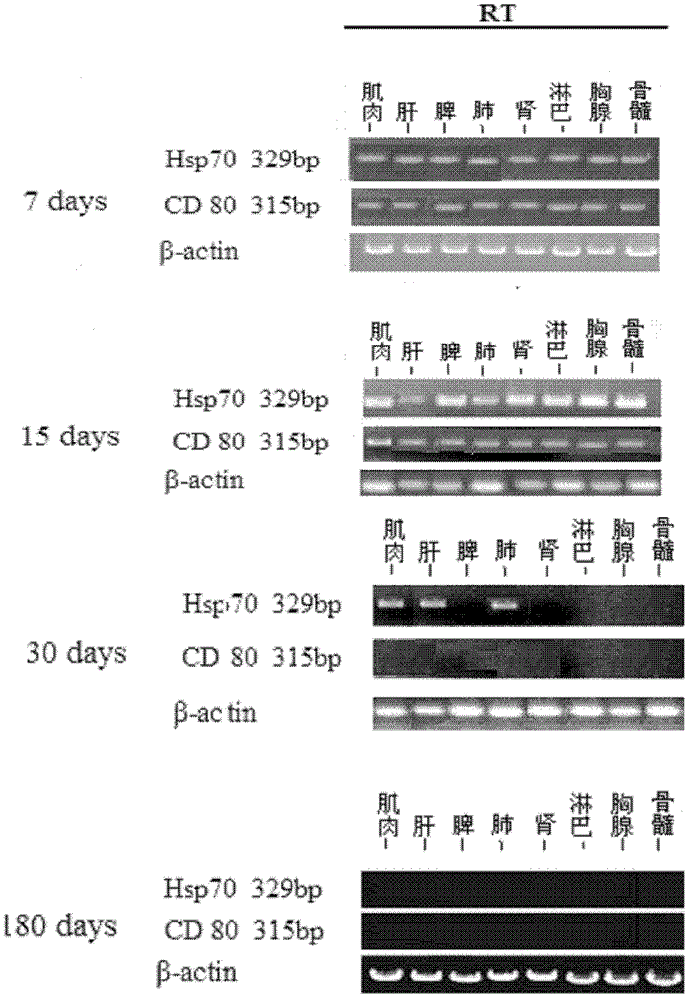
[0044] (2) In vivo expression and distribution detection
[0045] Exp...
Embodiment 3
[0058] Example 3 Study on the Immunoprotective Effect and Mechanism of pvAXI-Hsp70 / CD80 Chimeric DNA Vaccine on Asthma
[0059] (1) Test method.
[0060] 1. Experimental materials:
[0061] Rabbit anti-mouse PCNA (proliferating cell nuclear antigen) monoclonal antibody (mAb, Beijing Boaosen Company). Endotoxin-free plasmid purification system (Qiagen). IFN-γ, IL-4, IL-5, IL-9, IL-10, IL-13, IL-17, IL-23, TGF-β, OVA-sIgE ELISA kit (ADL company), CD4 + CD25 + Regulatory T cell isolation kit (Miltenyi Biotec). SPF grade female BALB / c mice. Chicken ovalbumin (OVA, product of Sigma). 402A nebulizer (Jiangsu Yuyue Medical Equipment Co., Ltd.).
[0062] Preparation of DNA plasmids: Preparation of pVAXI-Hsp70 / CD80 and PcDNA with an endotoxin-free plasmid purification system 3.1 - Hsp70 / CD80 plasmid.
[0063] CD4 + CD25 + Regulatory T cell preparation: after the mice were anesthetized with ether, supercoiled plasmid DNA was prepared (5-iagen company), dissolved in physiologi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com