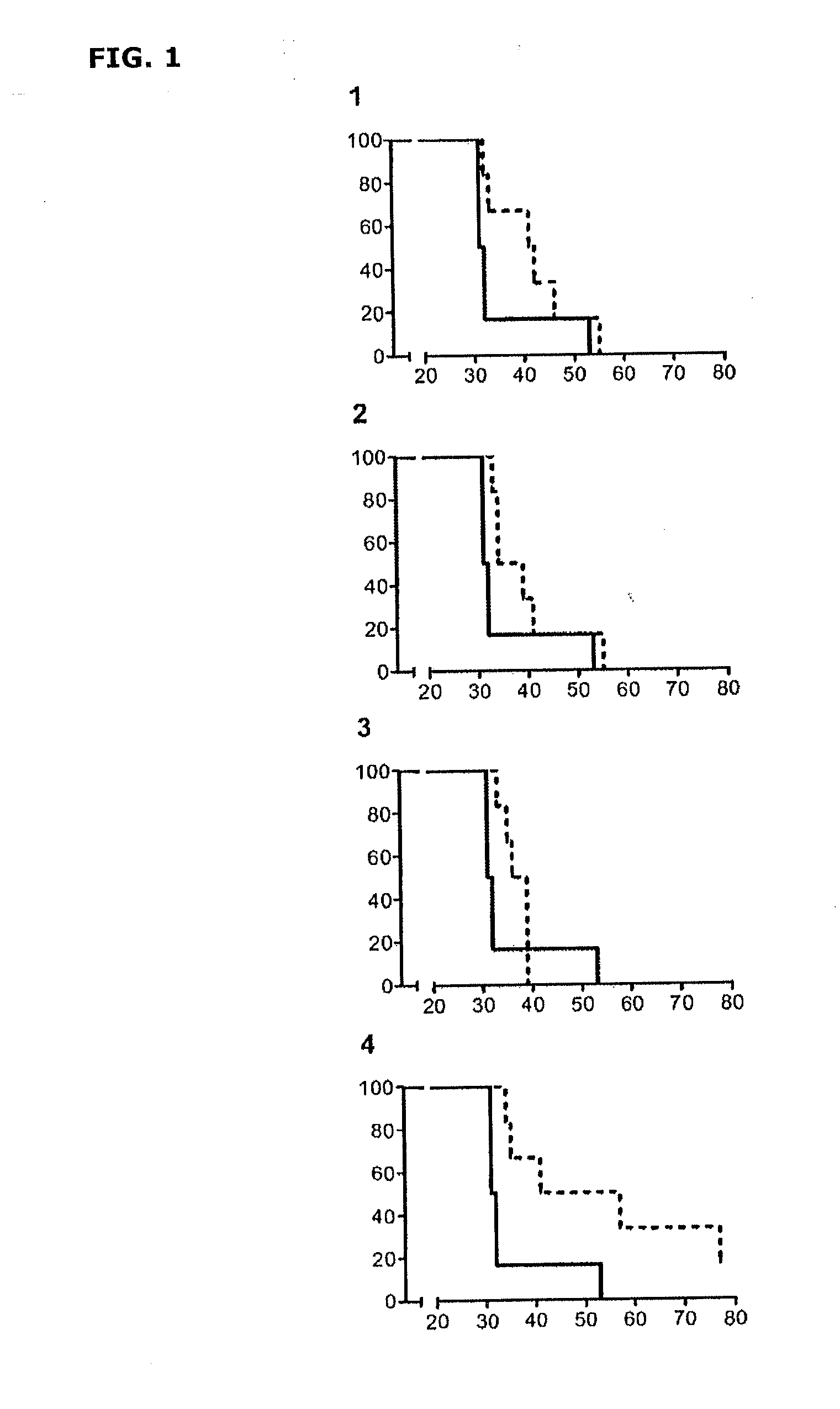
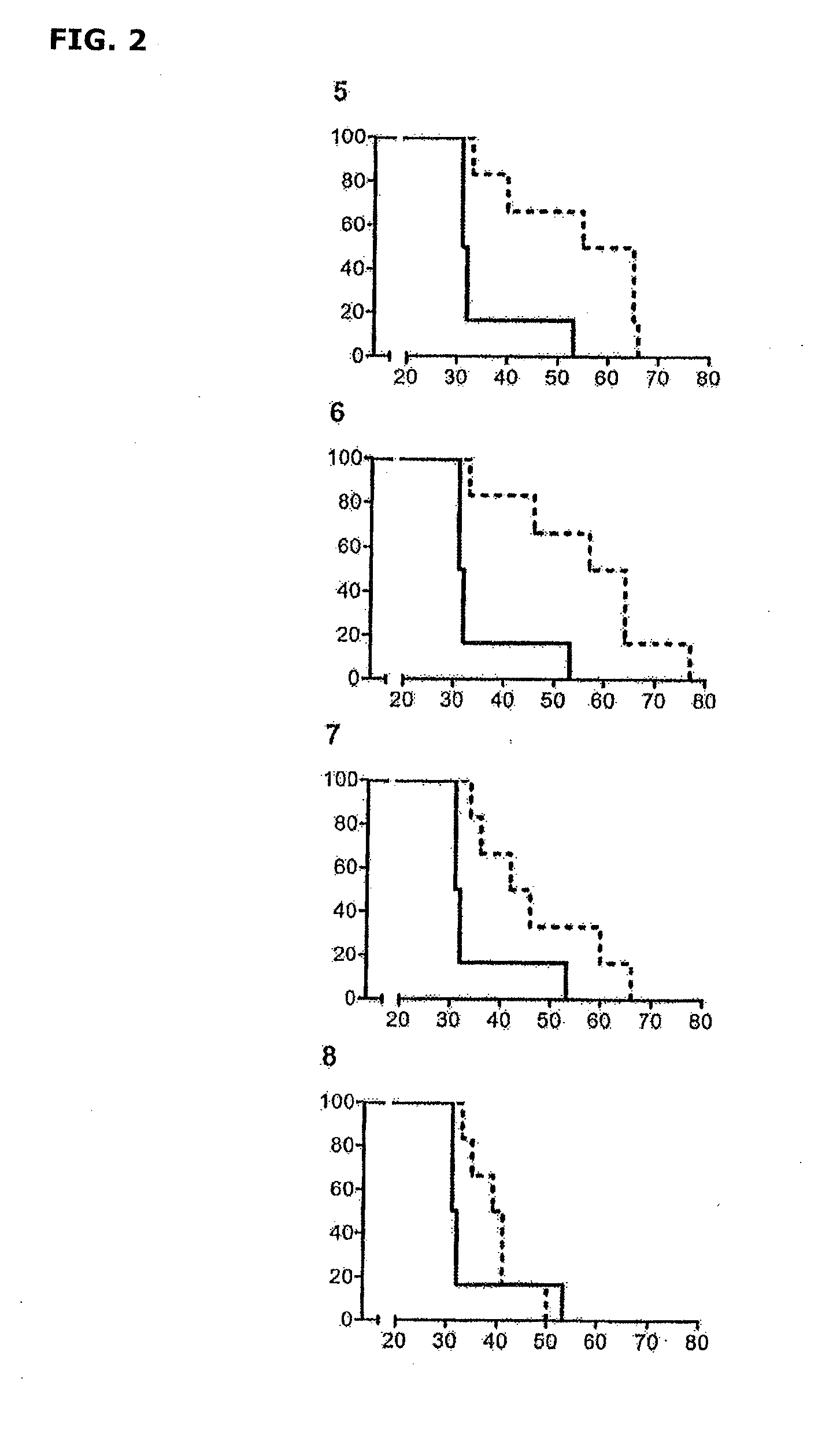
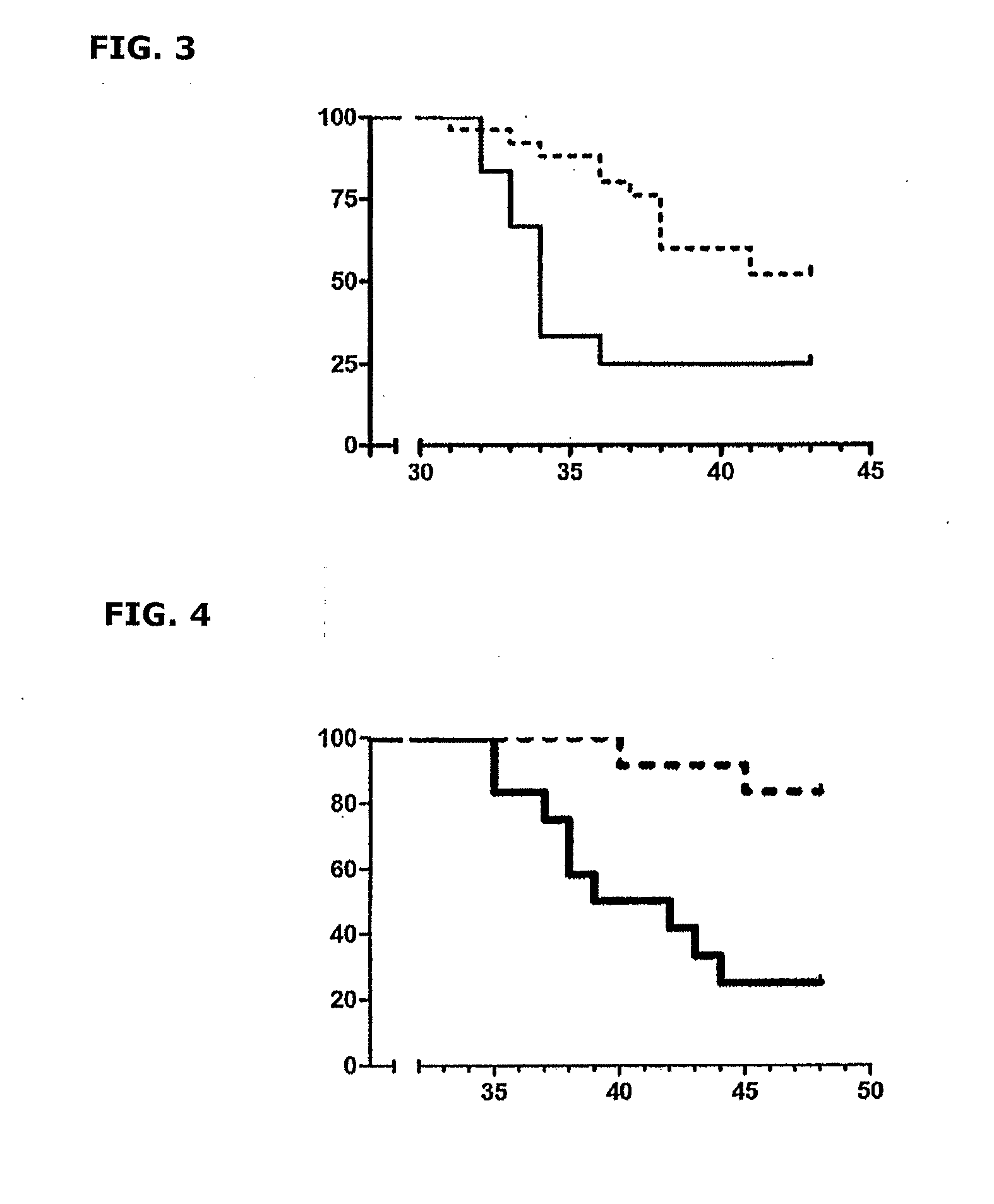
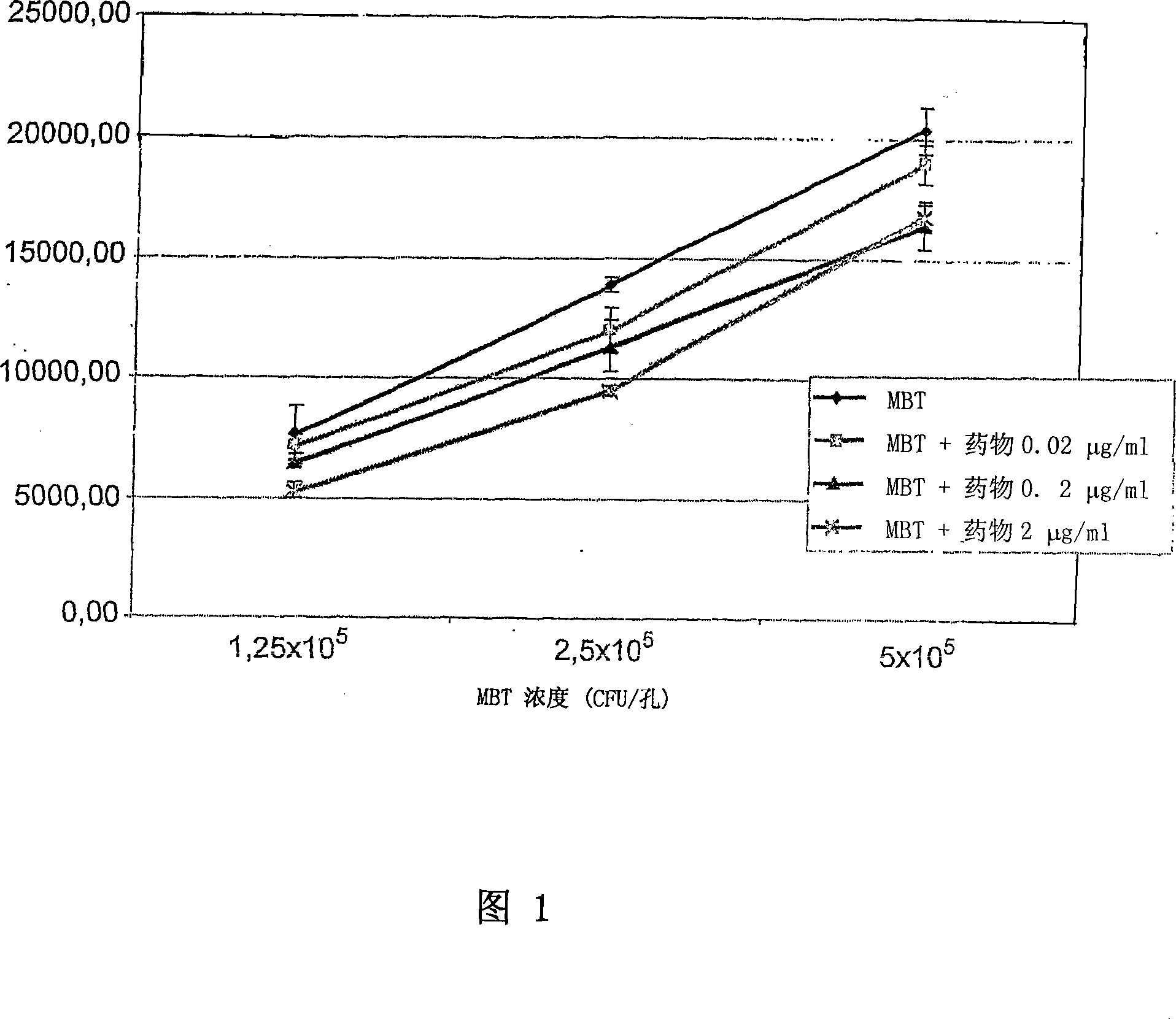
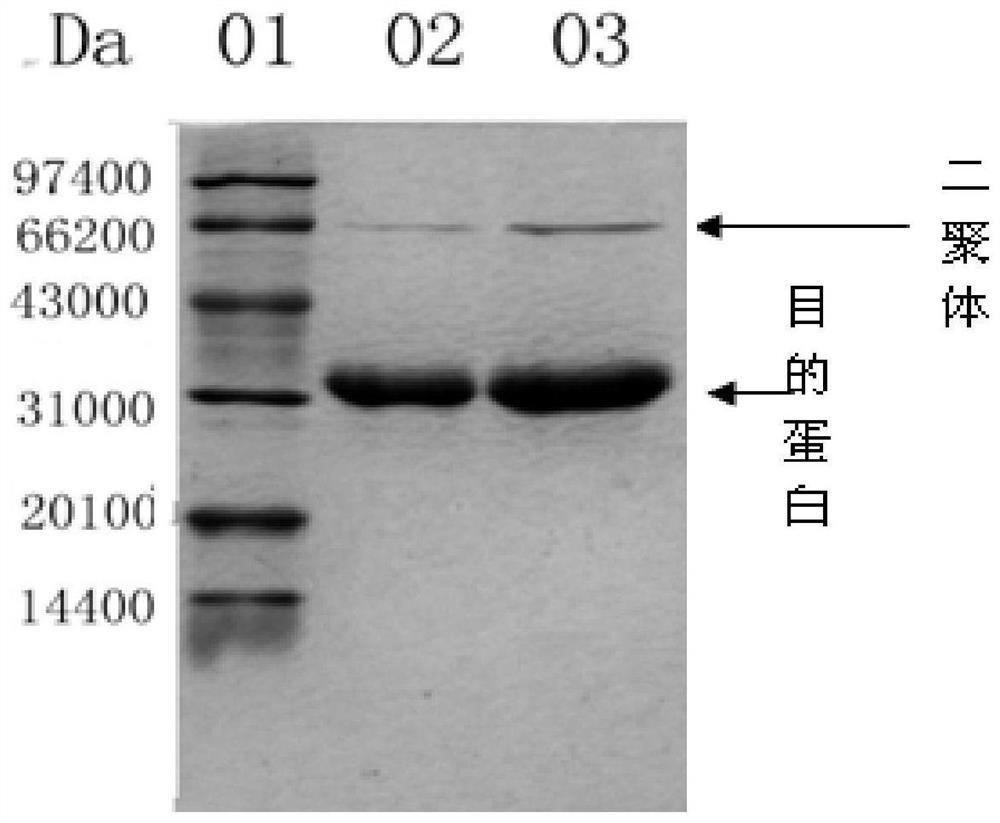
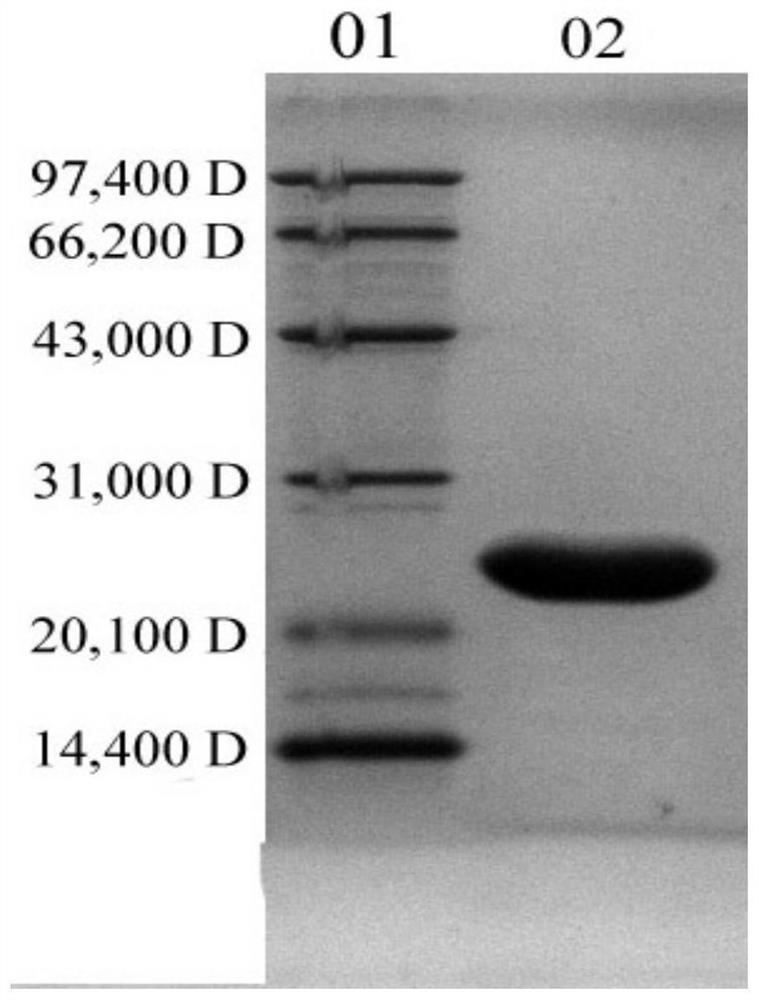
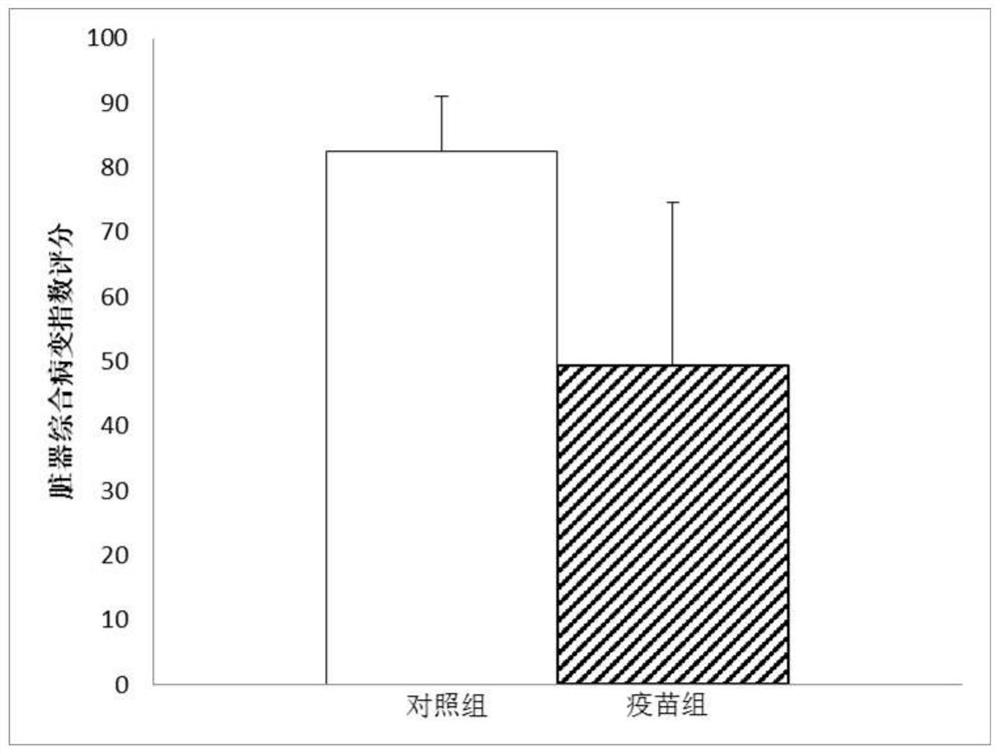
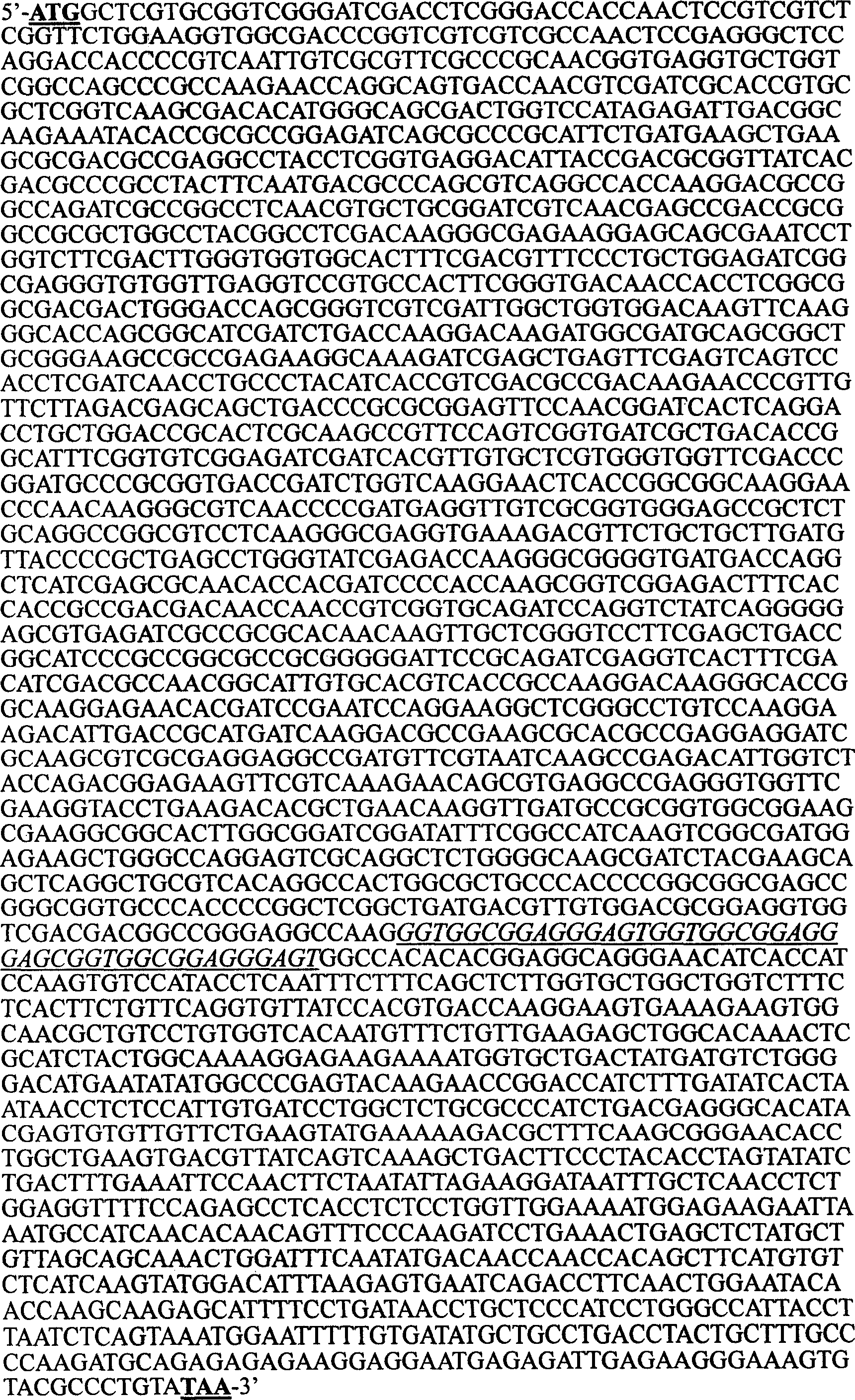Patents
Literature
61 results about "Tuberculosis prophylaxis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
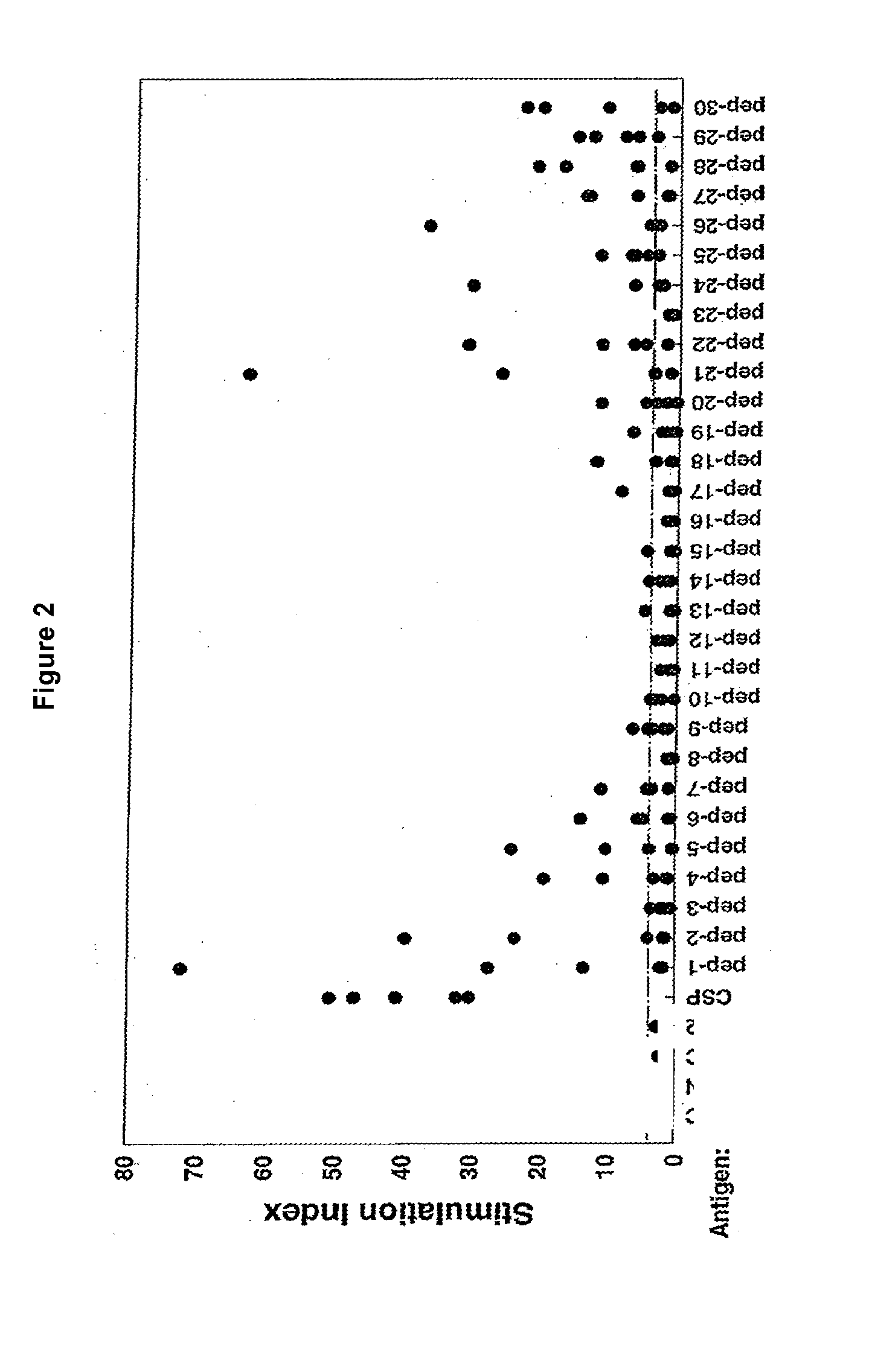
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS6544522B1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAntigenMycobacterial antigen
The present invention relates to fusion proteins of Mycobacterium tuberclosis antigens. In particular, it relates to two fusion proteins, each of which contains three individual M. tuberculosis antigens, and a fusion protein of two M. tuberculosis antigens, their coding sequences, and methods for their use in the treatment and prevention of tuberculosis.
Owner:CORIXA CORP
Fusion proteins of mycobacterium tuberculosis
InactiveUS7083796B2Good antigenicityHigh sensitivityAntibacterial agentsPeptide/protein ingredientsSerum igeBiology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Fusion proteins of Mycobacterium tuberculosis
InactiveUS7026465B2More immunogenicImprove the level ofAntibacterial agentsBacteriaSerum igeMycobacterium
