Vaccine and combination medicine for preventing tuberculosis, method for preparing vaccine and application
A tuberculosis and vaccine technology, applied in the field of combined drugs and applications for the prevention of tuberculosis, can solve the problems of hypersensitivity reaction and Koch reaction risk, many types of adjuvants, poor effect, etc., so as to reduce the risk of Koch reaction, reduce the treatment time, good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
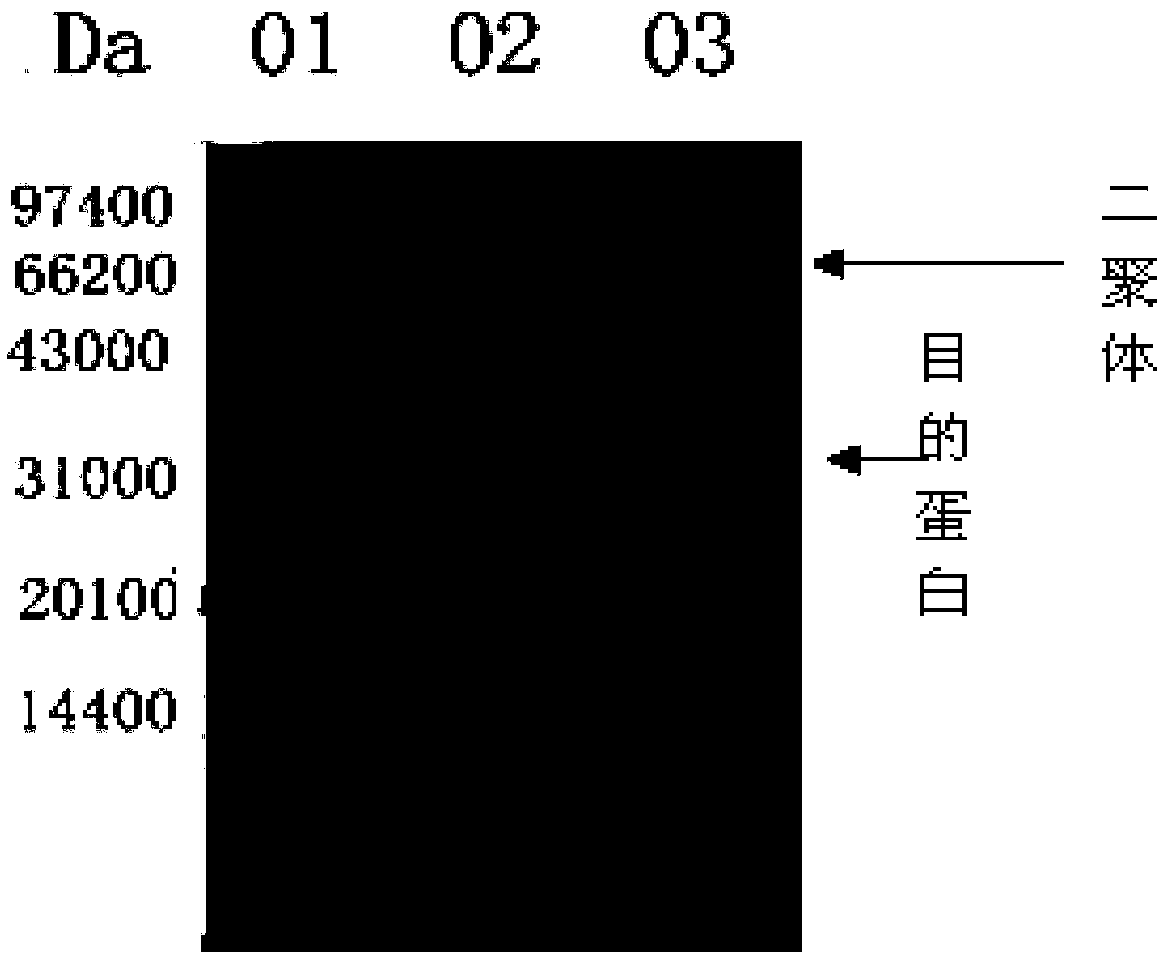
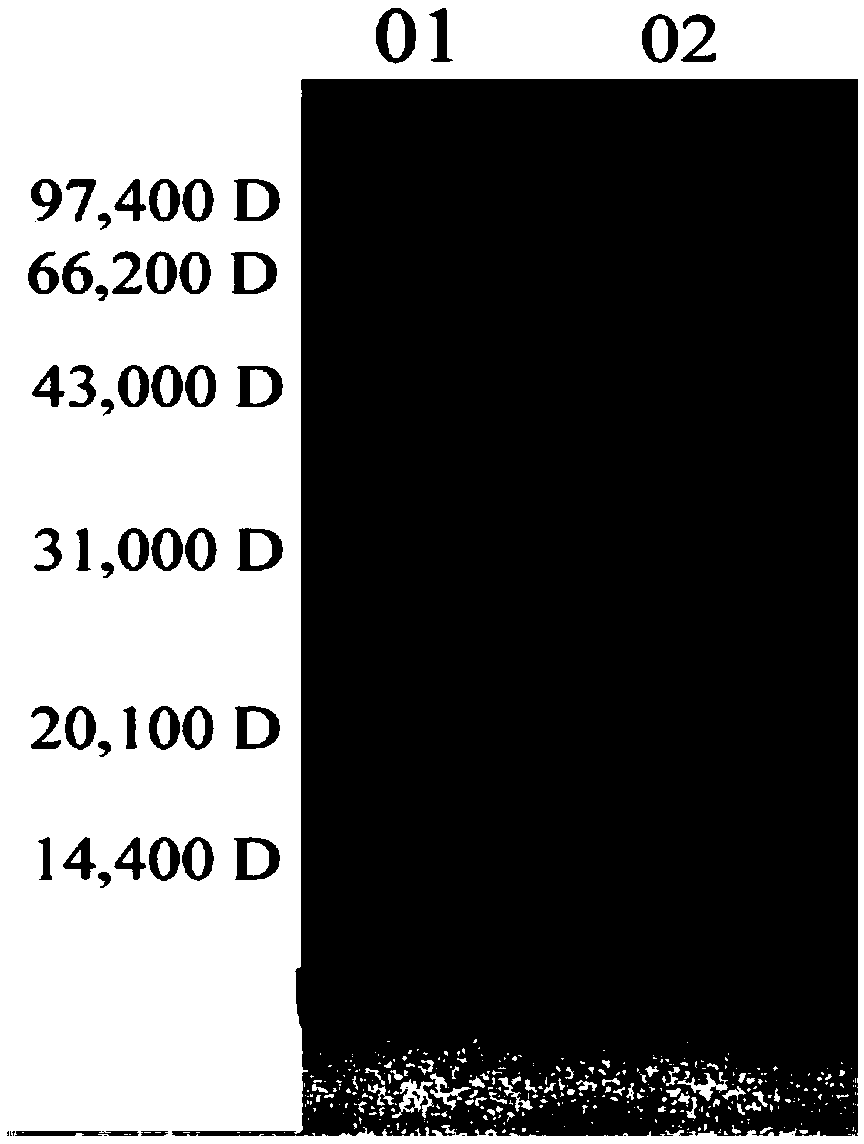
[0073] Preparation of Tuberculosis Vaccine Containing Mycobacterium-like Microorganism Compound Adjuvant
[0074] 1. Preparation process of Ag85b protein
[0075] Find the Ag85b protein gene sequence from GeneBank, and design primers as follows: See Table 1.
[0076] Table 1 Primer sequences, restriction sites and protective bases of the target gene of Ag85b
[0077]
[0078] Remarks: The underlines are the cleavage sites of the endonucleases, and the parts in italics are the start codons.
[0079] The Ag85b gene was amplified by PCR using the whole genome of Mycobacterium tuberculosis H37Rv as a template. The PCR reaction system was 50 μL (ddH 2 O 30 μL, 10×Buffer 5 μL, dNTP 4 μL, P1 primer 4 μL, P2 primer 4 μL, DNA template 2.5 μL, DNA polymerase 0.5 μL), the amplification conditions are: 96°C pre-denaturation for 3 minutes; 96°C denaturation for 45 seconds, 62 Annealing at ℃ for 45 seconds, extending at 72℃ for 1 minute, a total of 30 cycles; extending at 72℃ for 7 m...
