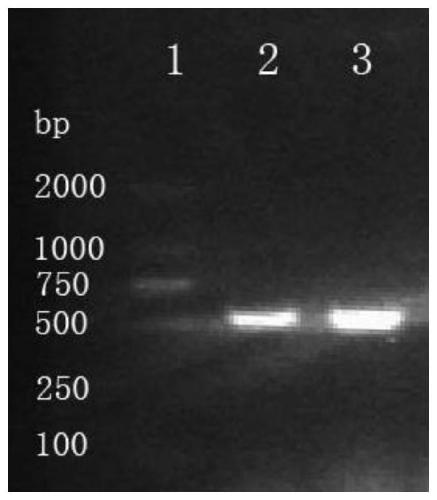
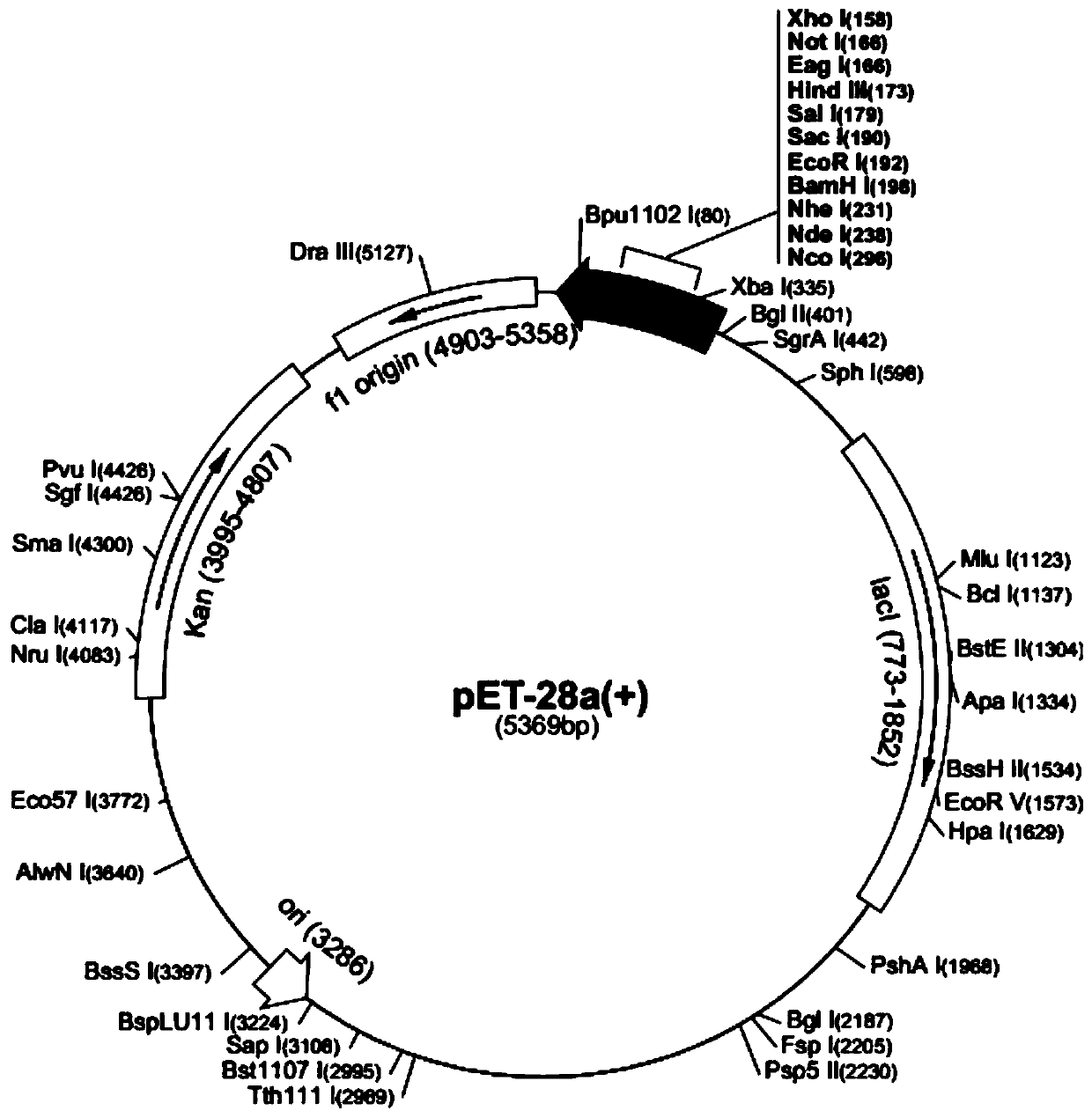
Tuberculosis candidate vaccine fusion protein
A technology of recombinant protein and Mycobacterium tuberculosis, applied in the field of fusion protein, can solve the problems of unstable immune protection effect and great difference in immune protection effect, and achieve the effect of excellent immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] 1. Experimental main reagents
[0020] Medium reagents: tryptone (Tryptone) and yeast extract (Yeast Extract) were purchased from Oxoid, UK;
[0021] Gene cloning reagents: NdeI, Hind III restriction endonuclease, and T4 DNA ligase were purchased from Thermo Company, Germany; dNTP and rTaq enzymes were purchased from Takara Company; E.Z.N.A. Plasmid Mini Kit I, E.Z.N.A. GelExtraction Kits were purchased from Omega Company in the United States; PCR primers were synthesized by Shanghai Sangon Biotechnology Company; pet28a vector and H37Rv strain genome were preserved in our laboratory; BL21 (DE3) competent cells were purchased from Shanghai Sangon Biotechnology Company; RPMI1640 medium and Fetal bovine serum was purchased from Gibco. Esat6 monoclonal antibody was purchased from Santa Cruz Company of the United States; cytokine ELISA kit was purchased from Boxinsheng Company. The endotoxin removal kit ToxinEraser was purchased from GenScript;
[0022] Antibiotics: Ka...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com