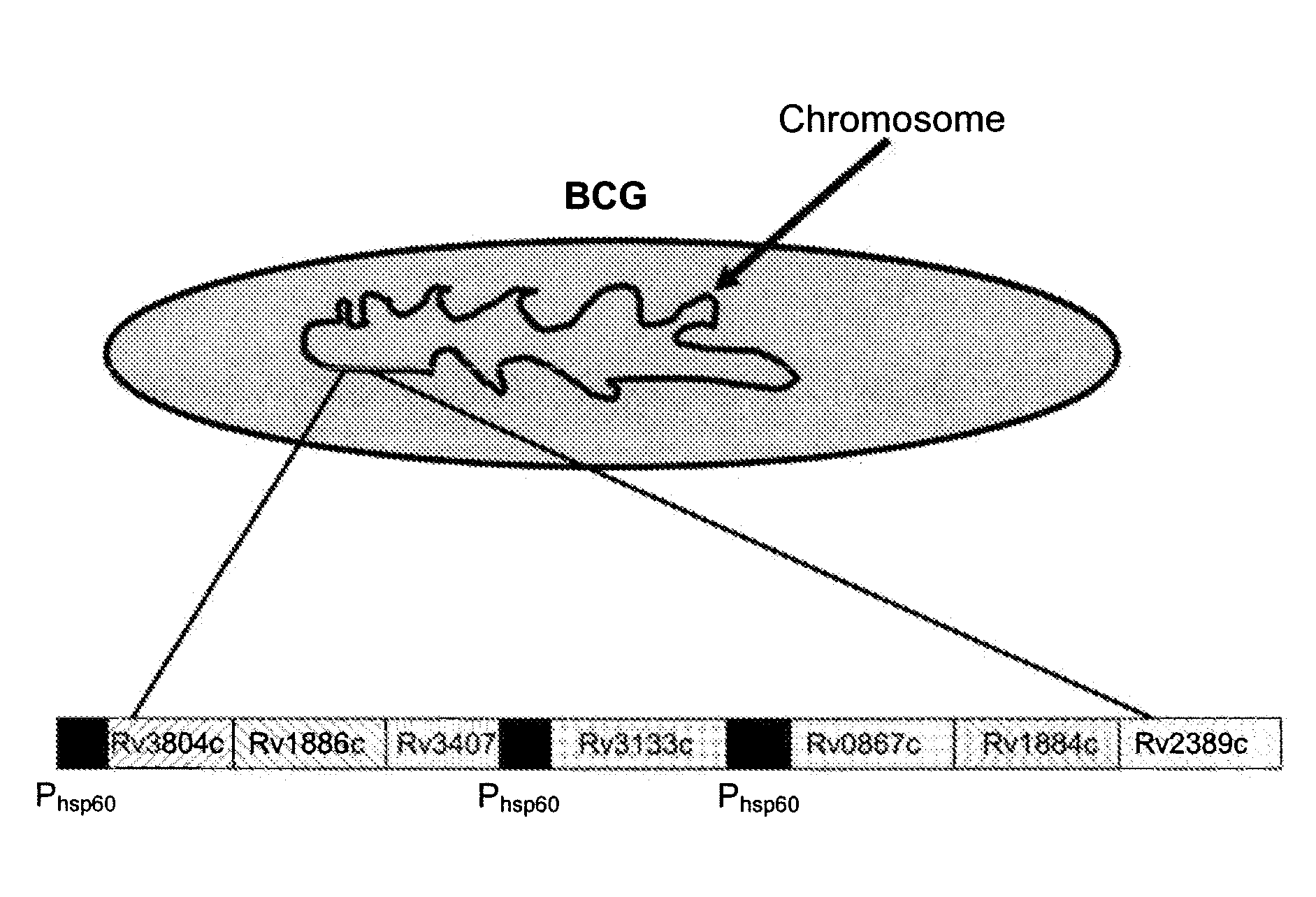