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Application of single nucleotide polymorphism rs3888188 to detection of tuberculosis susceptibility
InactiveCN102808030AMicrobiological testing/measurementTranscription initiation siteTranslation initiation sites
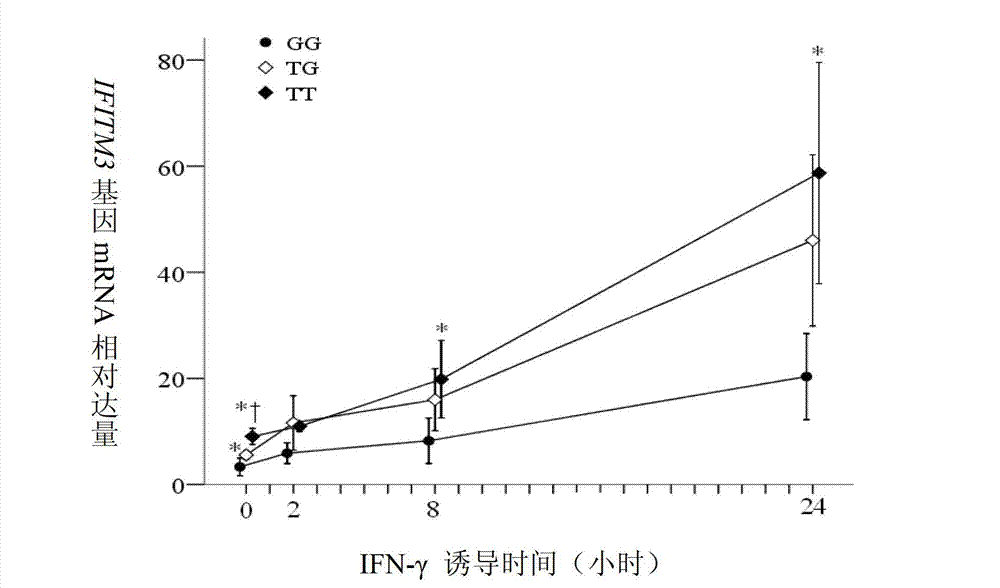
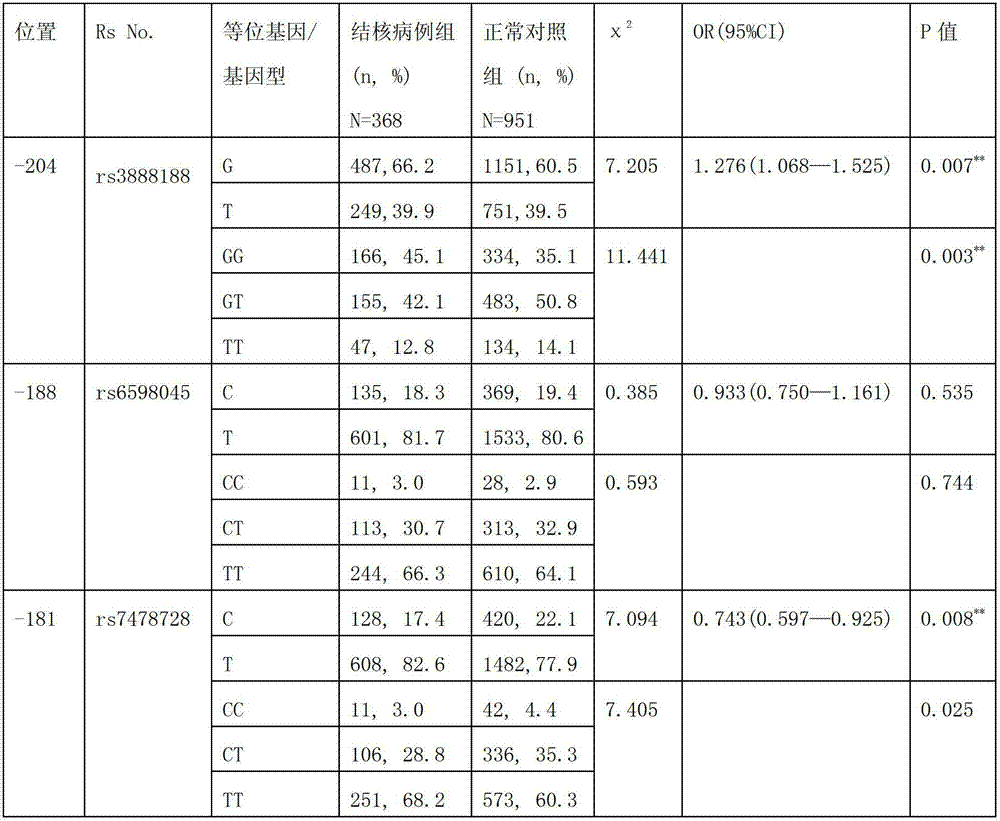
The invention discloses an application of single nucleotide polymorphism rs3888188 to detection of tuberculosis susceptibility. The SNP (Single Nucleotide Polymorphism) is the rs3888188 and is positioned in a human IFITM3 (interferon induced transmembrane protein) gene core promoter region (namely the upstream 103bp of a transcription initiation site / the upstream 204bp of a translation initiation site ATG), the rs3888188G is a risk factor of tuberculosis susceptibility, and the polymorphism of the rs3888188 is highly related with tuberculosis. When the genotype of the site is GG, the tuberculosis susceptibility or tuberculosis causing risk of an individual to be detected is increased. The application is of significant meaning and value in the aspects of diagnosing and treating the tuberculosis to reasonably prevent the tuberculosis.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
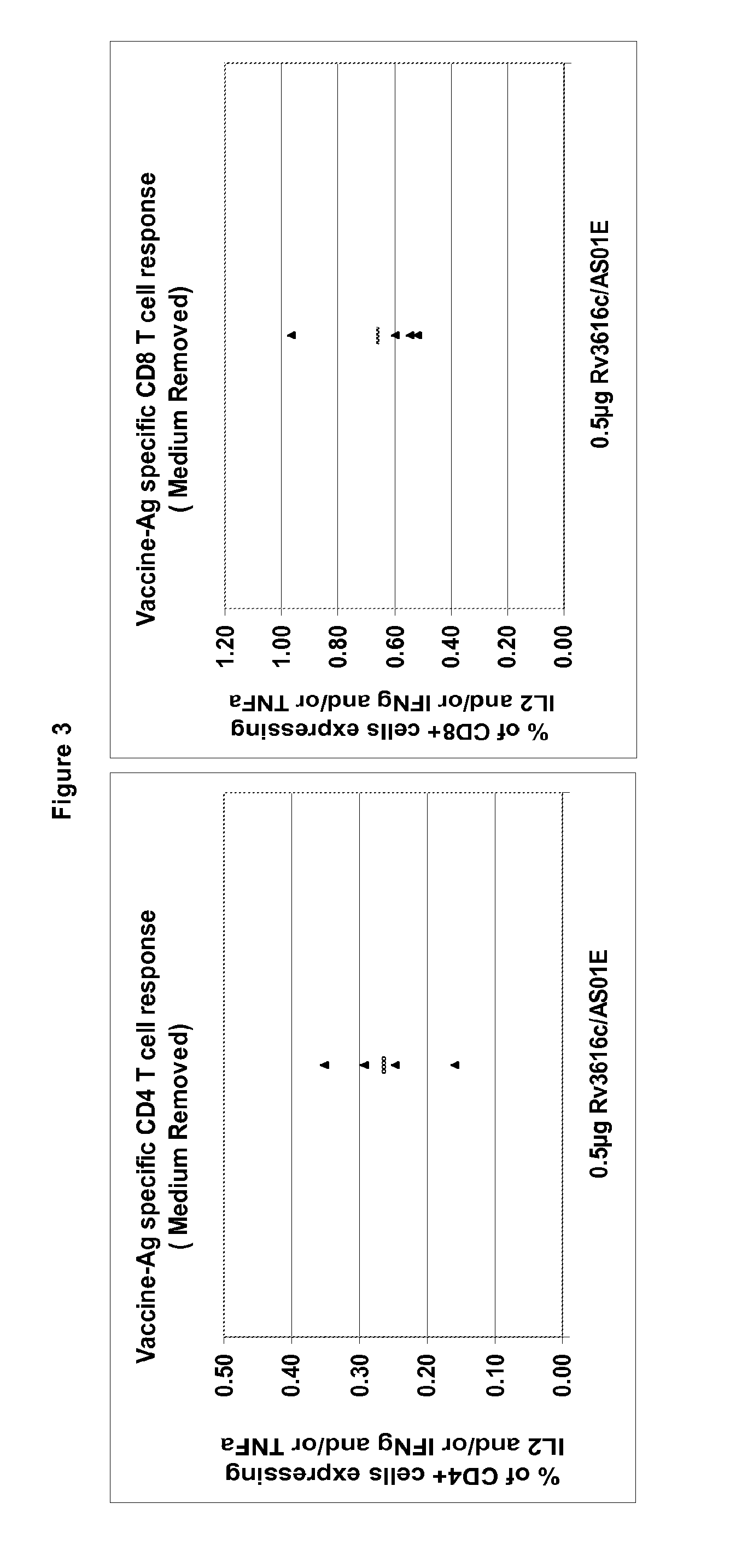
Modified tuberculosis antigens
ActiveUS20120294882A1High expressionAntibacterial agentsOrganic active ingredientsAntigenTuberculosis
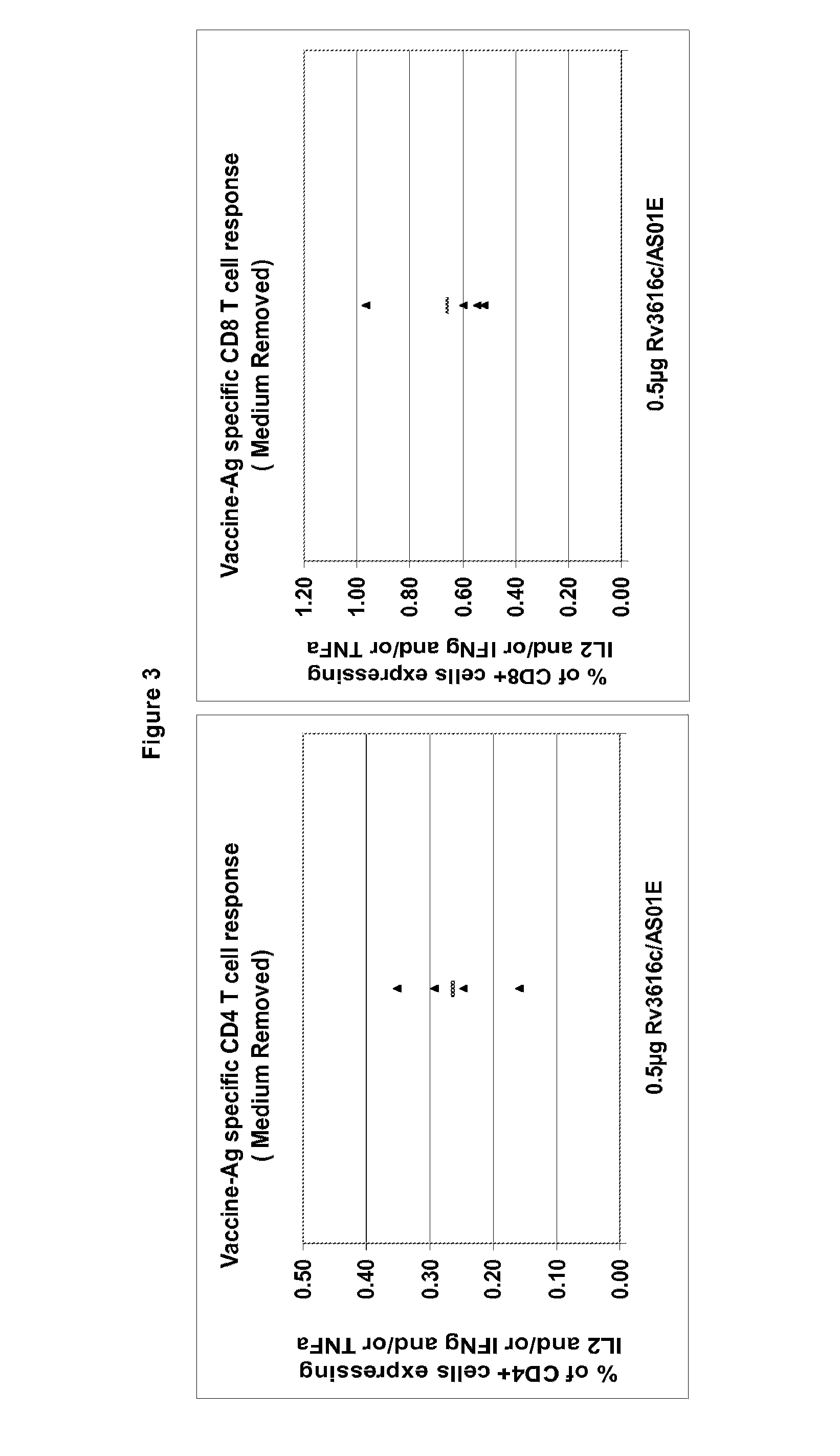
