Tuberculosis rv2386c protein, compositions and uses thereof
a technology of tuberculosis and rv2386c, applied in the field of polypeptides and polynucleotides, can solve the problems of fatigue, weight loss, fever and persistent cough, serious complications and death, and treatment is not sufficient to prevent the spread of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Rv2386c as a Latent TB Vaccine Target
[0595]The gene Rv2386c also known as Mbtl, encodes for a protein which is involved in the biogenesis of hydroxyphenyloxazoline-containing siderophore mycobactins where it converts chorismate to salicylate, the starter unit for mycobacterin siderophore synthesis.
[0596]Rv2386c was selected based on a genome-wide analysis of Mycobacterium tuberculosis genes associated with dormancy phase maintenance and infectivity as in Murphy and Brown BMC. Infect. Dis. 2007 7:84-99. Potential dormancy phase gene targets in Mycobacterium tuberculosis were prioritised through a bioinformatics meta-analysis of published genome-wide DNA microarray datasets of bacterial gene expression under simulated dormancy conditions. Subcellular localisation of M. tuberculosis proteins encoded by genes, was subsequently carried out on the entire genome to identify vaccine targets.
[0597]Briefly, experimental conditions in the dormancy models were quite varied so ...
example 2
Rv2386c Epitope Identification
Method
[0606]T cell epitope prediction was based on the following approaches:
PredictionNameURL / ReferencesCD4 andMultipredwebsite: antigen.i2r.a-star.edu.sg / multipred / CD8Zhang, G. L., Khan, A. M., Srinivasan, K. N., August, J. T. andBrusic, V. (2005) “MULTIPRED: a computational system forprediction of promiscuous HLA binding peptides” NucleicAcids Res. 33, W172-W179.SVMHCwebsite: www-bs.informatik.uni-tuebingen.de / SVMHC“Prediction of MHC class I binding peptides, using SVMHC.”Pierre Dönnes and Arne Elofsson in: BMC Bioinformatics2002 3: 25CD4ProPredwebsite: www.imtech.res.in / raghava / propred / Singh, H. and Raghava, G. P. S. (2001) “ProPred: Prediction ofHLA-DR binding sites.”Bioinformatics, 17(12), 1236-37.Tepitope2In house program based on:H. Bian, J. Hammer (2004) “Discovery of promiscuous HLA-II-restricted T cell epitopes with TEPITOPE.” Methods 34: 468-75CD8nHLAwebsite: www.imtech.res.in / raghava / nhlapred / Bhasin M. and Raghava G P S (2006) “A hybrid appr...