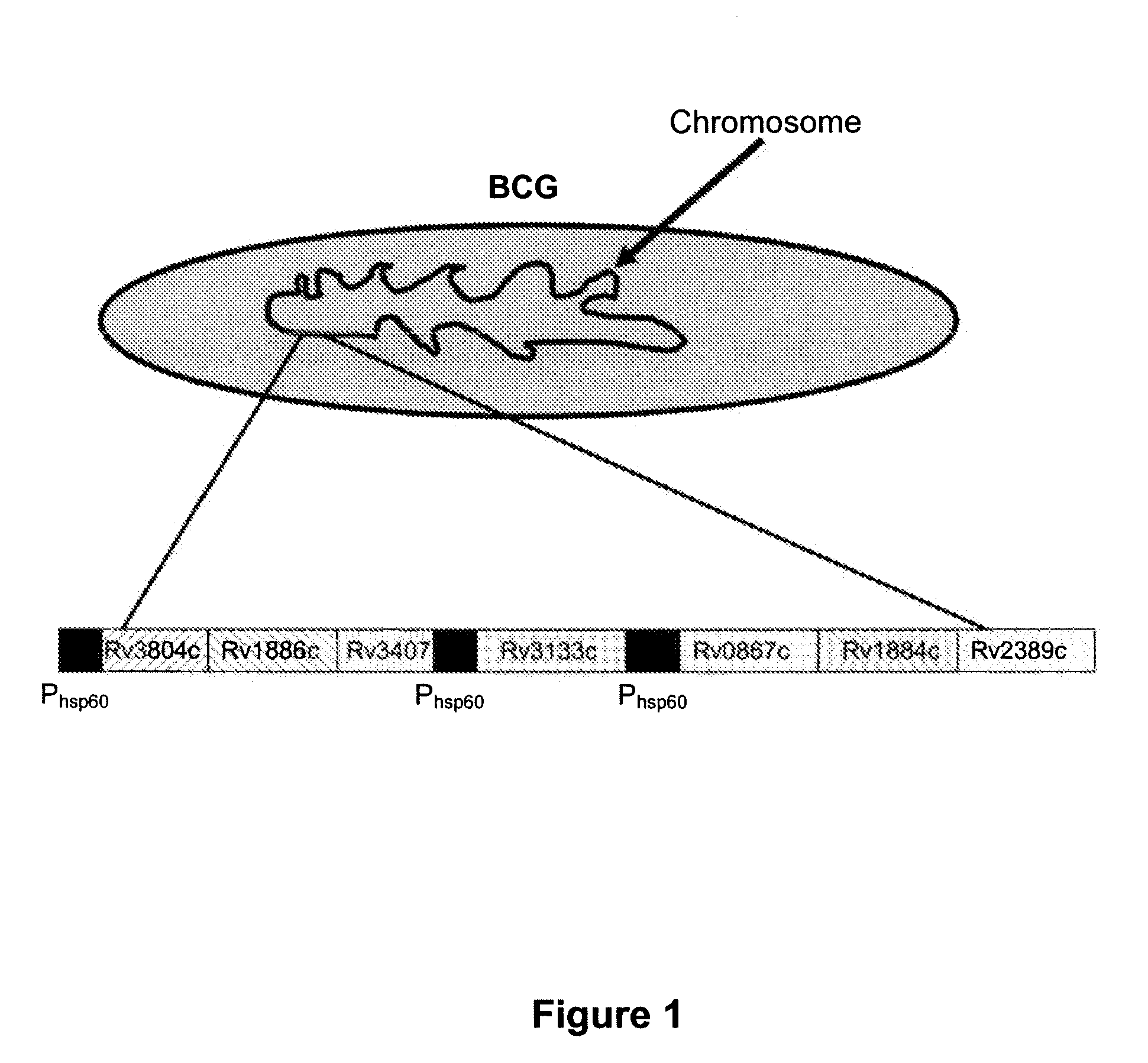