Modified Rv3616c proteins and their use as medicaments, particularly for the prevention of reactivation of tuberculosis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Novel recombinant vaccine used for preventing tuberculosis
InactiveCN101850112AStable expressionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to the construction of a human GM-CSF gene and Mycobacterium tuberculosis ESAT6 gene chimerically expressed GMCSF-ESAT6 protein recombinant Bacillus Calmette Guerin (BCG) vaccine and immunogenicity research thereof, namely the sequence of the human GM-CFS gene and the sequence of the Mycobacterium tuberculosis ESAT6 gene are inserted into the sequence of the same escherichiacoli-Mycobacterium tuberculosis shuttle plasmid pMV361 by gene engineering technology so as to construct a recombinant shuttle plasmid rpMV361GMCSF-ESAT6; and a vector is introduced into a BCG vaccine by an electroporation method so as to construct a recombinant BCG vaccine rBCG:GMCSF-ESAT6. The recombinant BCG vaccine can express the human GM-CSF and Mycobacterium tuberculosis ESAT6 gene chimeric protein GMCSF-ESAT6 stably, and has an immunogenicity superior to that of the conventional BCG vaccine. The invention provides a process for preparing the recombinant BCG vaccine, researches the immunity thereof, and belongs to the field of gene engineering and the field of tuberculosis vaccine. The novel recombinant vaccine prevents the generation and the propagration of tuberculosis more effectively.