example 3
H37Rv Homologues
[0609]Rv2386c sequences from a number of M. tuberculosis strains and BCG were identified using BLASTP searches of GenBank (H37Rv reference sequence accession number YP—177877.1
StrainAccession Number% identityCDC1551NP_336935.1100F11YP_001288342.1100HaarlemZP_02247771.1100CZP_00878160.199BCGCAL72388.1100
[0610]Alignment of the homologue sequences indicates a high level of identity.
Biological Assays
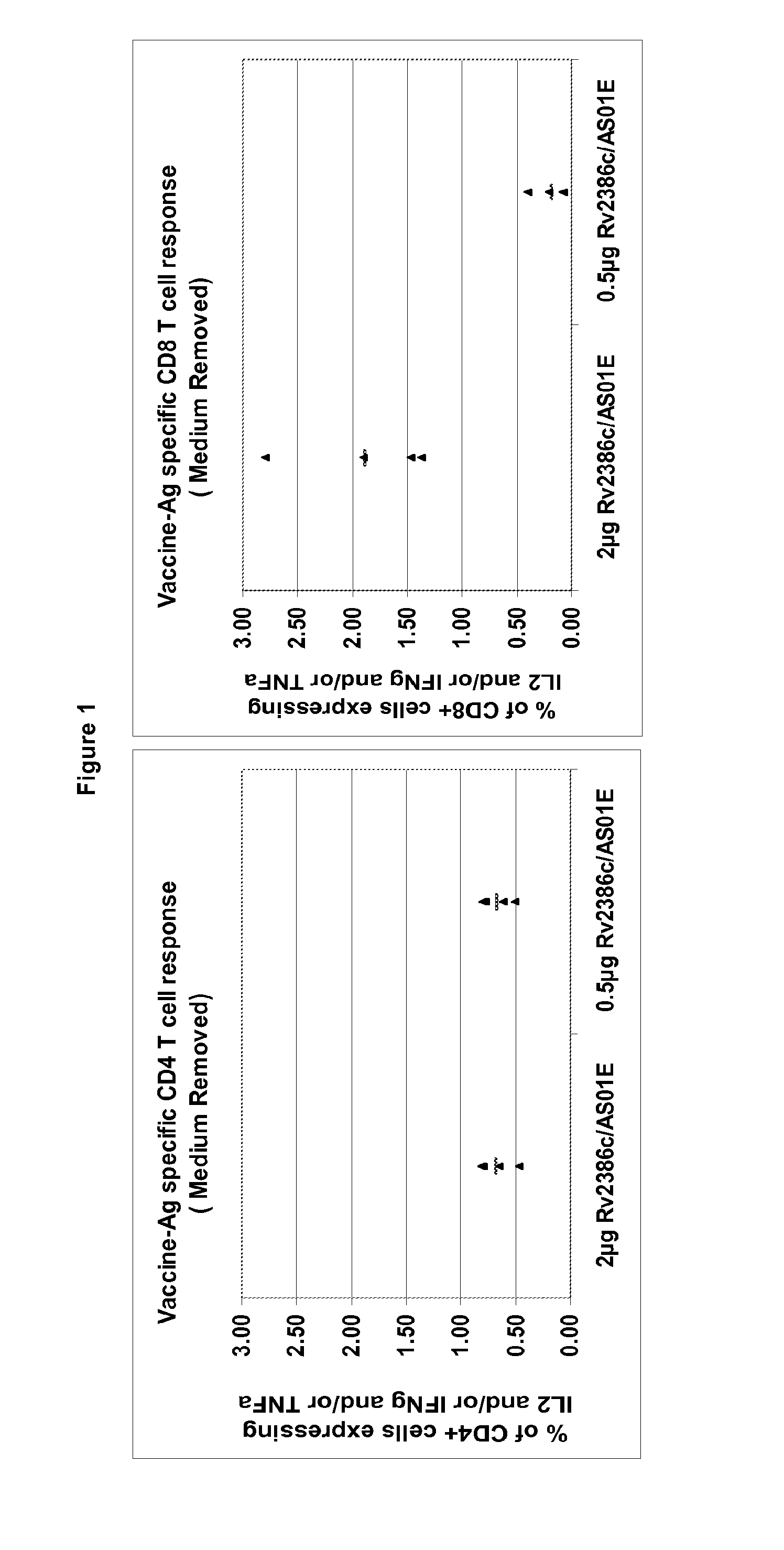
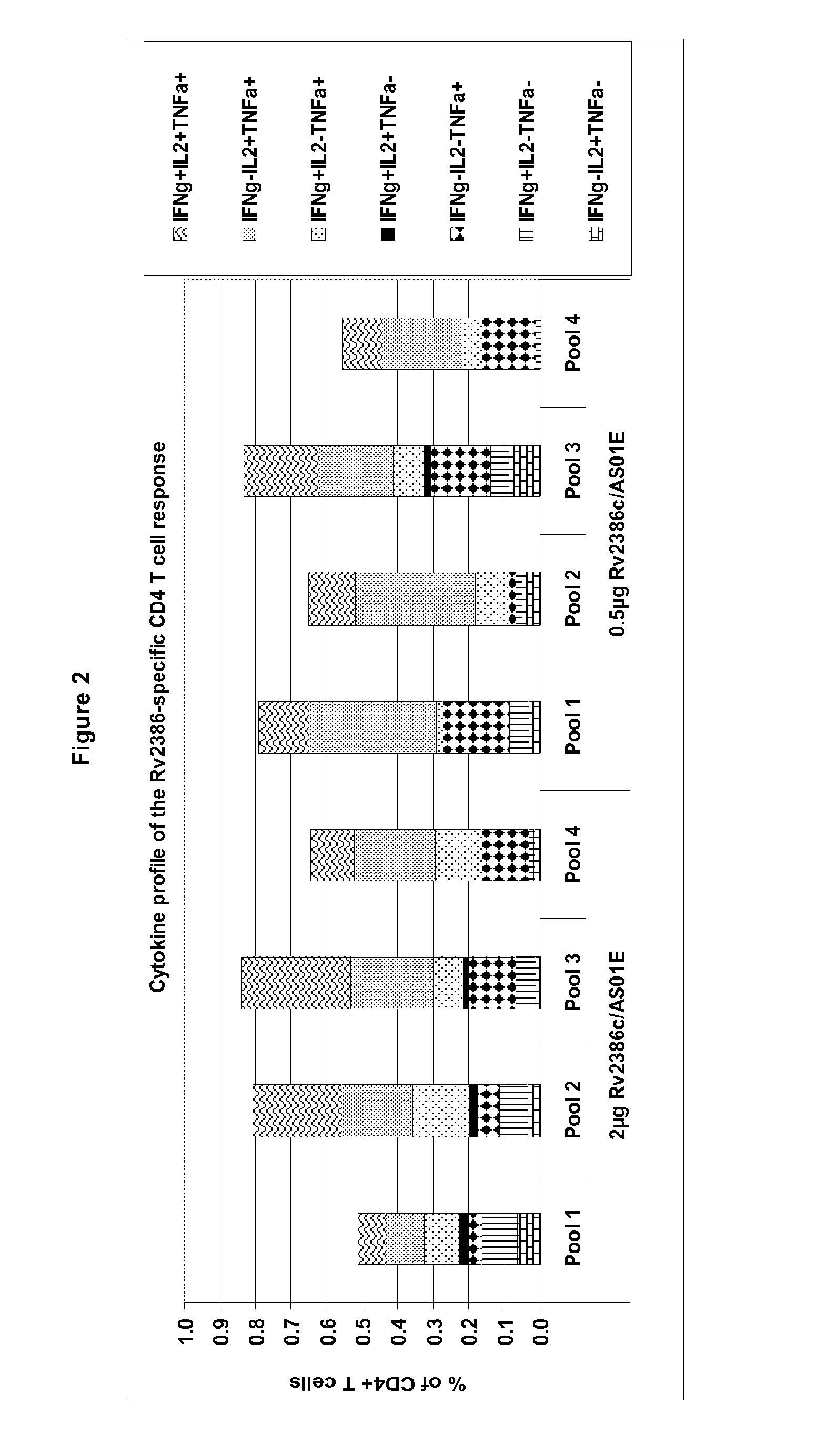
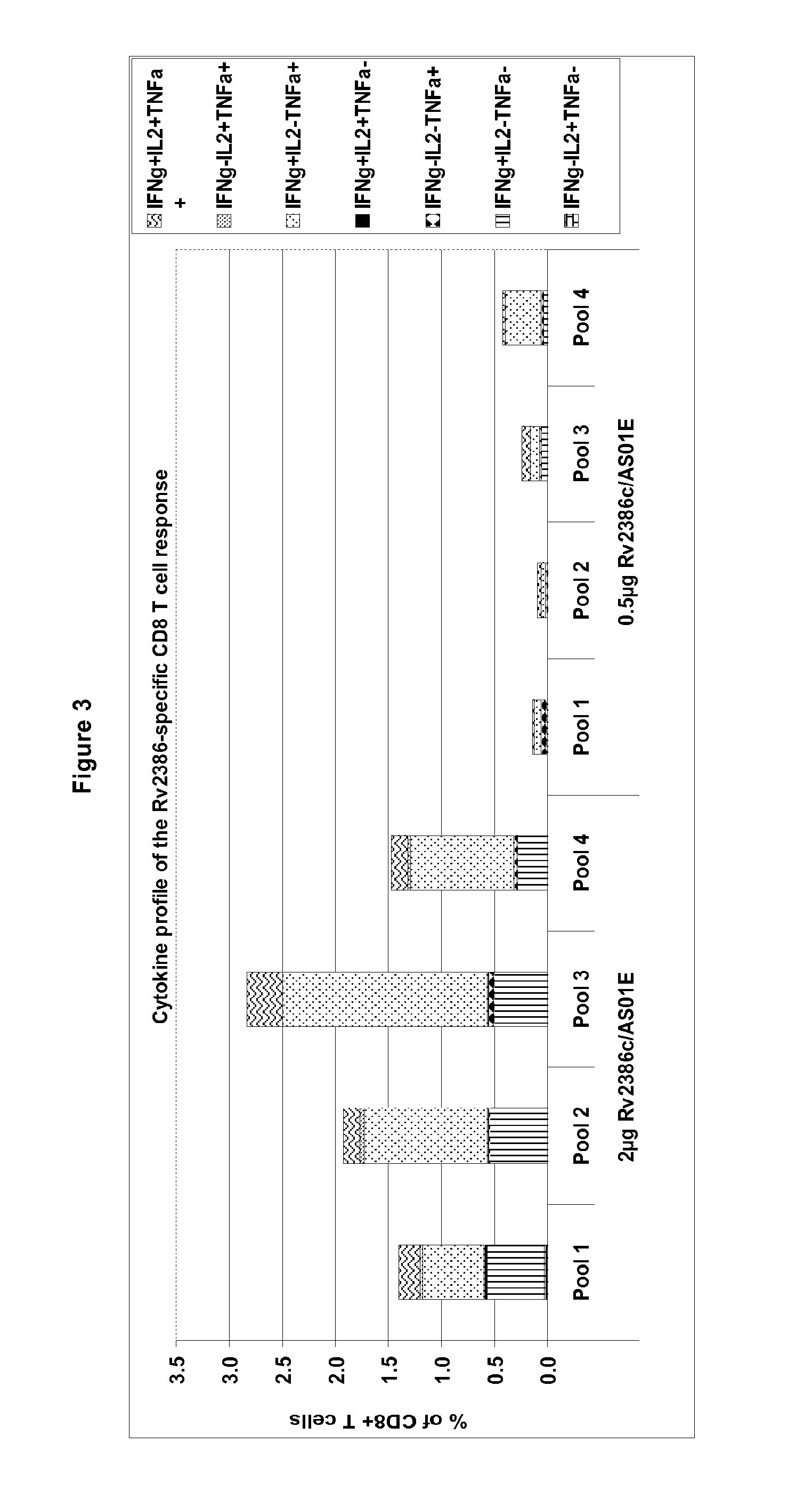
Quantification of T Cell Responses to Rv2386c
[0611]Polypeptides may be screened for their ability to activate T-cells (induction of proliferation and / or production of cytokines) in peripheral blood mononuclear cell (PBMC) or in whole blood preparations from infected (such as latently infected) individuals.
[0612]Latently infected individuals are usually identified by a skin test that has a diameter above 10 mm and without symptoms, with no Mtb (M. tuberculosis) positive culture, with a negative sputum negative and with no lesion (as detected by chest X-Ray).
[0613]A range of in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com