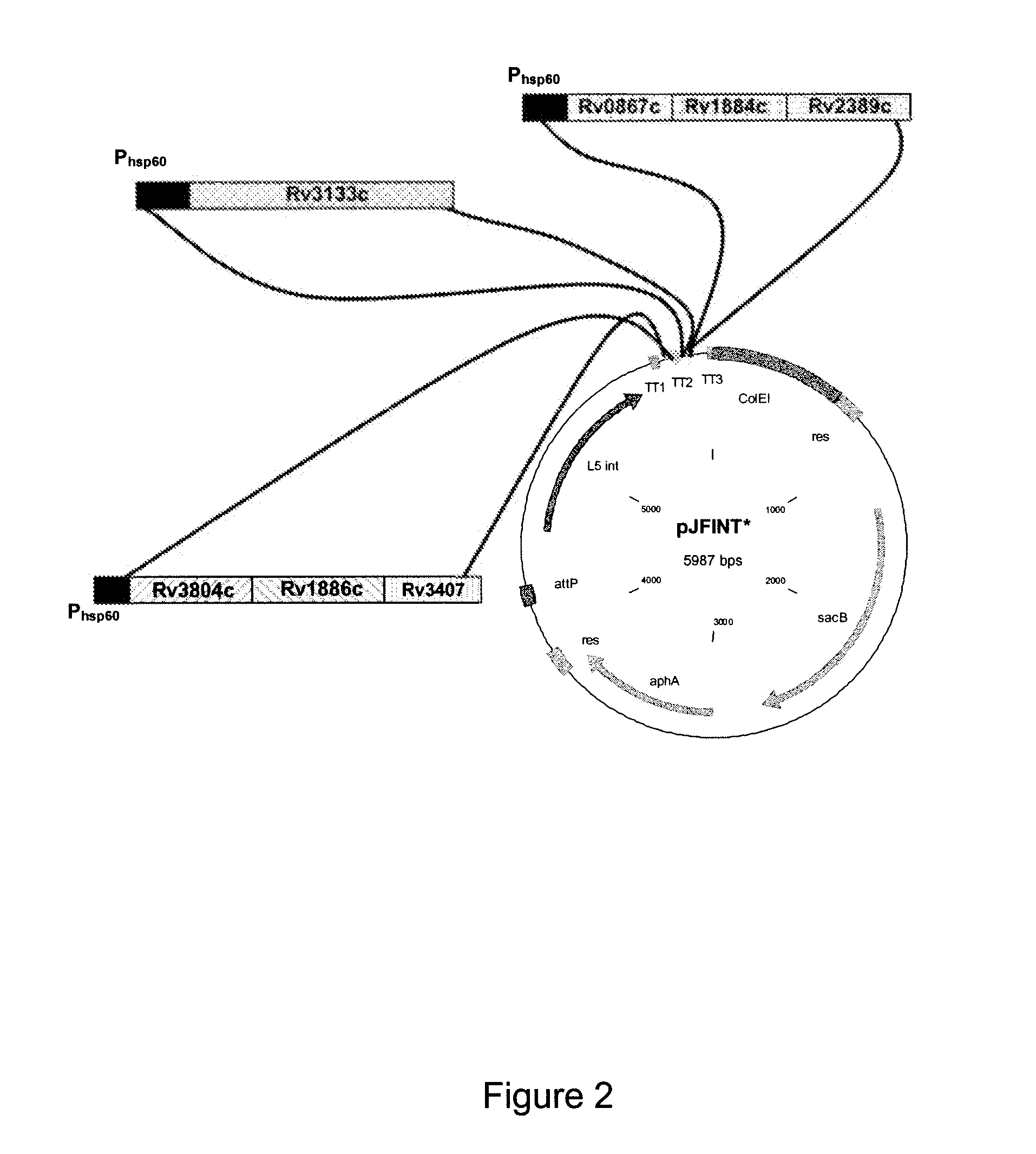