Owner:SICHUAN UNIV
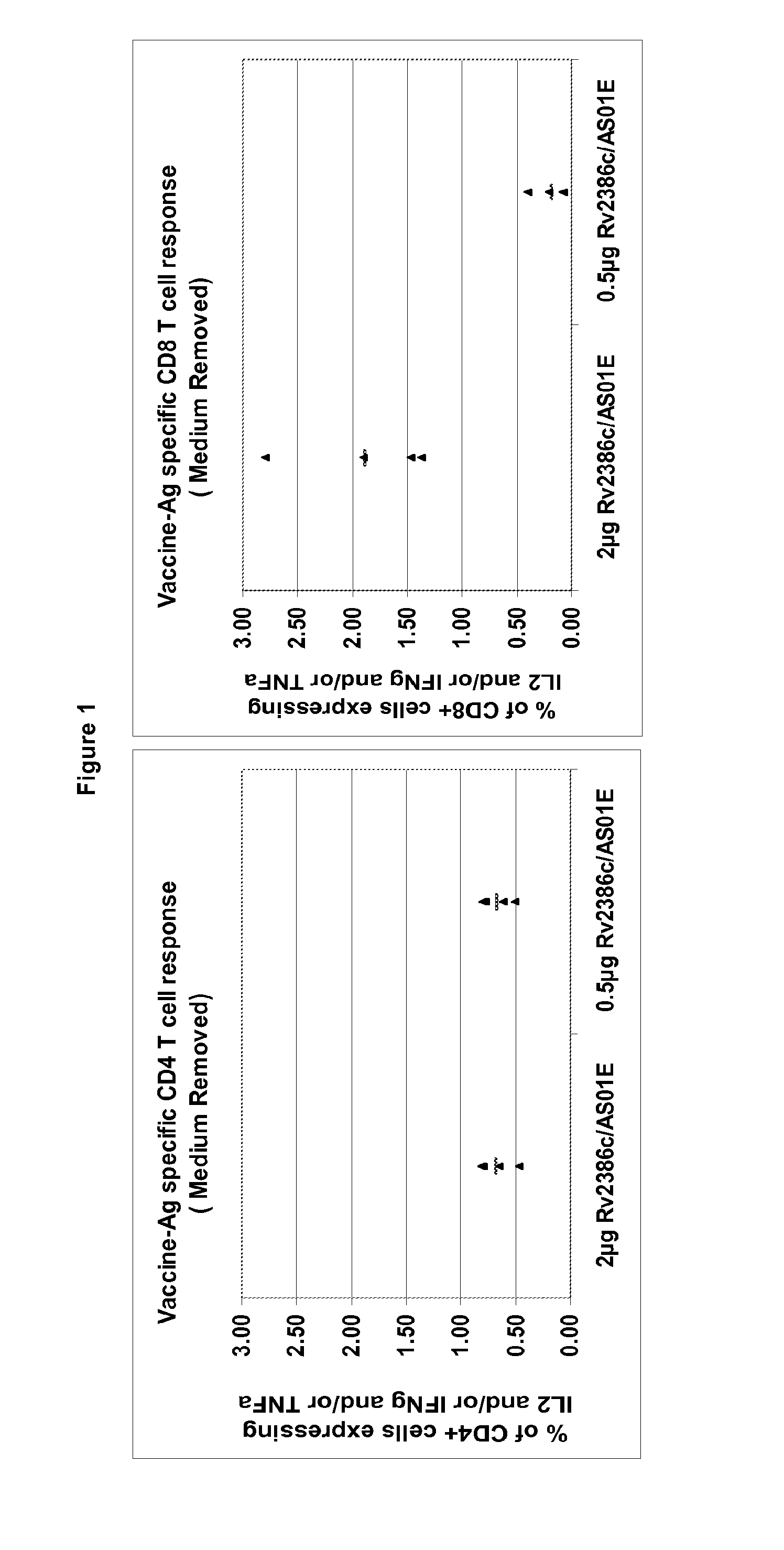
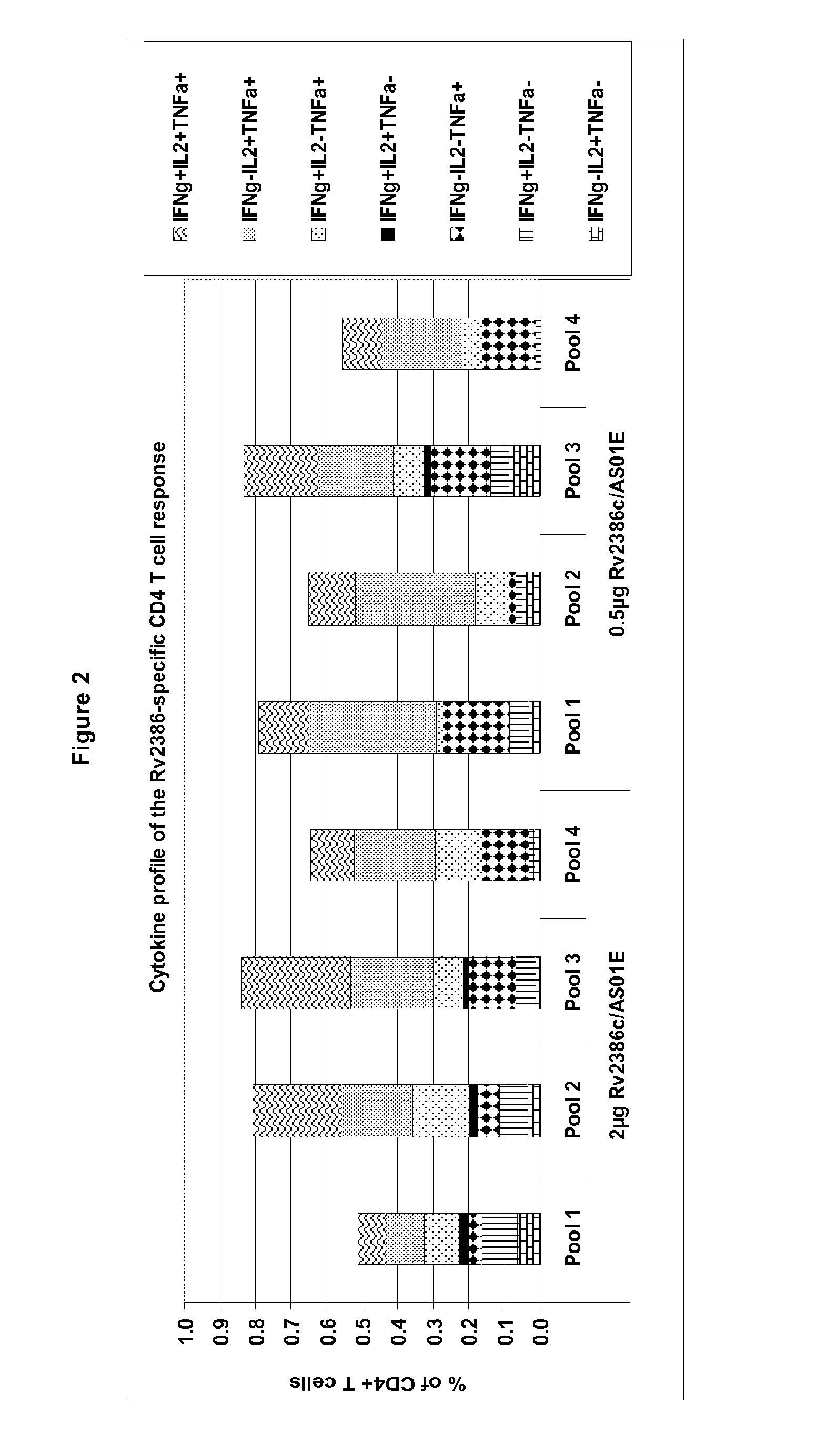
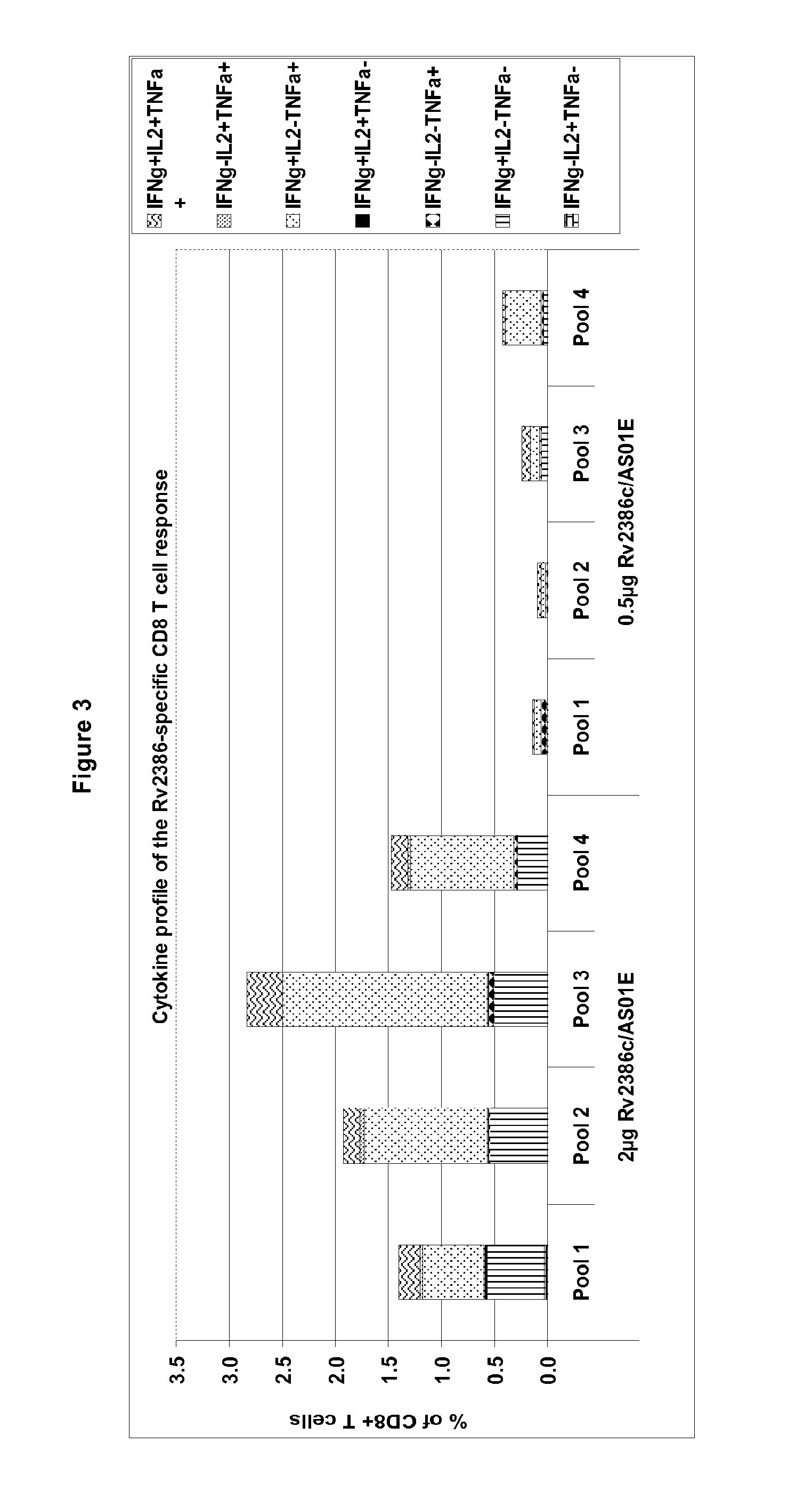
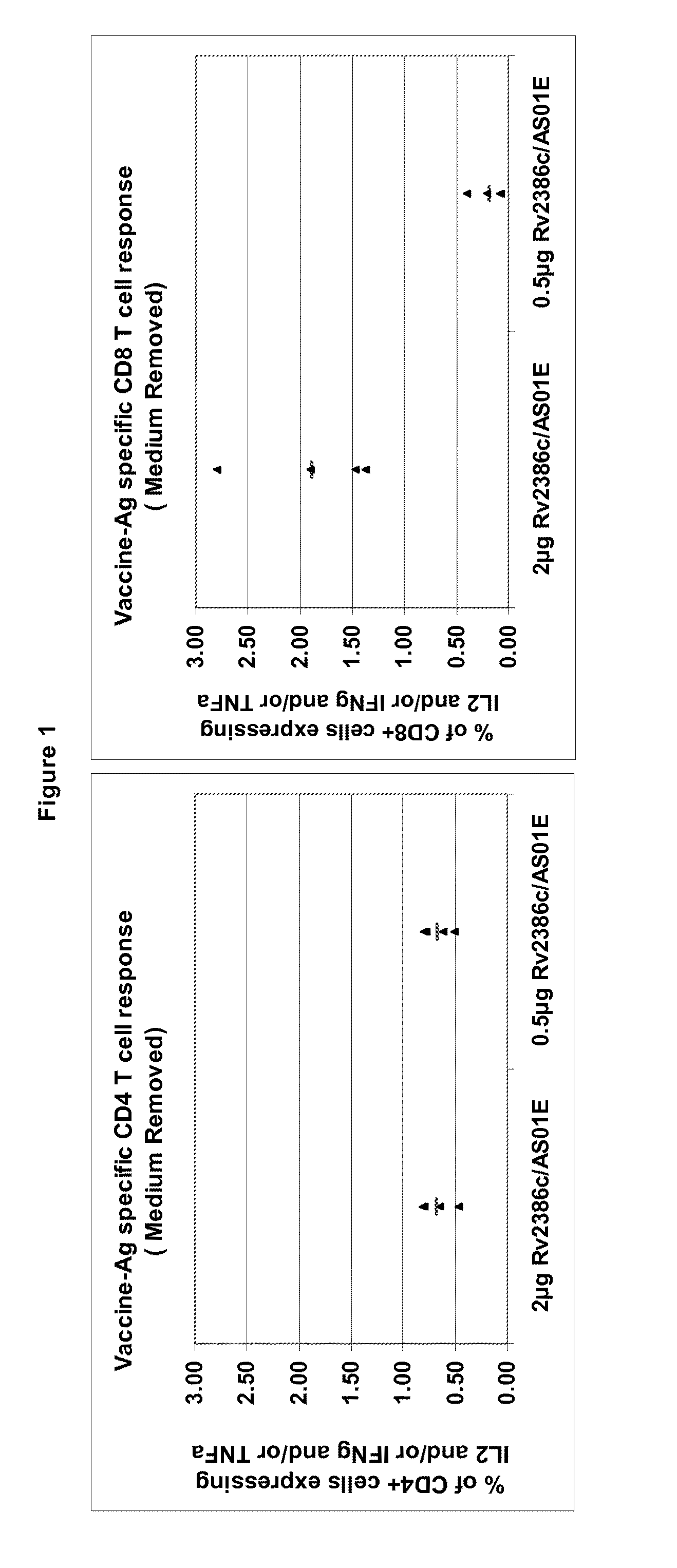
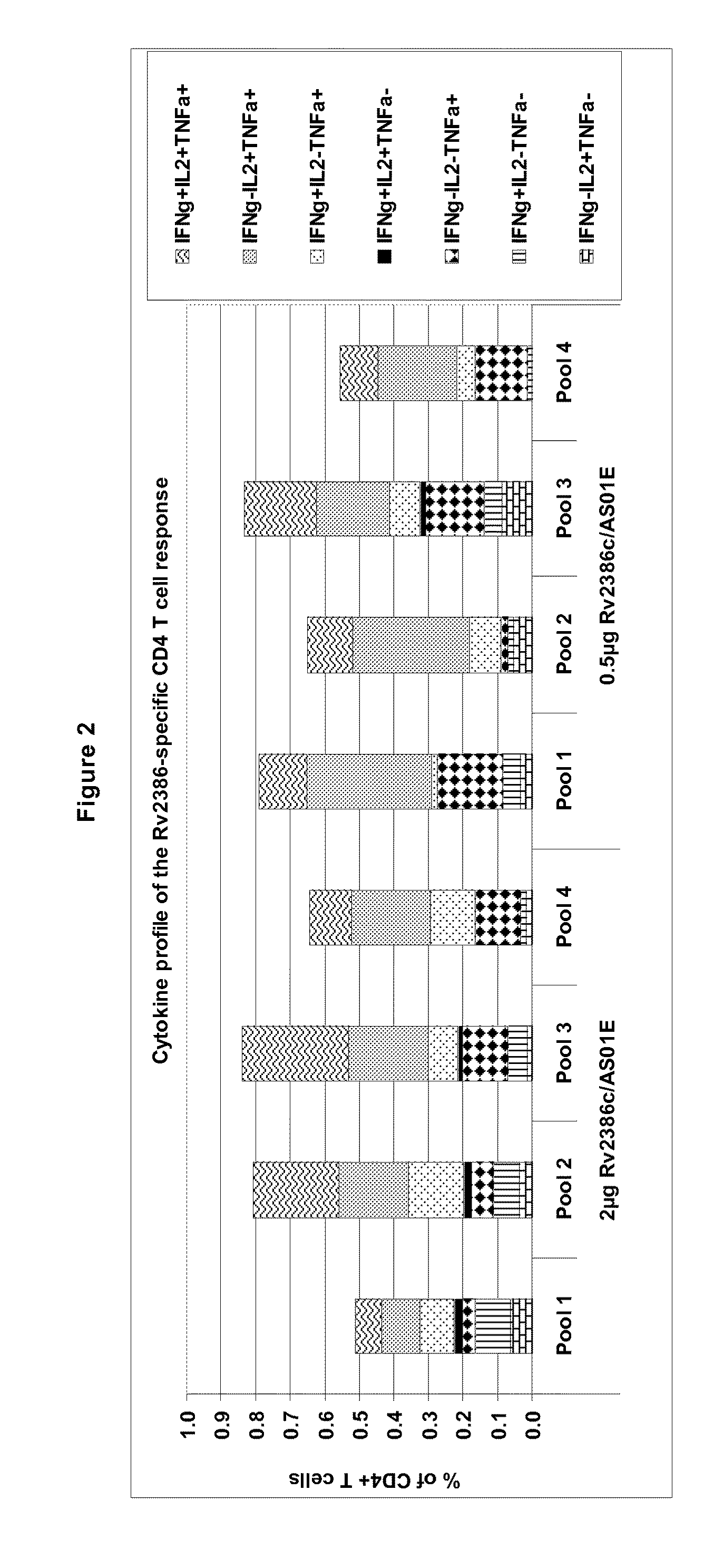
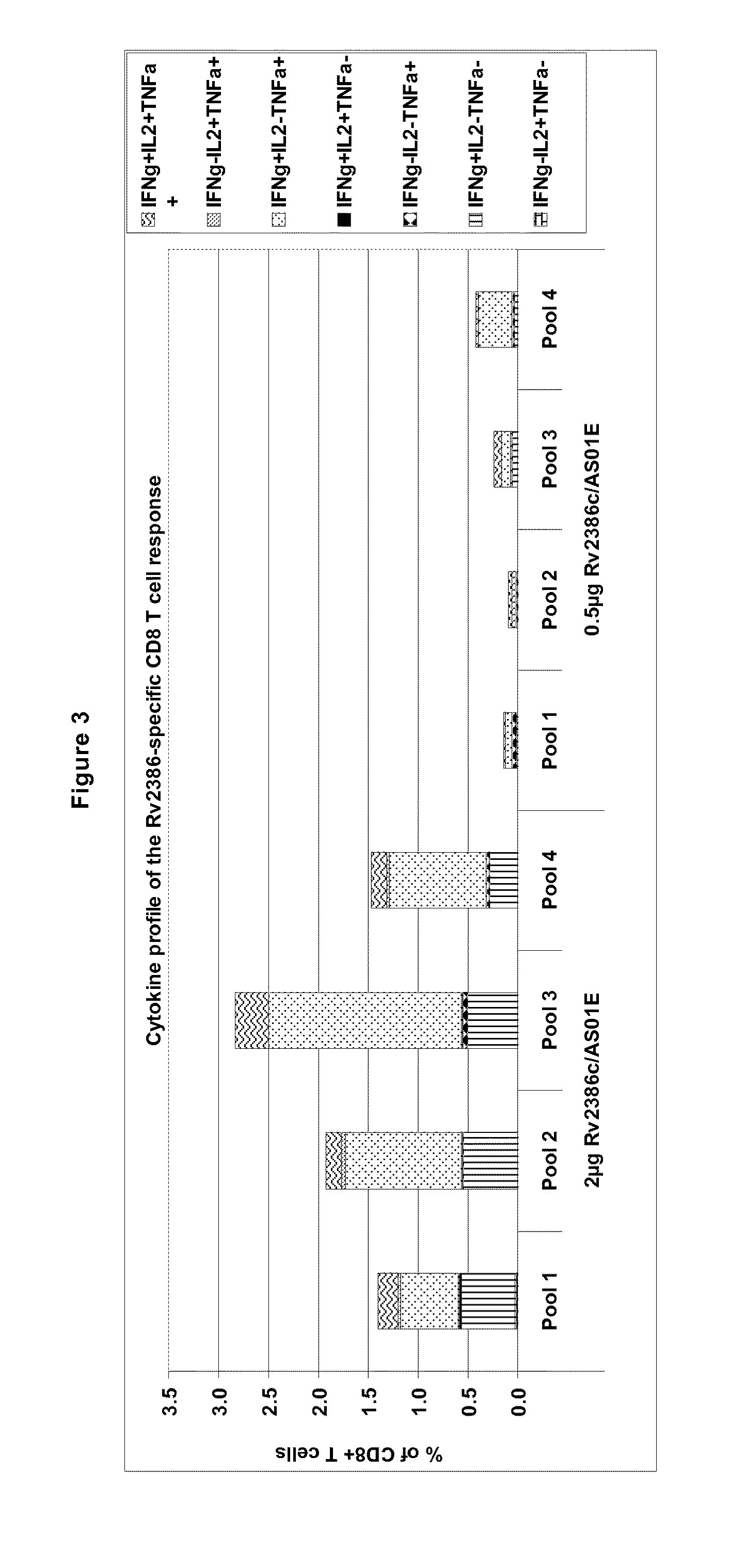
Tuberculosis rv2386c protein, compositions and uses thereof
The present invention is directed to a polypeptide which comprises: (i) an Rv2386c protein sequence; (ii) a variant of an Rv2386c protein sequence; of (iii) an immunogenic fragment of an Rv2386c protein sequence. In other aspects the invention is directed to associated polynucleotides, fusion proteins and methods for the treatment or prevention of tuberculosis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions and methods for the treatment or prevention of tuberculosis
ActiveUS20160213767A1Prevent TBAntibacterial agentsBacterial antigen ingredientsYeastTherapeutic treatment
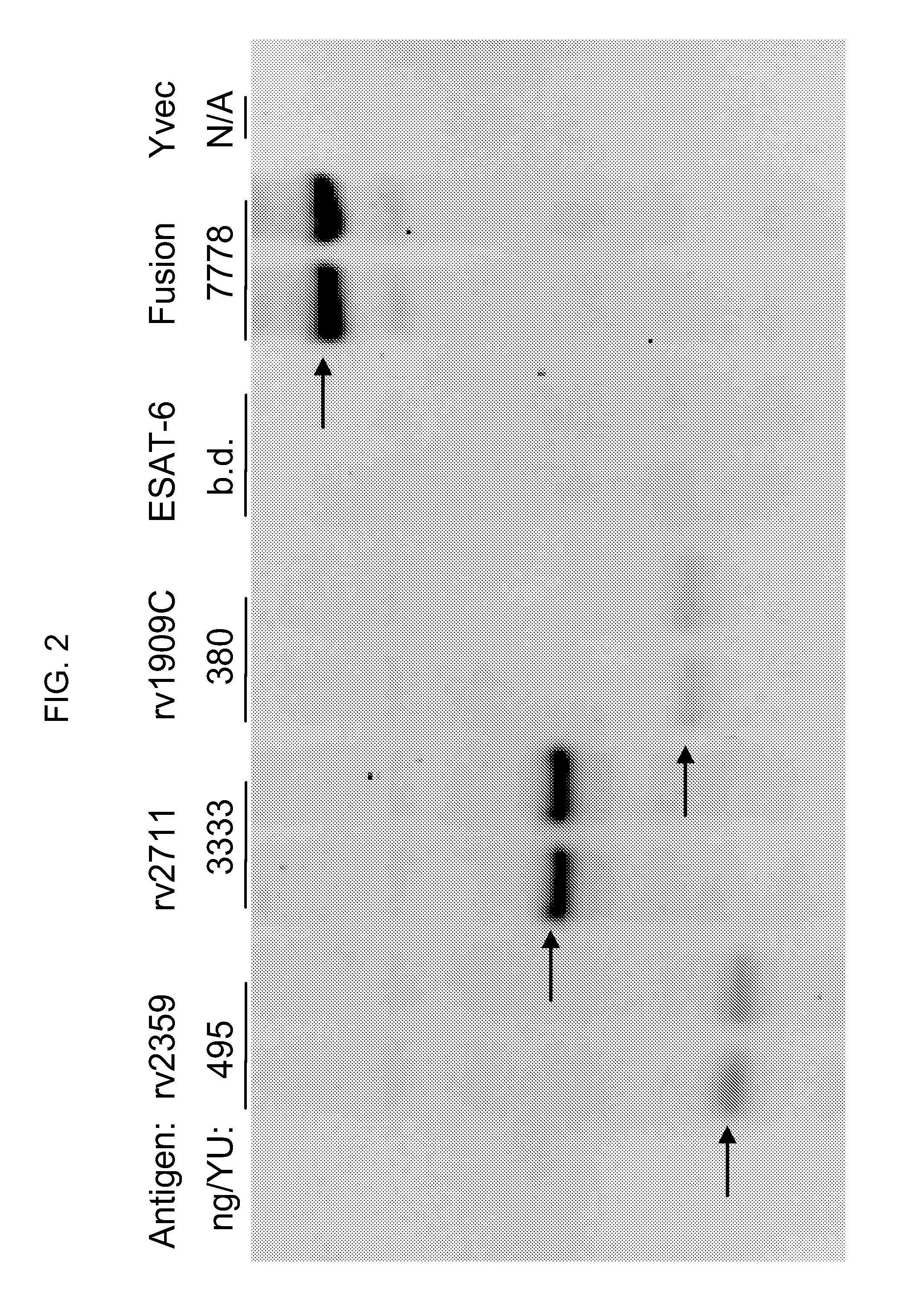
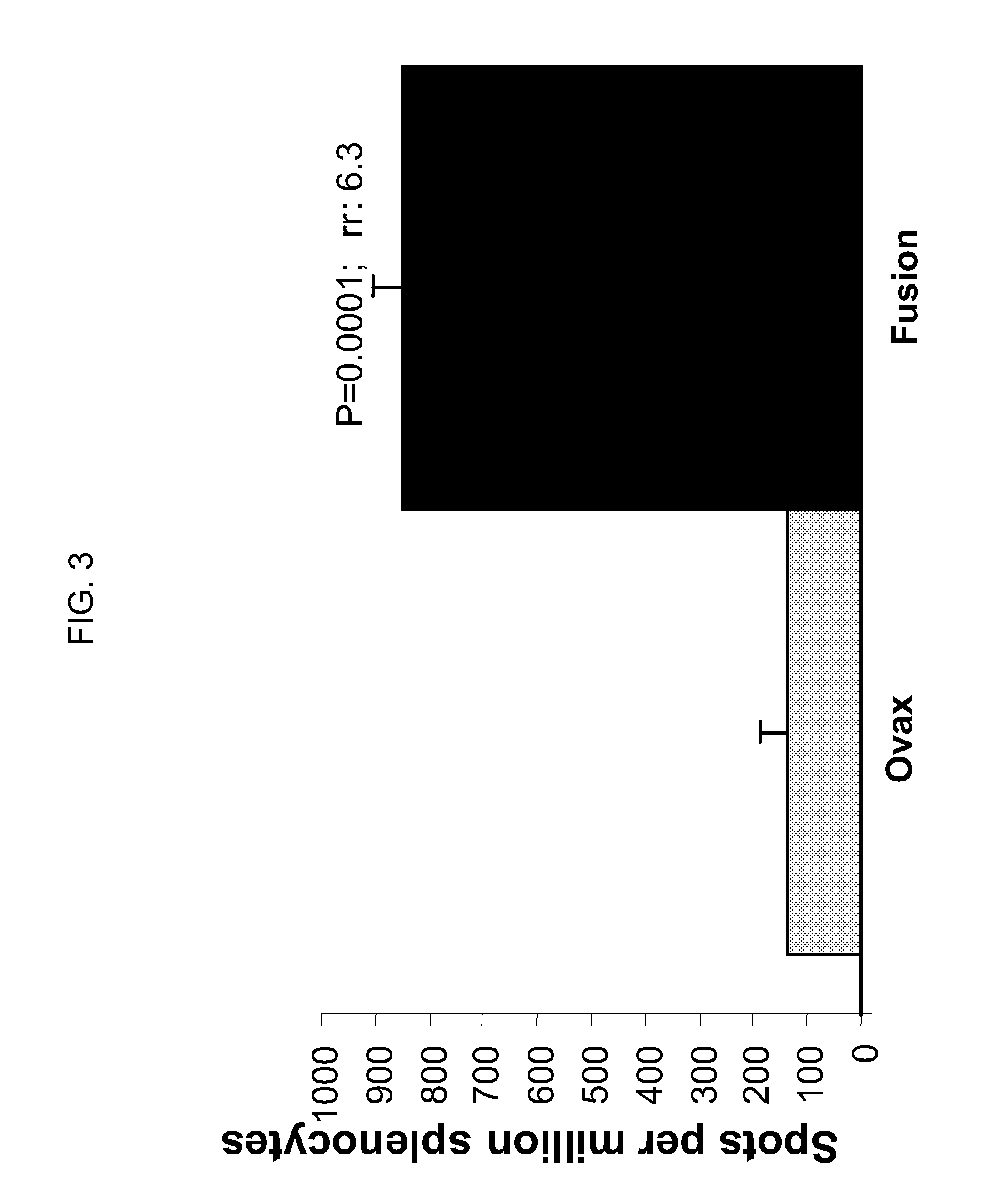
Disclosed are yeast-based immunotherapeutic compositions and fusion proteins for the treatment and / or prevention of TB infection and symptoms thereof, as well as methods of using the yeast-based immunotherapeutic compositions and fusion proteins for the prophylactic and / or therapeutic treatment of TB and / or symptoms thereof.