Embodiment 2
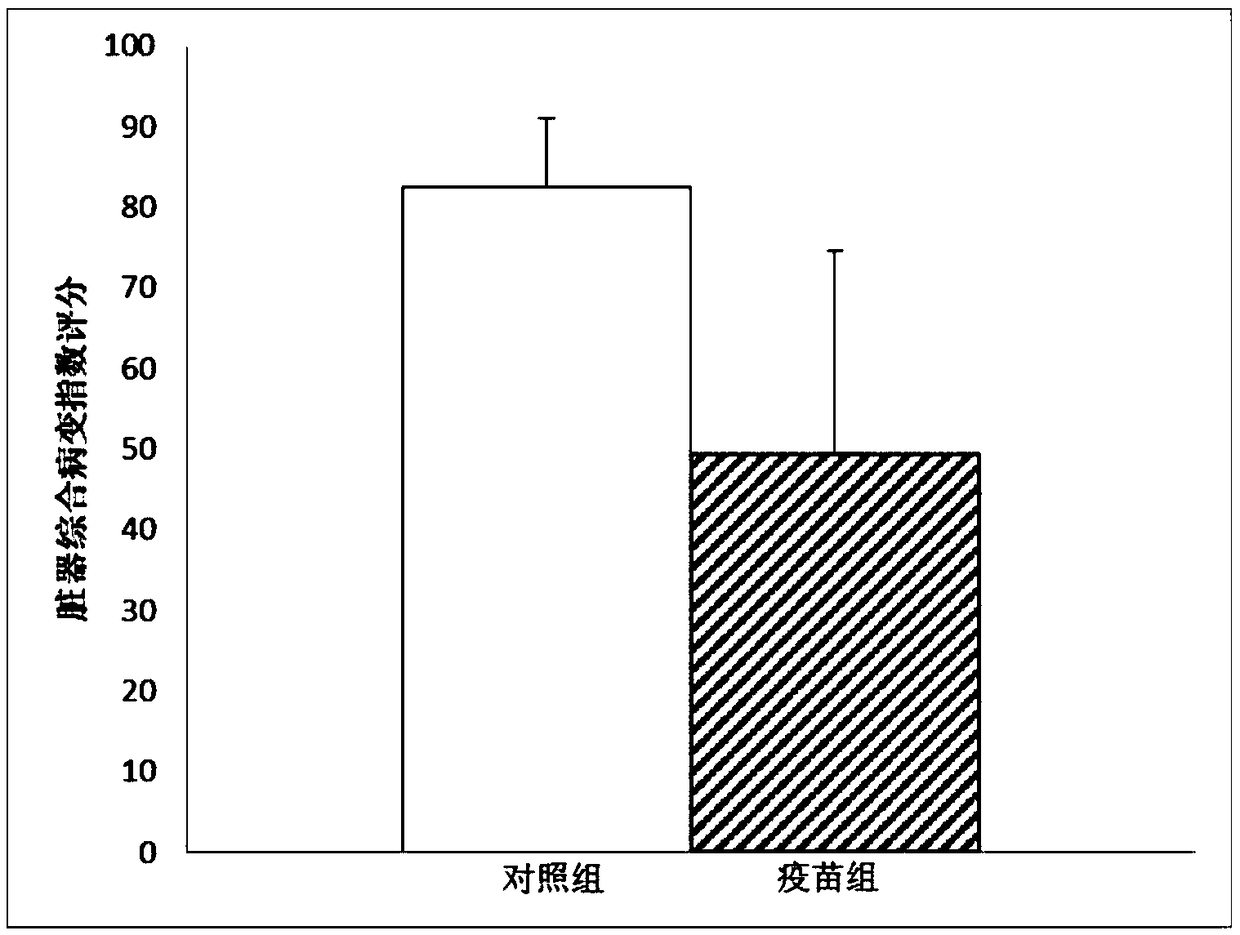
[0109] Animal Protection Test of Tuberculosis Vaccine Containing Mycobacterium-like Microorganism Compound Adjuvant
[0110] 1. Experimental method
[0111] Immunotherapy of guinea pigs infected with Mtb
[0112] Sixteen Hartley guinea pigs of SPF grade (300-350 g / guinea pig), half male and half male, were divided into two groups (vaccine group and control group), with 8 guinea pigs in each group. The guinea pigs of the vaccine group and the control group were subcutaneously challenged with 5.0×10 3 CFU Mtb, 4 weeks later, the vaccine group was injected with this vaccine for treatment, a total of 6 injections, with an interval of 1 week between each injection, and the dose of each injection was [Ag85b(10μg)+EC(10μg)+MV(22.5μg)] / 0.5mL / Only. The guinea pigs in the control group were injected with the same amount of normal saline as a control. Guinea pigs were dissected 12 weeks after Mtb infection. The comprehensive lesion index of liver, spleen and lung organs and the bac...
Embodiment 3
[0124] Comparative test of animal protection between tuberculosis vaccine containing mycobacterial compound adjuvant and subunit tuberculosis vaccine
[0125] 1. Experimental method
[0126] Immunotherapy of guinea pigs infected with Mtb
[0127] Sixteen Hartley guinea pigs of SPF grade (300-350 g / guinea pig), half male and half male, were divided into two groups (vaccine group and control group), with 8 guinea pigs in each group. The guinea pigs of the vaccine group and the control group were subcutaneously challenged with 5.0×10 3 CFU Mtb, 4 weeks later, the vaccine group was injected with this vaccine for treatment, a total of 6 injections, with an interval of 1 week between each injection, and the dose of each injection was [Ag85b(10μg)+EC(10μg)+MV(22.5μg)] / 0.5mL / Only. Guinea pigs in the control group were injected with the same amount of subunit tuberculosis vaccine ([Ag85b (10 μg) + EC (10 μg) + BCG-CpG-DNA (75 μg) + Al(OH) 3 (200μg)] / 0.5mL / only) as a control. Guinea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com