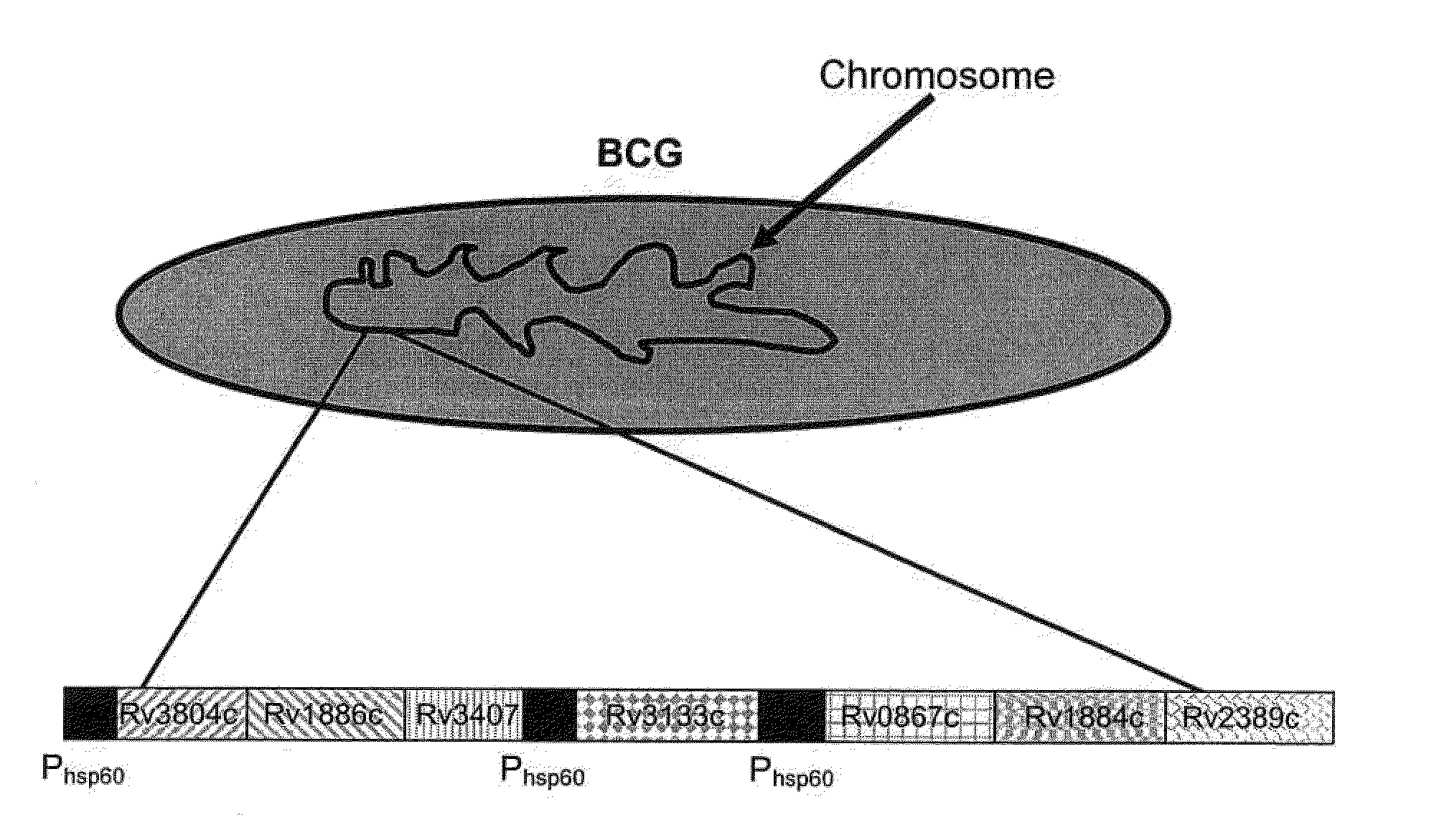