Owner:GLOBE IMMUNE INC +1
Treatment and prevention of tuberculosis
InactiveUS20110081382A1Conveniently combinedIncrease attractivenessAntibacterial agentsBacterial antigen ingredientsDiseaseImmunotherapy
The invention is within the field of immunology and microbiology, more specifically the field of mycobacteriology and is related to immunotherapy and prophylaxis of tuberculosis and related diseases. The composition useful for these purposes is disclosed, including the methods of using said composition.
Owner:IMMUNITOR USA
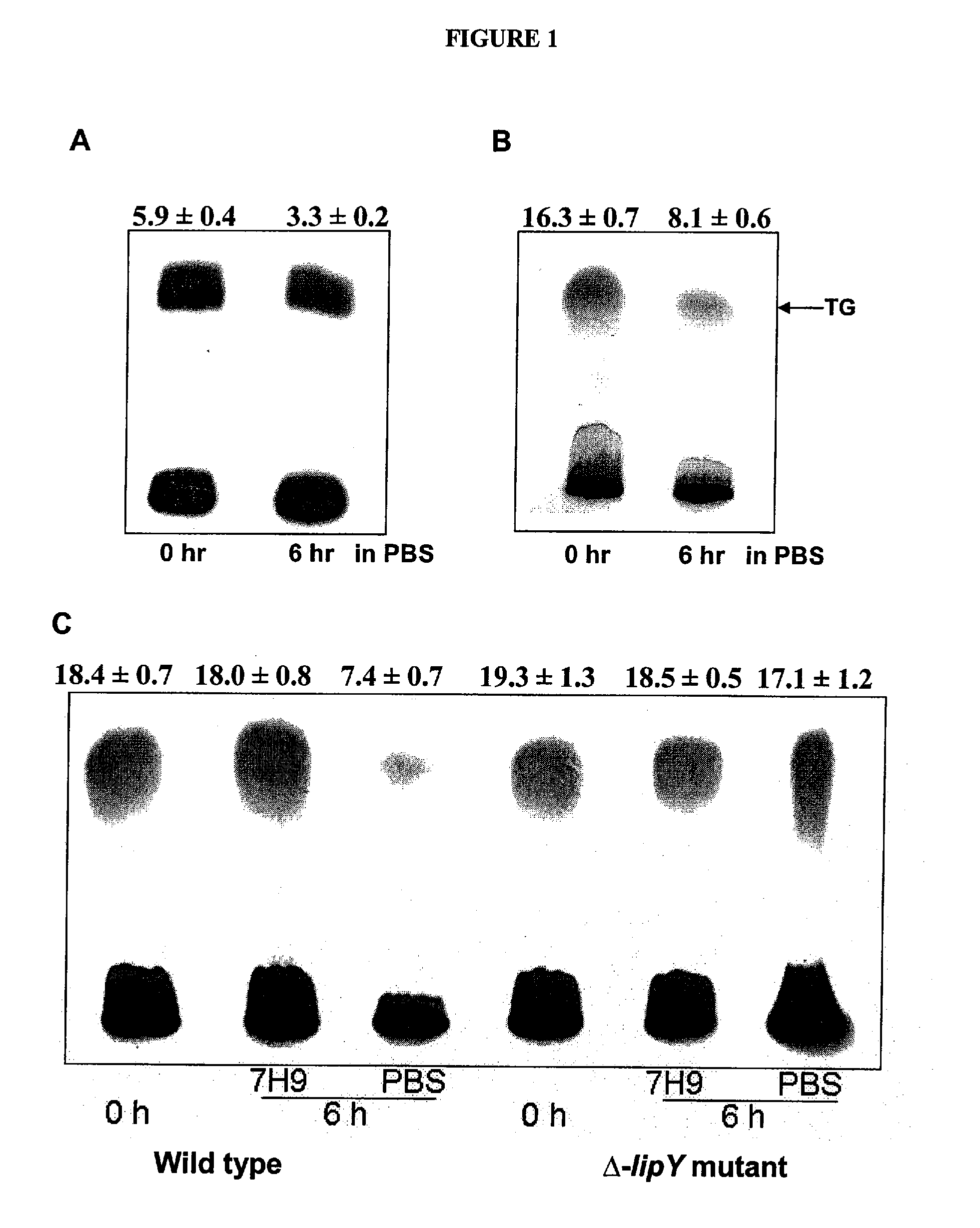
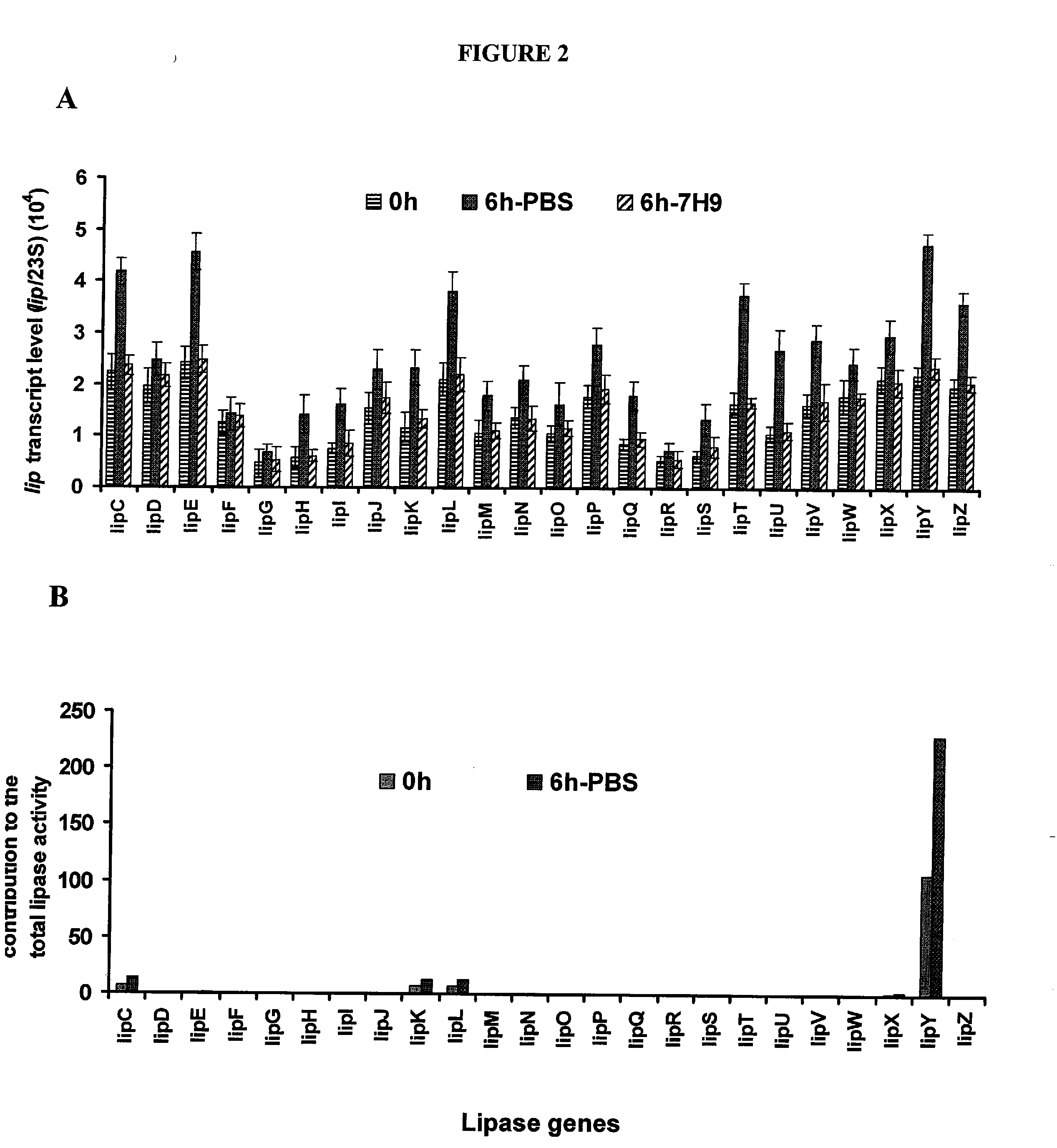
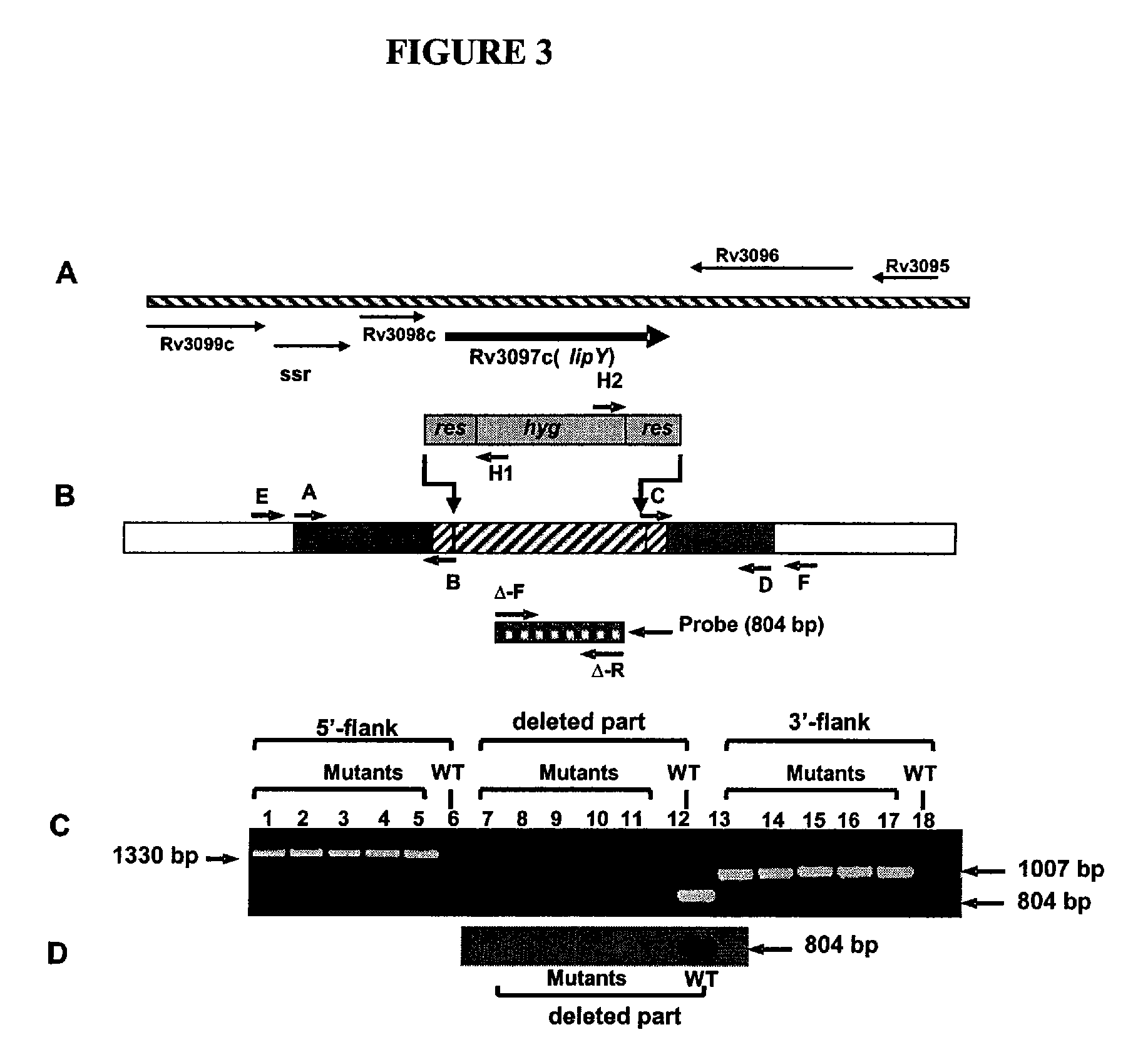
Targeting of long chain triacylglycerol hydrolase gene for tuberculosis treatment
Disclosed herein are novel methods for screening for compounds useful in treating or preventing tuberculosis. In exemplary embodiments, screening methods are based on the implementation or manipulation of triacylglycerol hydrolase like polypeptides or polynucleotides encoding the same. The methods are useful in identifying agents active against TB infection.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
4-Pyrimidinylamino-benzenesulfonamide derivatives and their use for the inhibition of polo-like kinase 1 (PLK1) for the treatment of cancer and their use for the treatment of bacterial infections
InactiveUS20150368209A1Antibacterial agentsOrganic active ingredientsActive agentResistant tuberculosis
The present invention relates to 4-pyrimidinylamino-benzenesulfonamide derivatives of general formula (I) and pharmaceutically acceptable salts, solvates, hydrates, regioisomeric and polymorphic forms thereof, processes for manufacturing of them, the use of them, as well as pharmaceutical compositions containing at least one of them as pharmaceutically active agent(s) together with pharmaceutically acceptable carrier, excipient and / or diluents, especially for the inhibition of polo-like kinases (PLKs) and the treatment of cancer. Said 4-pyrimidinylamino-benzenesulfonamide compounds have been also identified as new drug candidates for the prevention and / or treatment of infectious diseases like bacterial diseases e.g. tuberculosis, including the currently multidrug-resistant tuberculosis (MDR-TB), extensively drug-resistant tuberculosis (XDR-TB) as well as for preventing tuberculosis.