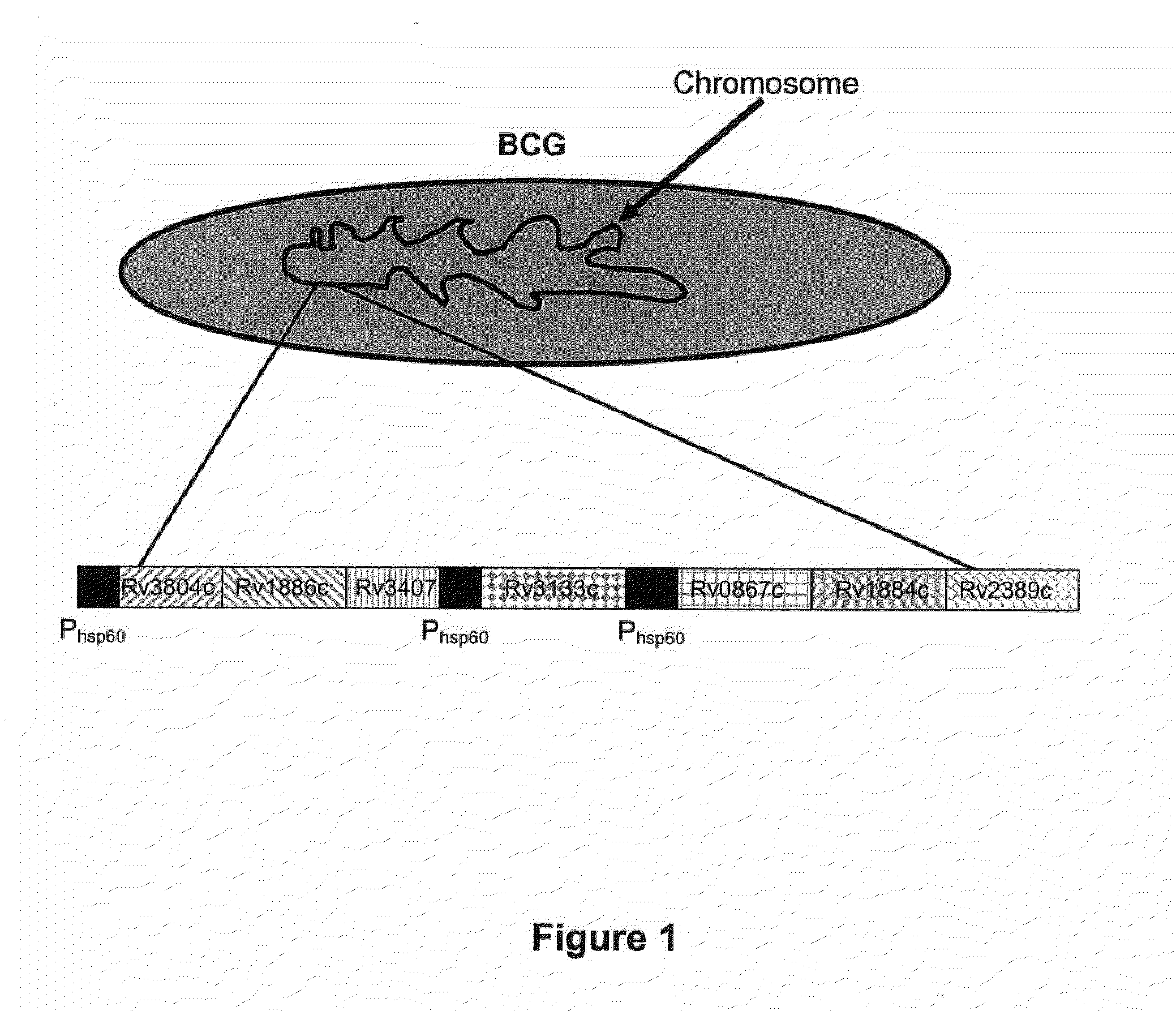
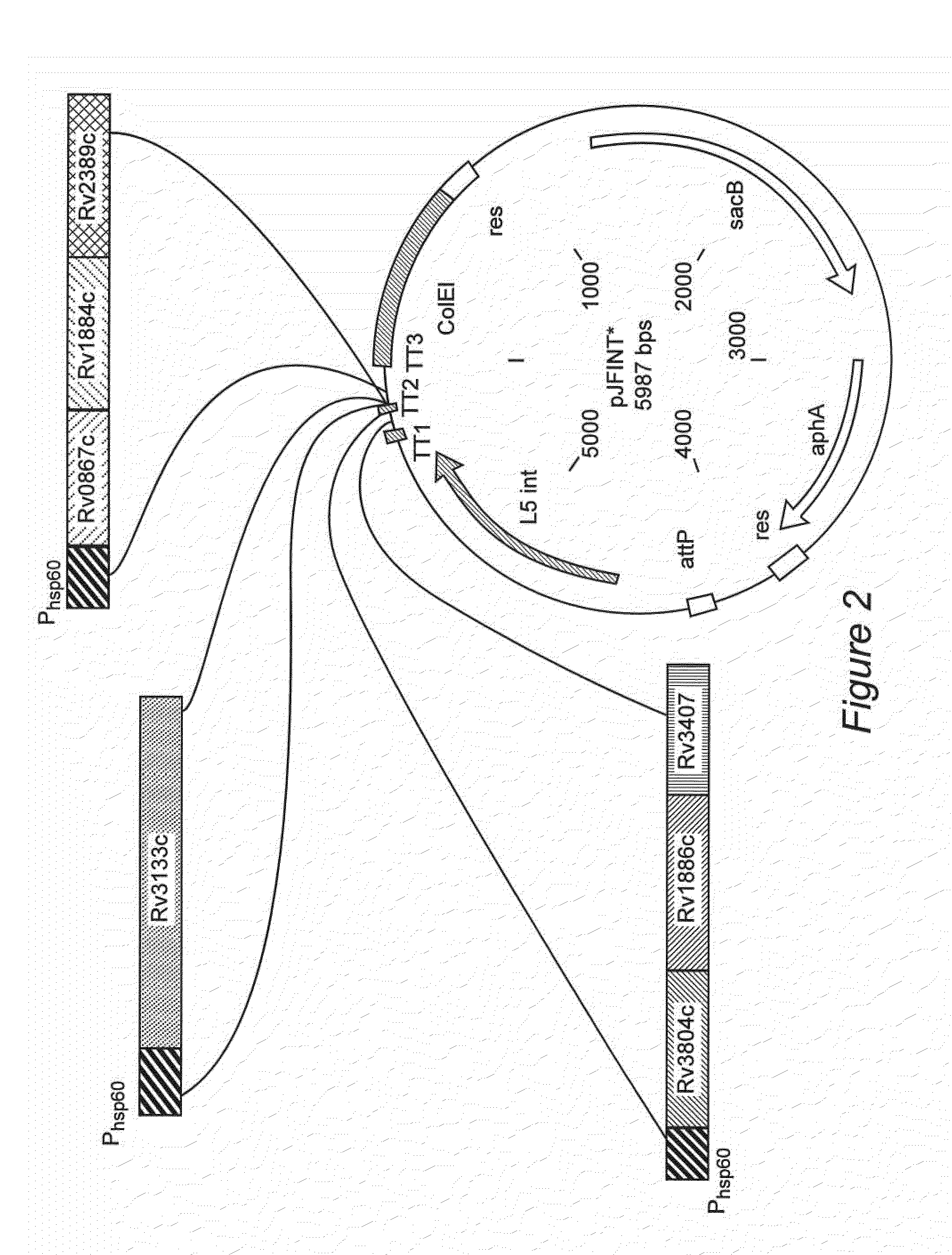