Owner:VICHEM CHEM KUTATO
Compositions and methods
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Method for the treatment or prophylaxis of tuberculosis
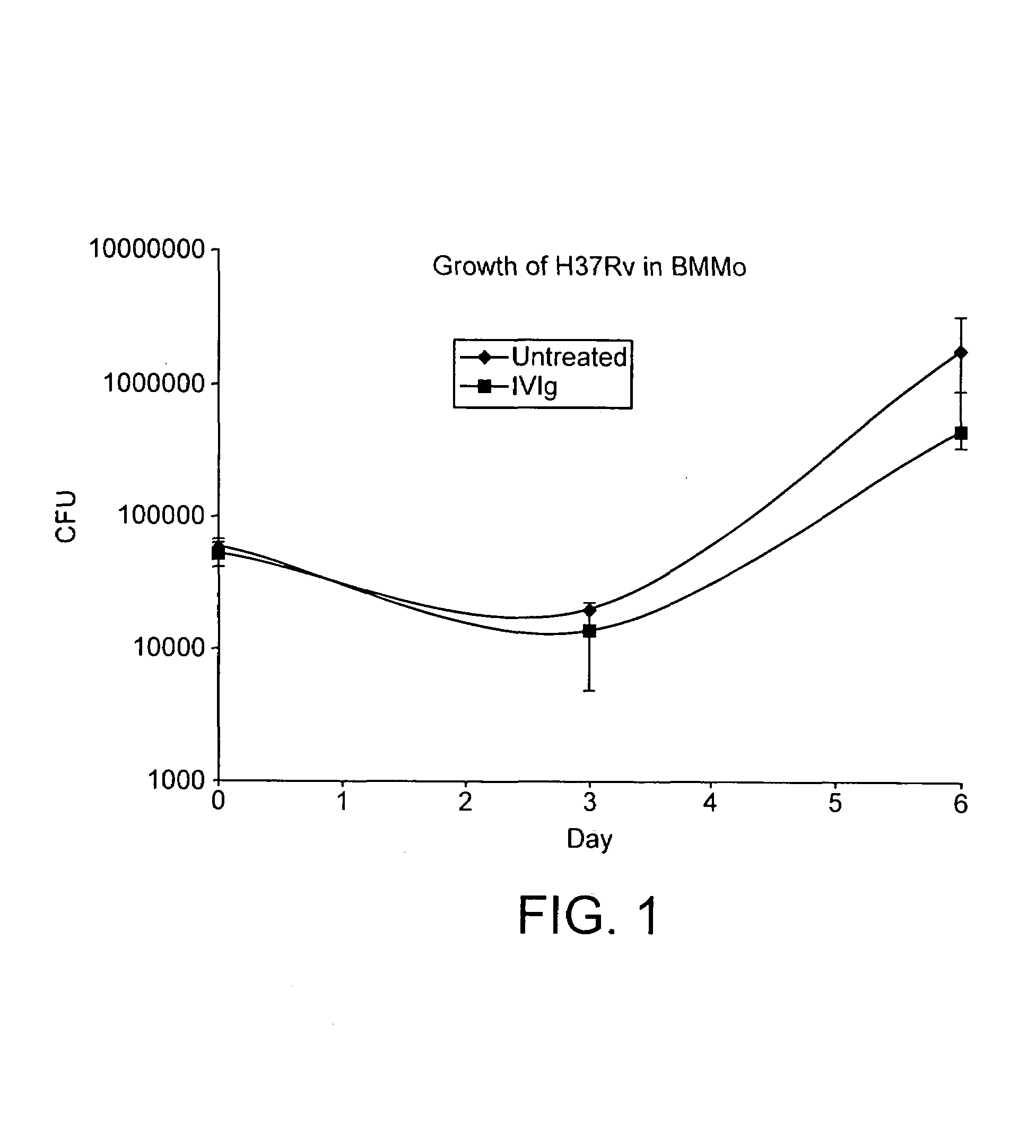
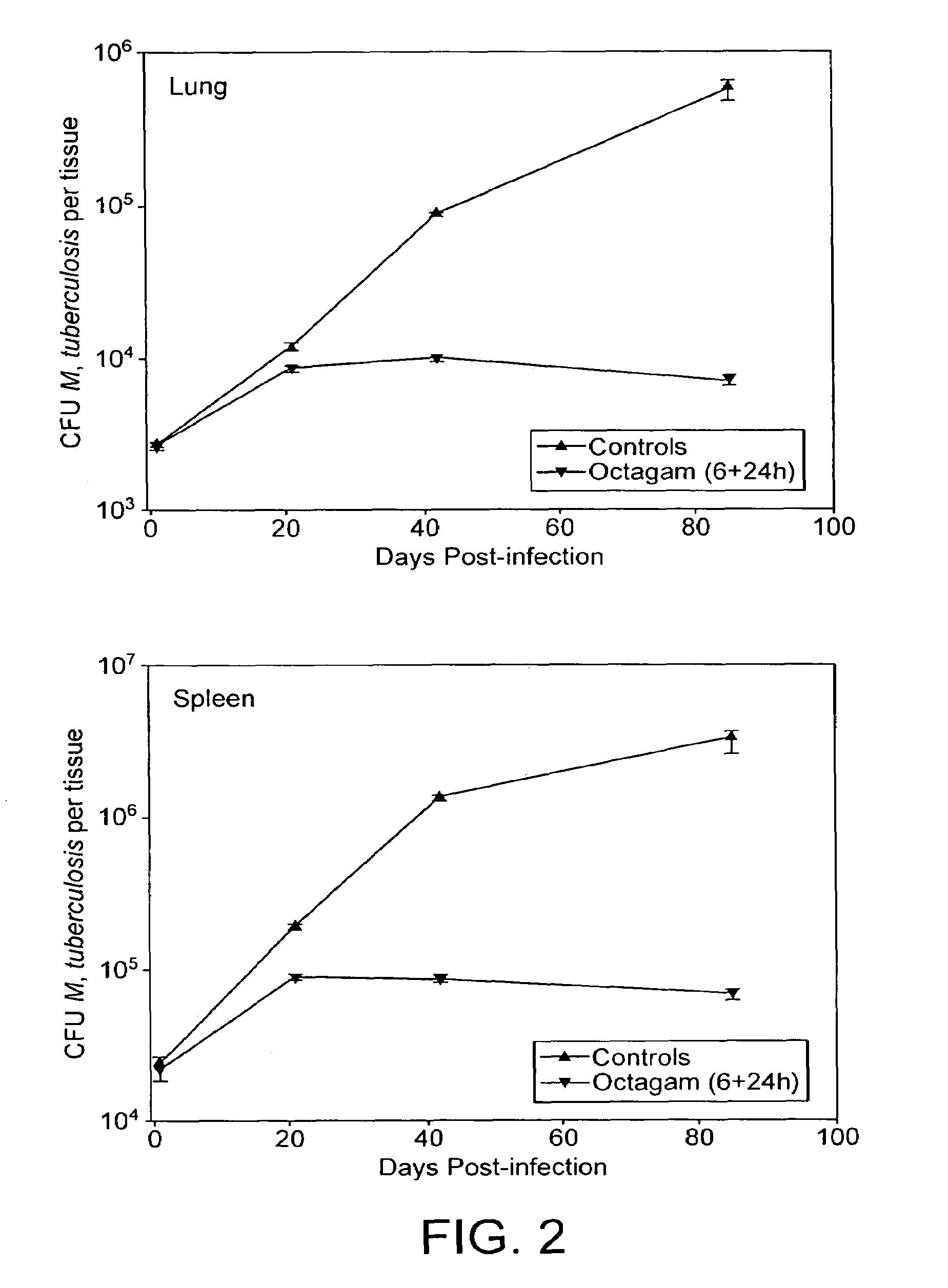
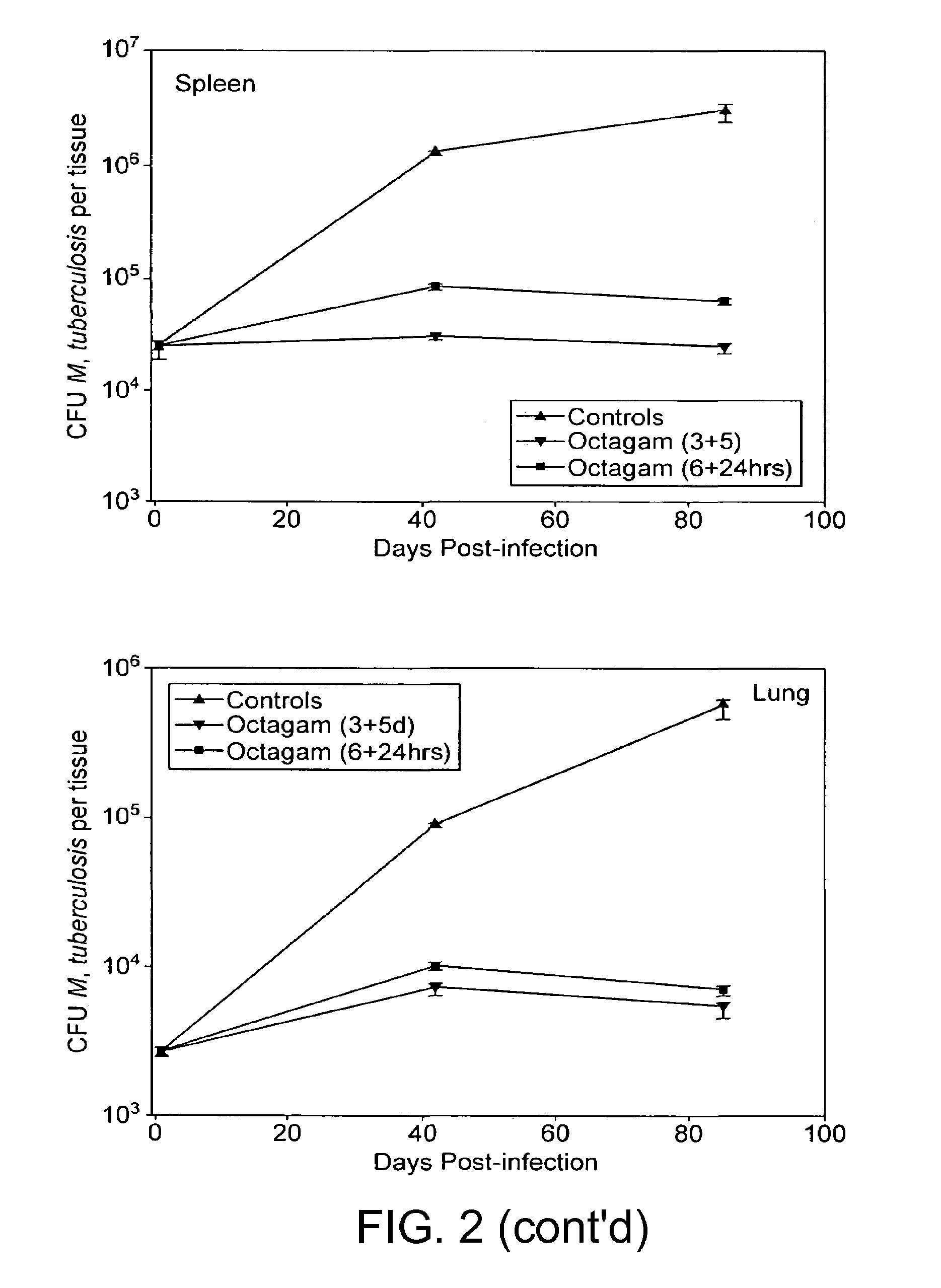
InactiveUS7687060B2Colony countStimulate immune responseSerum immunoglobulinsImmunoglobulins against bacteriaTuberculosisTuberculosis prophylaxis
Owner:MEDICAL RESEARCH COUNCIL
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS20040013677A1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAntigenMycobacterial antigen
The present invention relates to fusion proteins of Mycobacterium tuberculosis antigens. In particular, it relates to two fusion proteins, each of which contains three individual M. tuberculosis antigens, and a fusion protein of two M. tuberculosis antigens, their coding sequences, and methods for their use in the treatment and prevention of tuberculosis.
Owner:CORIXA CORP
Inactivated mycobacteria for oral use in the prevention of tuberculosis
An inactivated mycobacteria for oral use in the prevention of tuberculosis, which are administered using a multi-dose regimen and with a reduced time interval between doses, such as to induce a tolerance-building to infection by the tubercle bacillus. The inactivated bacteria can be used with the aforementioned regimen to control the progression of the infection from a latent state to active tuberculosis.
Owner:FUNDACIO INST PER A LA INVESTIGACIO & CONCIES DE LA SALUT GERMANS TRIAS I PUJOLIGTP +1
Tuberculosis treating and preventing agent
InactiveCN101010089AAntibacterial agentsHydroxy compound active ingredientsTuberculosis treatedOncology
The possibility of using betulin and birch bark containing said betulin for treating and preventing tuberculosis is disclosed.
Owner:OOO百日瑞有限公司
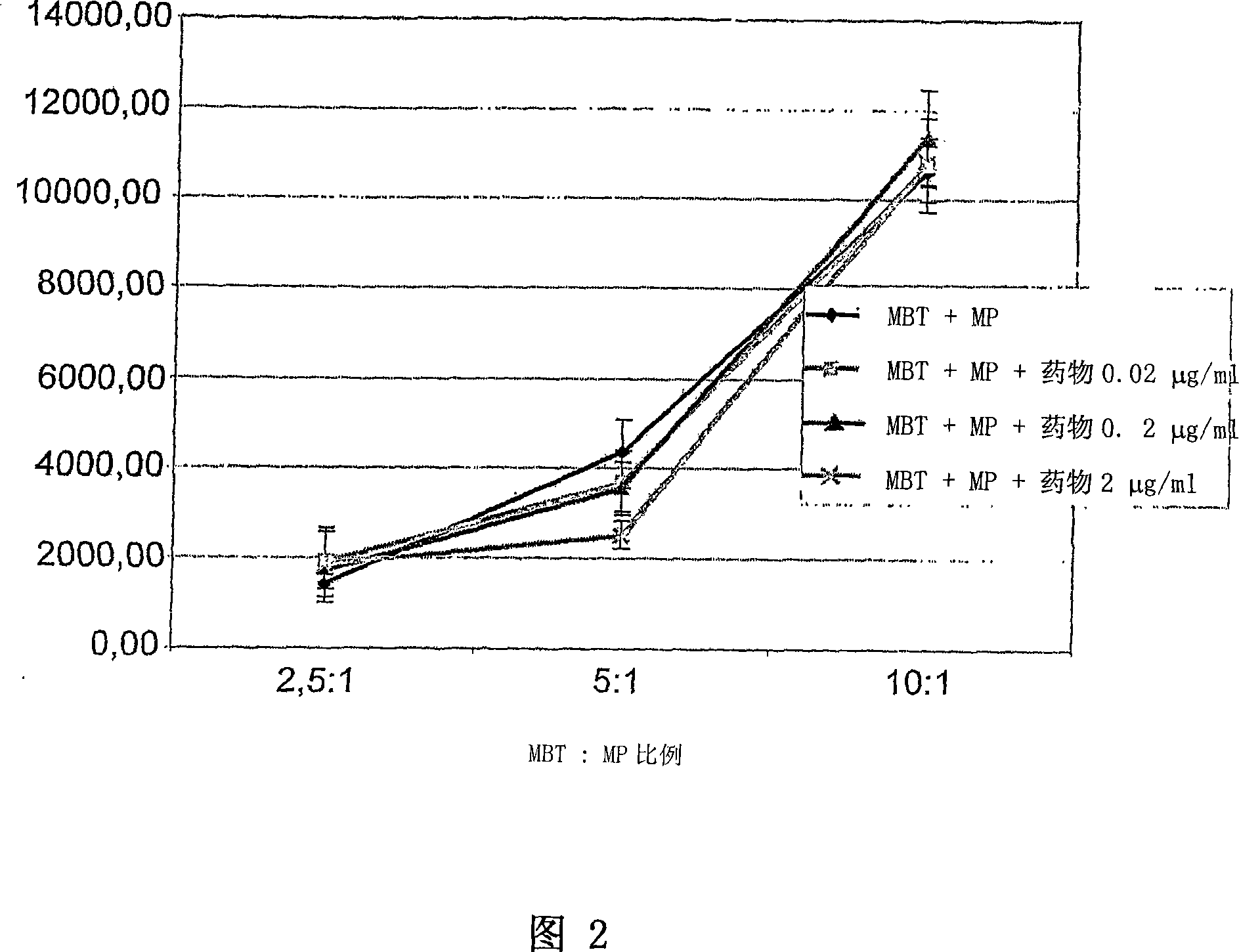
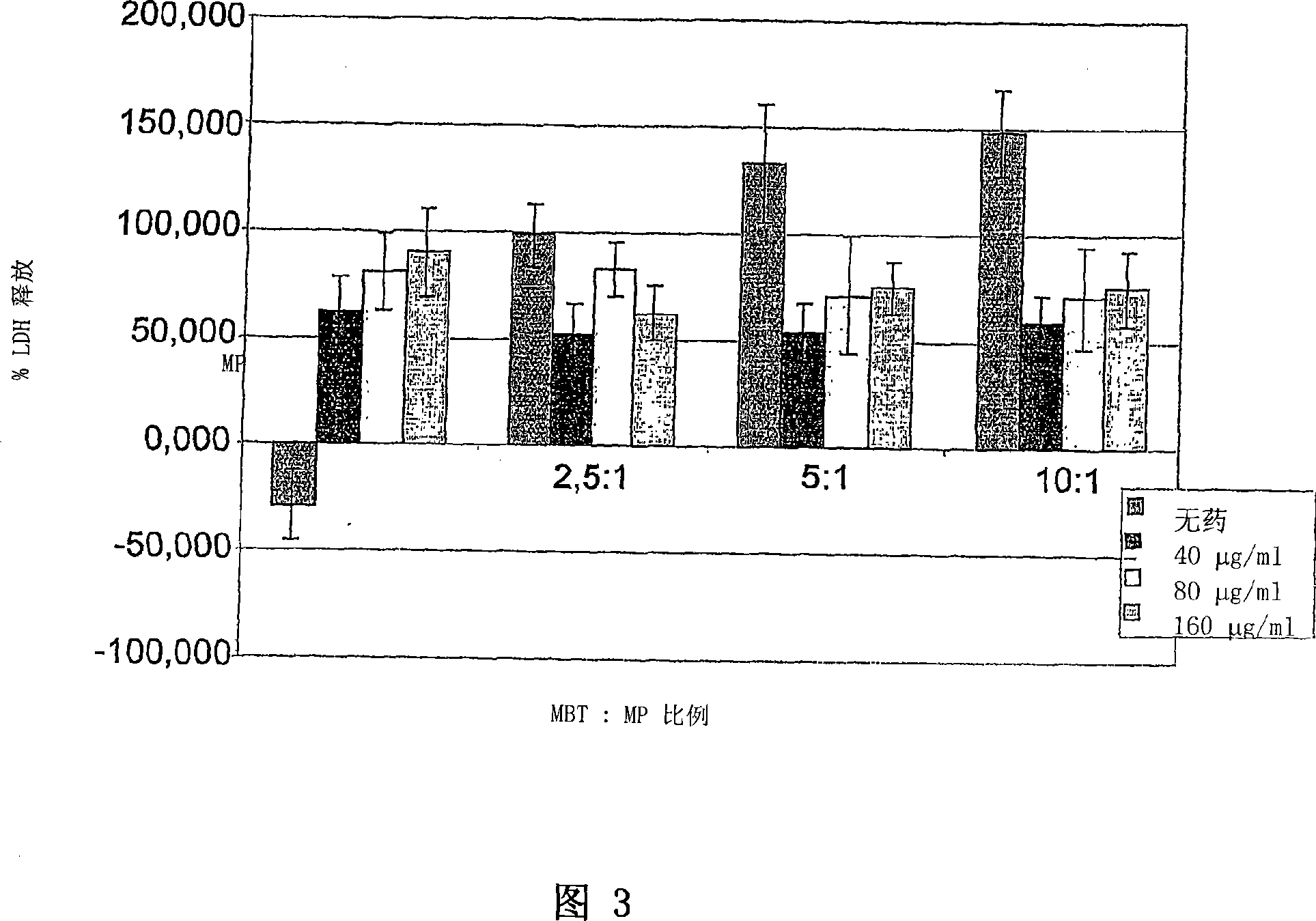
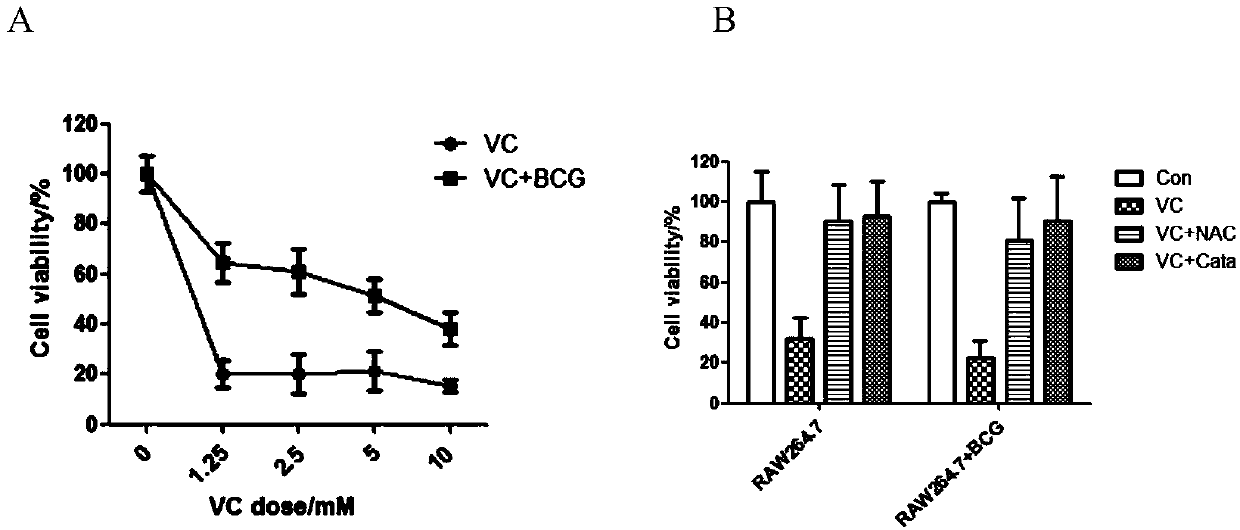
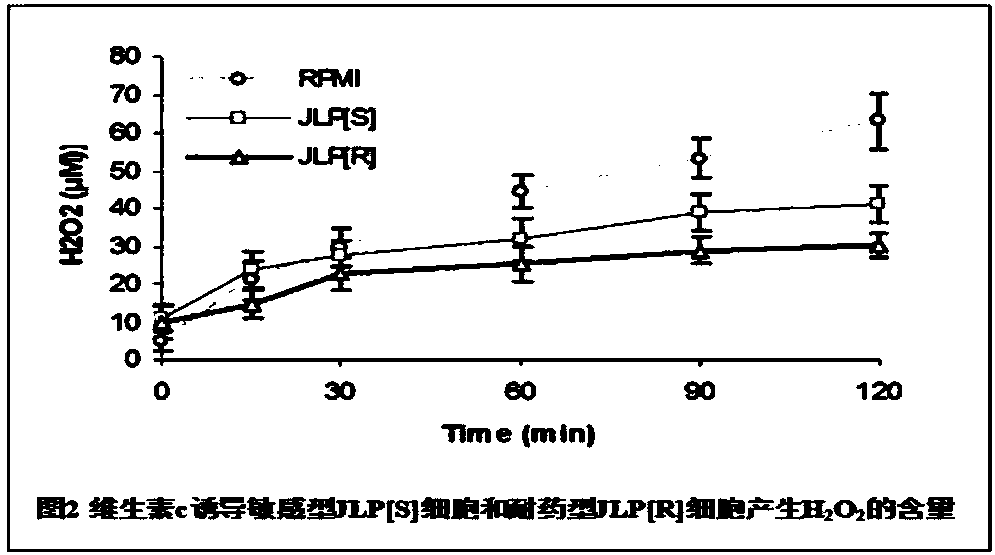
Application of vitamin C in preparation of medicines for treating and preventing tuberculosis
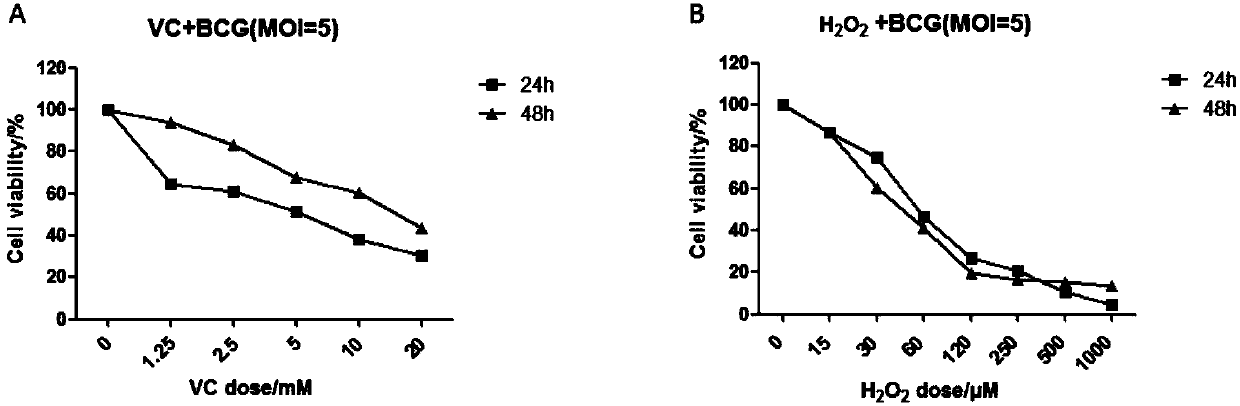
PendingCN108324712AAchieve therapeutic effectAntibacterial agentsOrganic active ingredientsIntervention trialPectobacterium
The invention belongs to the field of biomedicines, and relates to an application of vitamin C (ascorbic acid), as a single component, in preparation of medicines for treating and preventing tuberculosis. Through cytobiology and pharmacology experiments with combination of animal experiments, anti-tuberculosis pharmaceutical concentration of the vitamin C is determined; and on the basis of regulatory mechanism on oxidative stress status of mycobacterium tuberculosis after infection by macrophages, interference test is carried out, wherein a result proves that high-dose vitamin C can kill the mycobacterium tuberculosis, so that inhibition effect is achieved through NAC and catalase; the vitamin C treats the macrophages to generate H2O2, wherein functional effect is achieved with the signalchannel being same as H2O2; large-dose vitamin C kills mycobacteria, wherein inhibition function is achieved through a glutathione precursor NAC and CAT; high-dose vitamin C can infect the TNF-alpha signal pathway induced by RAW 264.7 cells via mediation of Bacillus Calmette-Guerin vaccine and H37Rv, thus achieving sterilization effect and influence on body immunity. The invention provides effective treatment strategy for clinical therapy and drug resistance of tuberculosis and tuberculosis in incubation period.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Novel compositions and methods
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
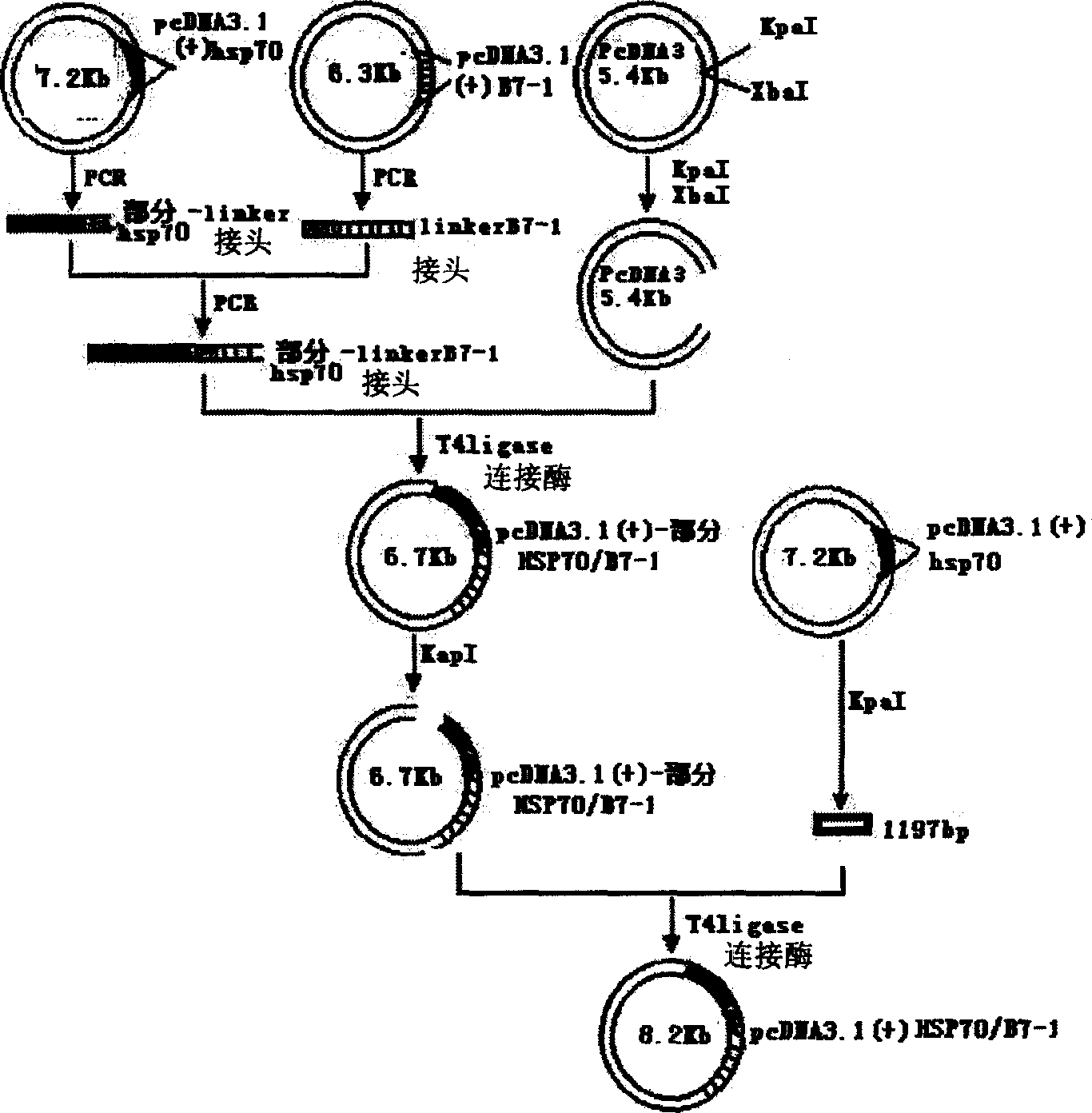
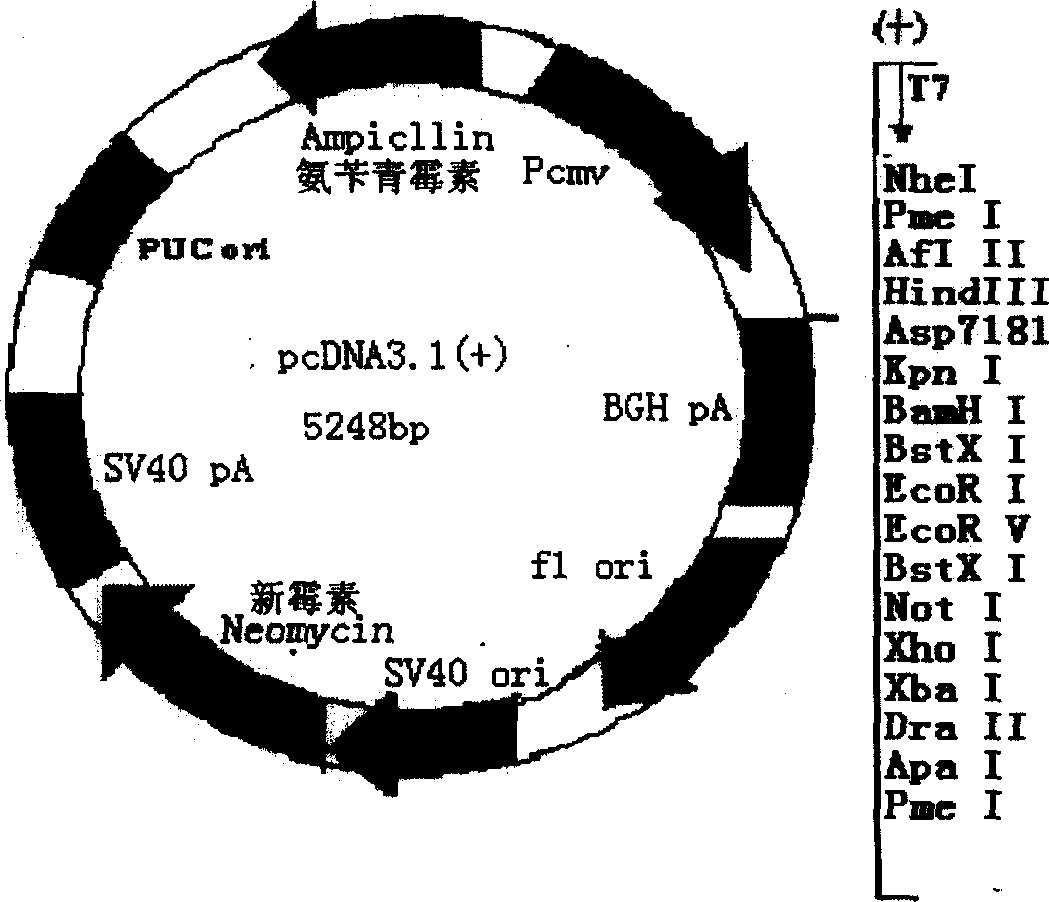
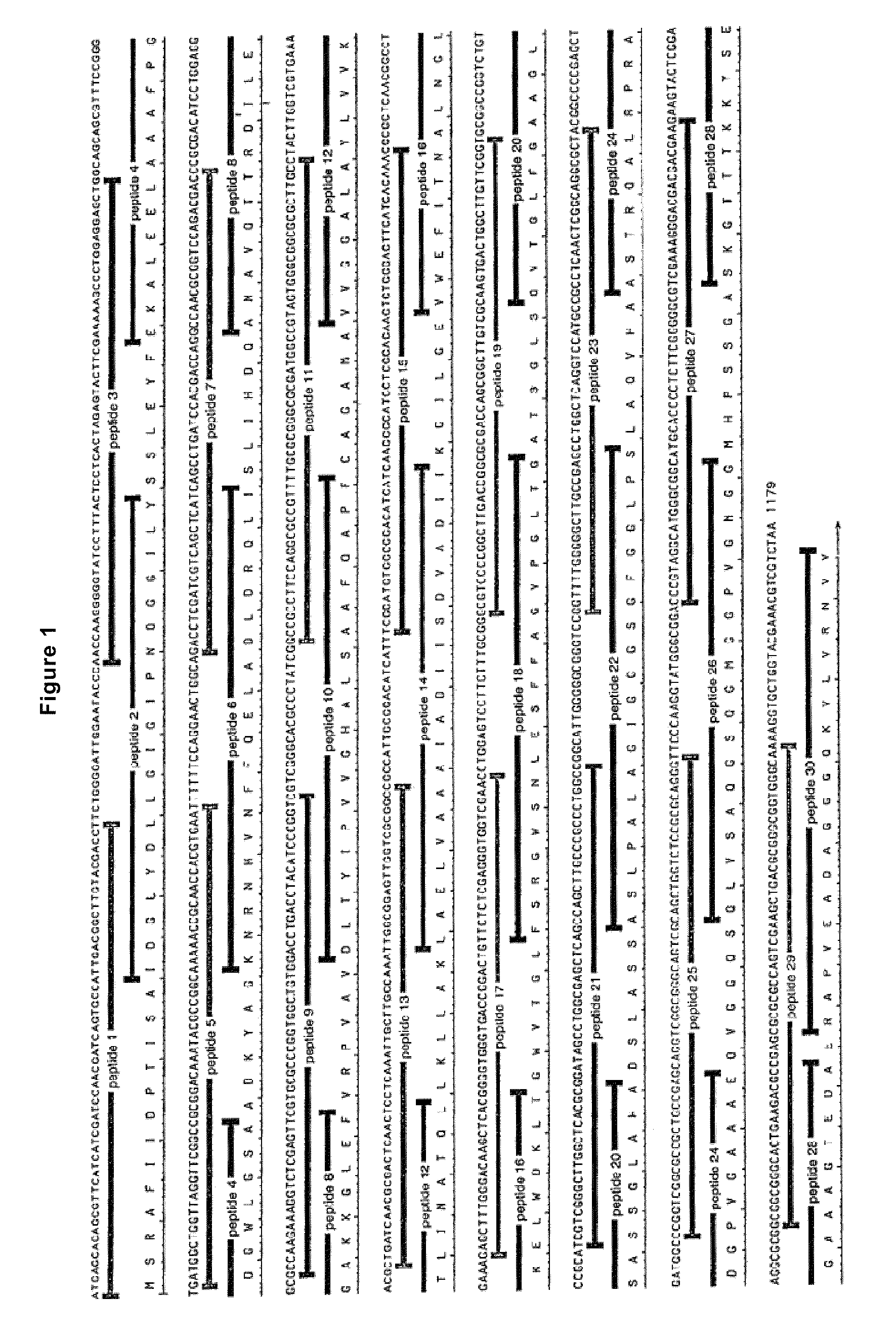
Mosaic type DNA vaccine in use for preventing tuberculosis and immunological therapy
InactiveCN1284854CExert biological functionAntibacterial agentsBacterial antigen ingredientsOpen reading frameNucleotide