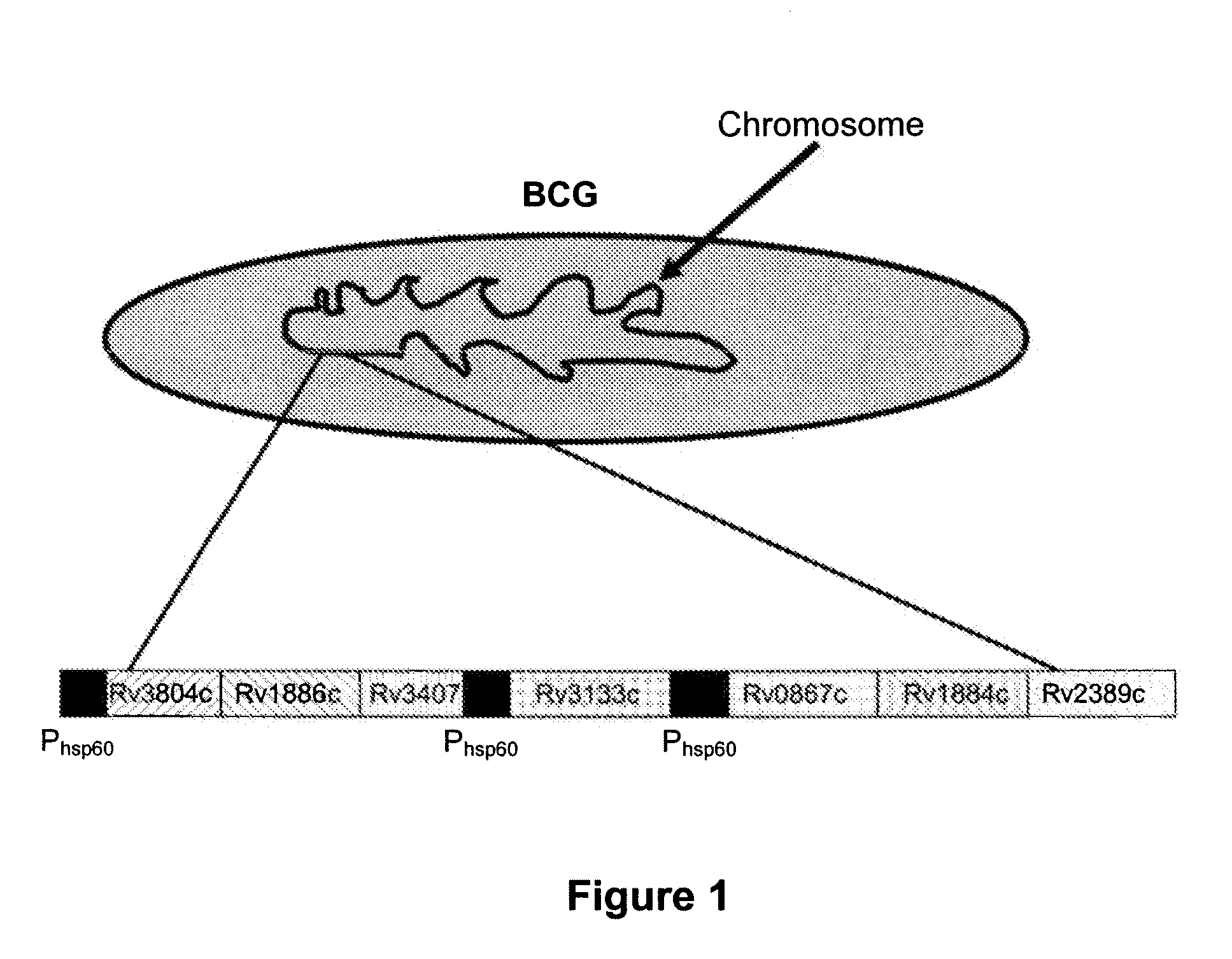