The present invention relates to a chimeric DNA vaccine for preventing tuberculosis and immunotherapy, comprising a full-length open reading frame of the nucleotide sequence shown in SEQ ID NO.1, or a conservative variant thereof, in SEQ ID NO.1 It comprises mycobacterium hsp70 gene and human B7-1 gene, and a linker sequence is designed between the two genes to connect them. The vaccine of the invention can be used for tuberculosis caused by Mycobacterium tuberculosis; or express the corresponding protein in eukaryotic cells in vitro for preventing and treating tuberculosis. It can also be used as an immune enhancer for corresponding viral diseases and tumor immunotherapy.
Owner:钟森 +3
Modified antigens
Modified Rv3616c proteins and their use as medicaments, particularly for the prevention of reactivation of tuberculosis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Application of bovine beta defensin 5 as novel mucosal immunologic adjuvant
ActiveCN112891529AAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsTGE VACCINE
The invention provides application of bovine beta defensin 5 as a mucosal immunologic adjuvant in prevention of tuberculosis. A C57BL / 6 mouse is immunized by expressing and purifying recombinant protein bovine beta defensin 5, combining polyinosinic-polycytidylic acid (PolyI: C) and a mycobacterium tuberculosis protein subunit vaccine AH in a nasal drop mode, and the effect of promoting the immune effect of the protein subunit vaccine AH is proved. Compared with polyinosinic-polycytidylic acid and AH immunization, the bovine beta defensin 5 can promote antigen AH specific cellular immunization and humoral immunization levels and promote the protective effect of subunit vaccine AH on resisting bovine mycobacterium infection.
Owner:CHINA AGRI UNIV
Tuberculosis candidate vaccine fusion protein
ActiveCN111518180AImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to construction of a chimeric protein Dodecin-ESAT6 candidate vaccine of an ESAT6 gene and an RV1498a gene of mycobacterium tuberculosis, and immunogenicity research of the chimeric protein Dodecin-ESAT6 candidate vaccine, i.e., through a genetic engineering technique, sequences of the ESAT6 gene and the RV1498a gene of the mycobacterium tuberculosis are inserted into sequences of the same escherichia coli plasmid pET28a, and a recombinant plasmid rpET28a:RV1498a-ESAT6 is constructed; and by a heat shock transforming method, carriers are guided into escherichia coli, andfusion protein Dodecin-ESAT6 is expressed. The expressed fusion protein Dodecin-ESAT6 is constructed, and the immunogenicity is better than that of ESAT6. The invention provides a preparation processof the fusion protein Dodecin-ESAT6, researches the immunogenicity of the fusion protein Dodecin-ESAT6, and belongs to the field of genetic engineering and the field of tuberculosis vaccines. Occurrence and propagation of tuberculosis can be effectively prevented.
Owner:SICHUAN UNIV
Tuberculosis rv2386c protein, compositions and uses thereof
The present invention is directed to a polypeptide which comprises: (i) an Rv2386c protein sequence; (ii) a variant of an Rv2386c protein sequence; or (iii) an immunogenic fragment of an Rv2386c protein sequence. In other aspects the invention is directed to associated polynucleotides, fusion proteins and methods for the treatment or prevention of tuberculosis.
Owner:GLAXO GROUP LTD +1
Liposome formulation suitable for treating or preventing tuberculosis
ActiveUS20150050327A1Suitable for preparationAntibacterial agentsBacterial antigen ingredientsSucroseMedicine
The invention provides liposome formulations comprising fragments from a Mycobacterium tuberculosis-complex strain, it also provides a Mycobacterium tuberculosis-complex strain, fragments of which may be incorporated into selected embodiments of the liposome formulation. The invention further provides suspensions and pharmaceutical compositions comprising the liposome formulations. Furthermore, it discloses the use of the liposome formulations for use in a method of treatment of the human or animal body by therapy, in particular for use in a method of treating or preventing tuberculosis, such as in preventing latent tuberculosis or in tuberculosis prophylaxis, optionally in combination therapy. The formulation of this invention contains sucrose and / or has a lower average particle size than conventional liposome-based agents of tuberculosis therapy, resulting in higher bioavailability and efficiency.
Owner:ARCHIVEL FARMA SL
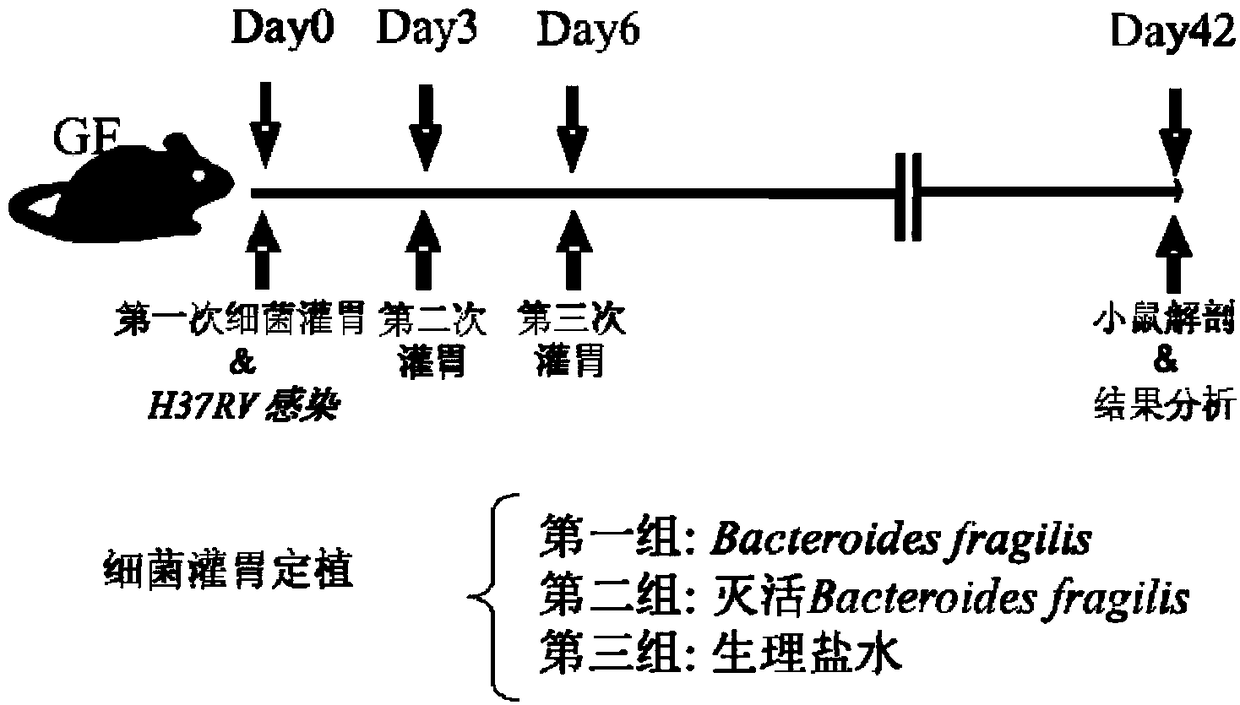
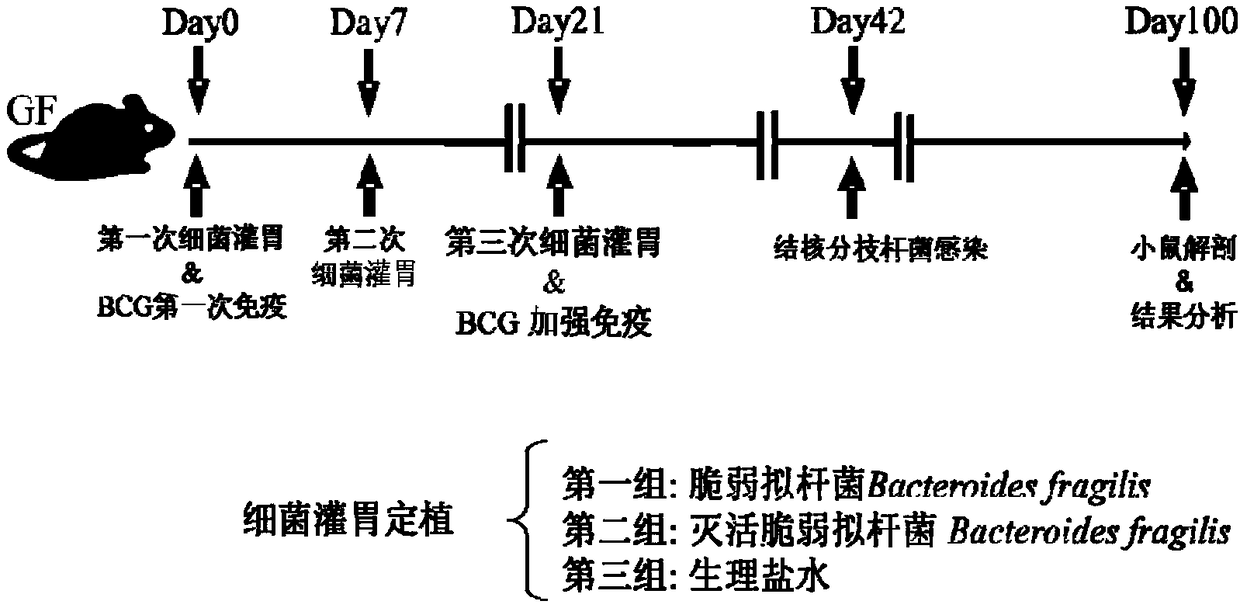
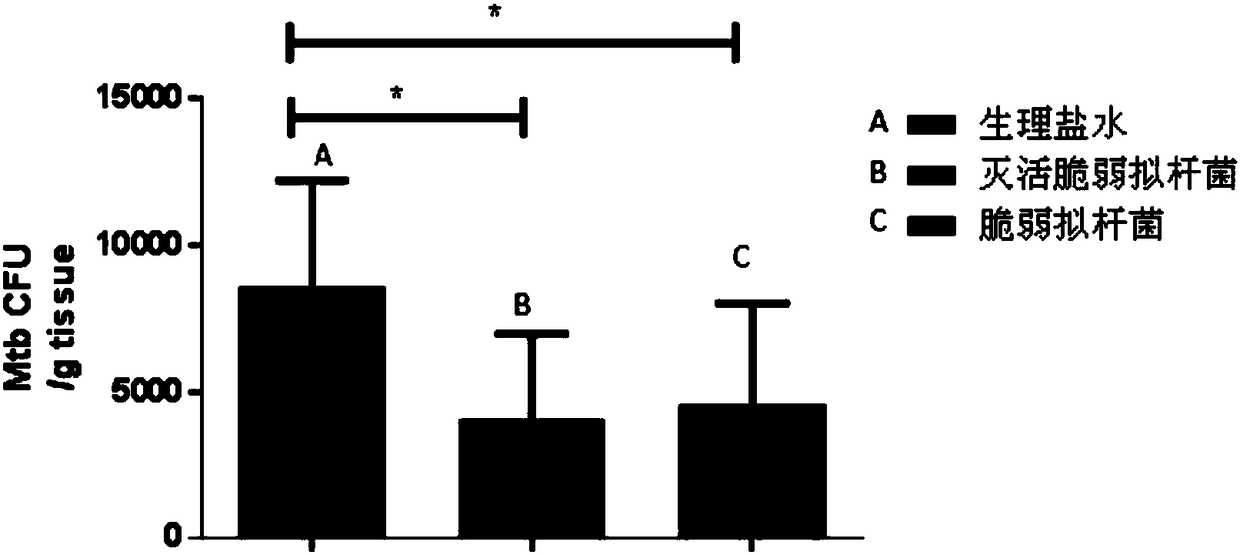
Use of Bacteroides fragilis in the preparation of drugs for the treatment and prevention of tuberculosis
InactiveCN109200063AGood immune protectionEnhanced inhibitory effectAntibacterial agentsUnknown materialsFood additiveMedicine
The invention belongs to the biomedical field, in particular to the use of Bacteroides fragilis in the preparation of drugs for the treatment and prevention of tuberculosis. The invention also provides the application of Bacteroides fragilis in the preparation of pharmaceutical compositions, foods, health products and food additives for treating and / or preventing tuberculosis. The Bacteroides fragilis provided by the invention can effectively treat and / or prevent tuberculosis and enhance the immune protection effect of tuberculosis bacillus Calmette-Guerin vaccine (BCG), and has wide application prospect.
Owner:REVAISSANT SHENZHEN BIOSCIENCES CO LTD
Vaccine and combination medicine for preventing tuberculosis, method for preparing vaccine and application
ActiveCN109078177AStrong ability to eradicate Mycobacterium tuberculosisImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsAdjuvantSingle type
The invention discloses vaccine and a combination medicine for preventing tuberculosis, a method for preparing the vaccine and application, and belongs to the field of medicines for treating tuberculosis latent infection. By the aid of the vaccine with mycobacterium microbial compound adjuvants for preventing the tuberculosis, the problems of ineffectiveness or poor effects of existing subunit vaccine for treating tubercle bacillus latent infected population, diversified adjuvants, hypersensitivity and Koch reaction risks can be pertinently solved. The vaccine with mycobacterium microbial compound adjuvants for preventing the tuberculosis comprises Ag85b proteins, ESAT6-CFP10 proteins and mycobacterium vaccae thallus extract. The vaccine, the combination medicine, the method and the application have the advantages that the vaccine with the mycobacterium microbial compound adjuvants for preventing the tuberculosis is used for treating latent tuberculosis infection, and obvious effects can be realized; the vaccine only comprises a single type of adjuvants, and accordingly hypersensitivity and Koch reaction risks can be prevented.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +3
Tuberculosis compositions and methods of treating or preventing tuberculosis
The present disclosure provides fusion proteins comprising Mycobacterium tuberculosis (Mtb) antigens, nucleic acid molecules encoding the same, vectors comprising nucleic acid molecules, compositionscomprising the same, and methods of eliciting an immune response against tuberculosis.
Owner:INT AIDS VACCINE INITIATIVE INC
A kind of vaccine for preventing tuberculosis and combination drug and preparation method and application
ActiveCN109078177BSide effects are rareMinor side effectsAntibacterial agentsBacterial antigen ingredientsAdjuvantLatent tuberculosis
The invention discloses a vaccine for preventing tuberculosis, a combined medicine, a preparation method and an application, and belongs to the field of medicines for treating latent infection of tuberculosis. Aiming at the problems that the existing subunit vaccines are ineffective or poor in treating people with latent tuberculosis infection, many types of adjuvants are used, and there are risks of hypersensitivity reactions and Koch reactions, the present invention provides a prevention method containing a mycobacterial microbial compound adjuvant Tuberculosis vaccine, which comprises Ag85b protein, ESAT6-CFP10 protein and Mycobacterium vaccae cell extract. The tuberculosis prevention vaccine containing the mycobacterial microbial compound adjuvant of the present invention is used for treating latent tuberculosis infection, and the effect is remarkable.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +3
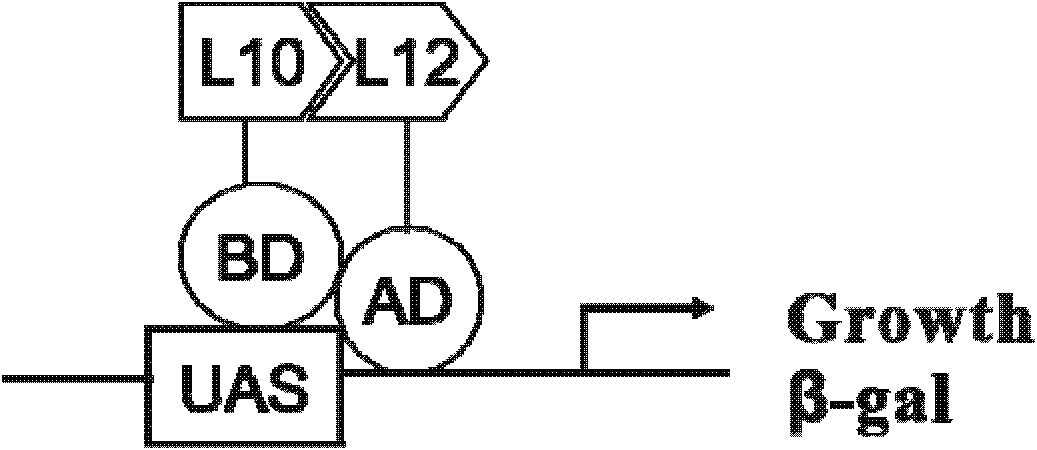
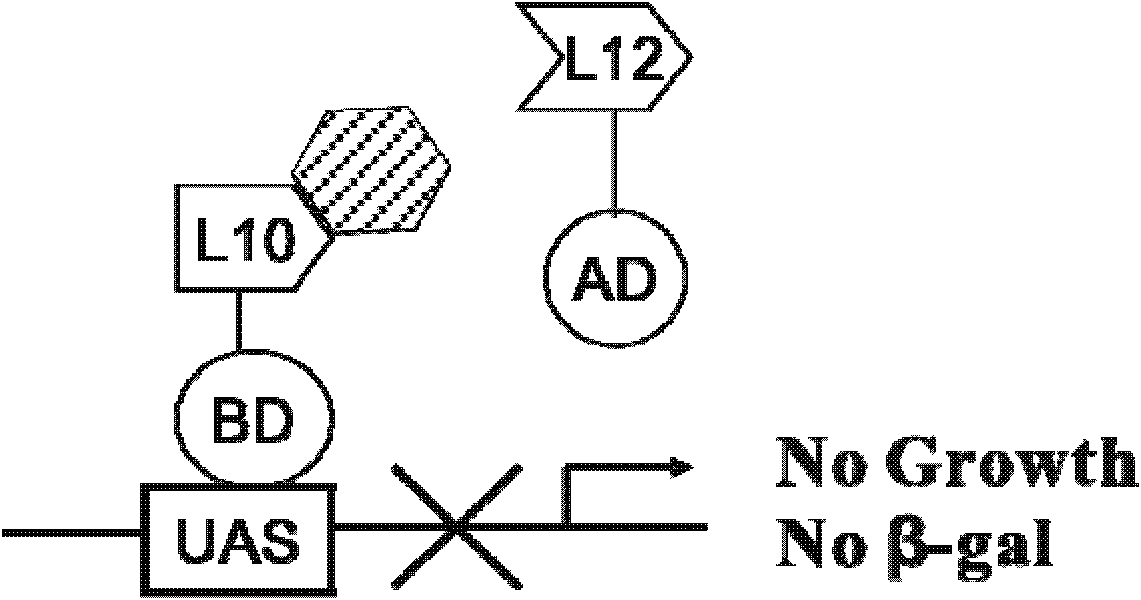
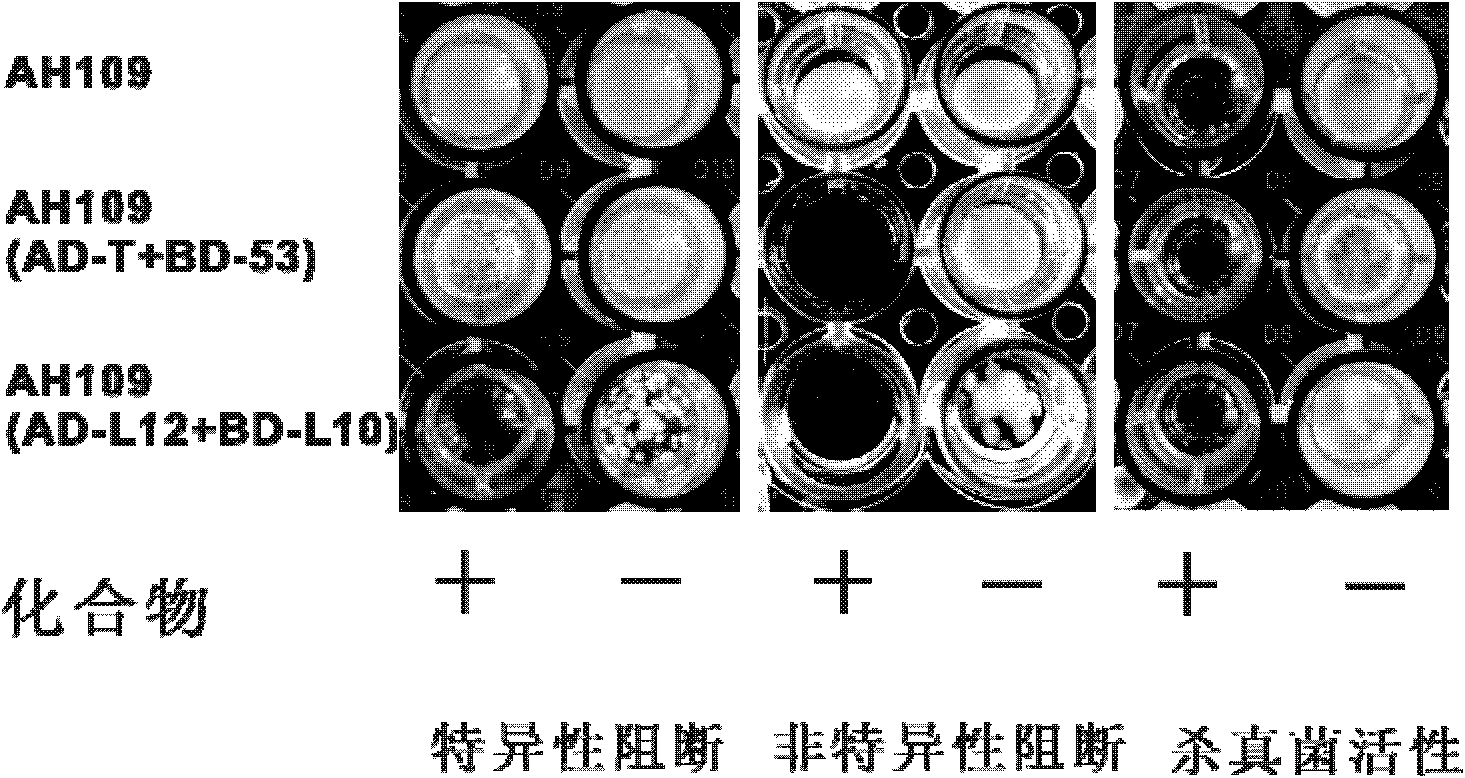
Cell model for screening inhibitor of mycobacterium tuberculosis ribosomal protein L12 and L10 and application thereof
ActiveCN102719368BCheap purificationSimplify the screening processAntibacterial agentsHalogenated hydrocarbon active ingredientsDNA-binding domainRibosomal protein
Provided are a cell model and screening method for screening inhibitors of the tubercle bacillus. Provided is a cell which expresses the tubercle bacillus proteins L12 and L10, wherein the L12 encoding gene and the transcription activation domain of its transcription factor are located in one expression vector, and the L10 encoding gene and the DNA binding domain of its transcription factor are located in another expression vector. The present invention also relates to the use of the compound shown in formulae I or II in preparing a blocker of the interaction between proteins L12 and L10 of tubercle bacillus, an anti-tubercle bacillus drug, or a drug for treating and / or preventing tuberculosis.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Candidate component of tuberculosis vaccine and vaccine containing the component
ActiveCN107281465BReflect release functionHigh compliance rateAntibacterial agentsBacterial antigen ingredientsPharmaceutical drugPulmonary tuberculosis
The invention belongs to the fields of immunology and molecular biology and relates to a tuberculosis vaccine candidate component and a vaccine containing the tuberculosis vaccine candidate component. The invention further relates to a molecular marker diagnosing tuberculosis. Specifically, the invention relates to application of a protein MYVA_1927 in preparation of drugs for treating and / or preventing tuberculosis or drugs for inhibiting mycobacterium tuberculosis, or application in preparation of drugs for diagnosing tuberculosis. The invention further relates to a vaccine containing the protein MYVA_1927. The protein MYVA_1927 has excellent coincidence rate and response intensity and has potential of being applied to preparing the tuberculosis vaccine. In addition, the protein MYVA_1927 can further serve as an immunologic diagnosis molecular marker of tuberculosis (such as pulmonary tuberculosis).
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com