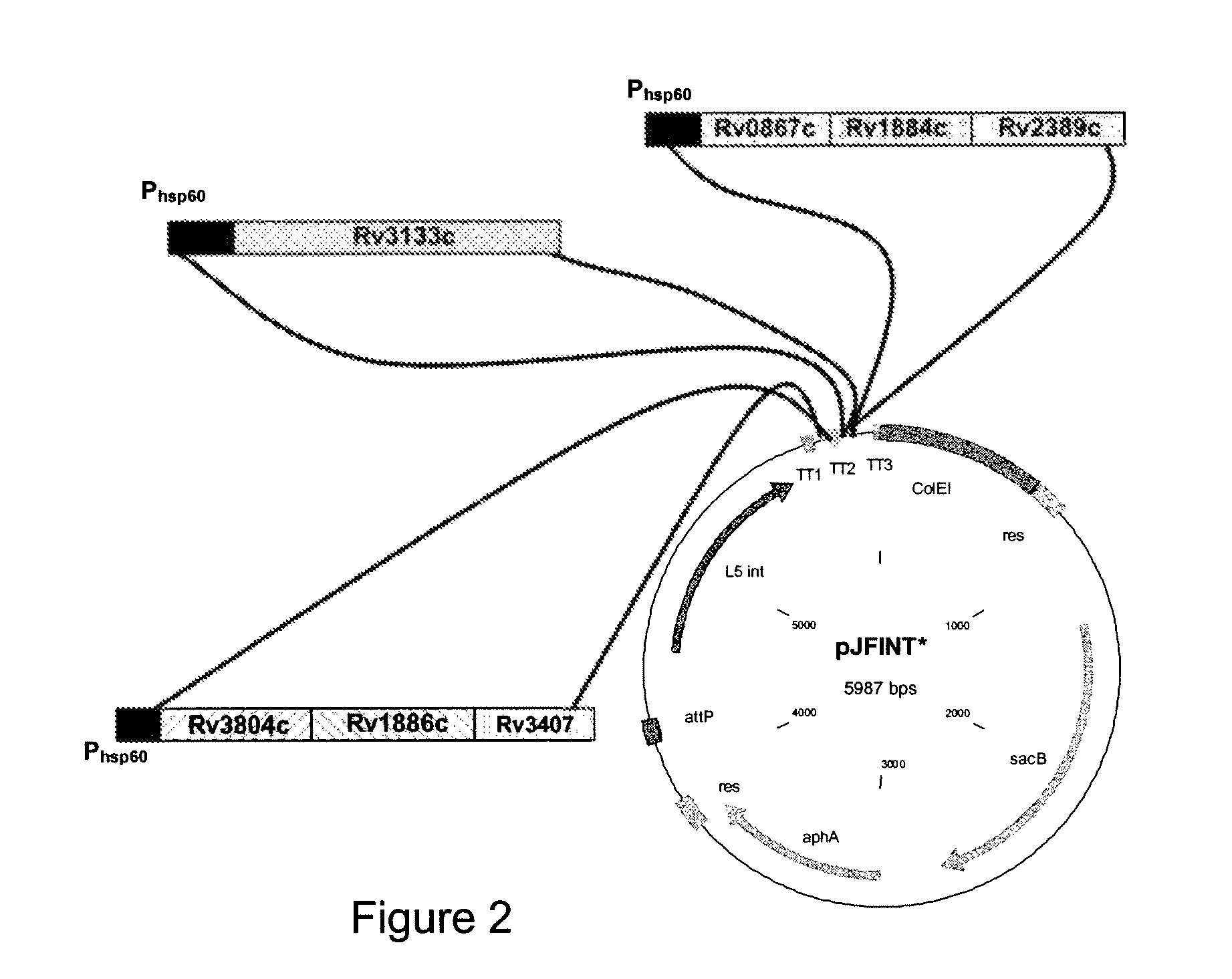
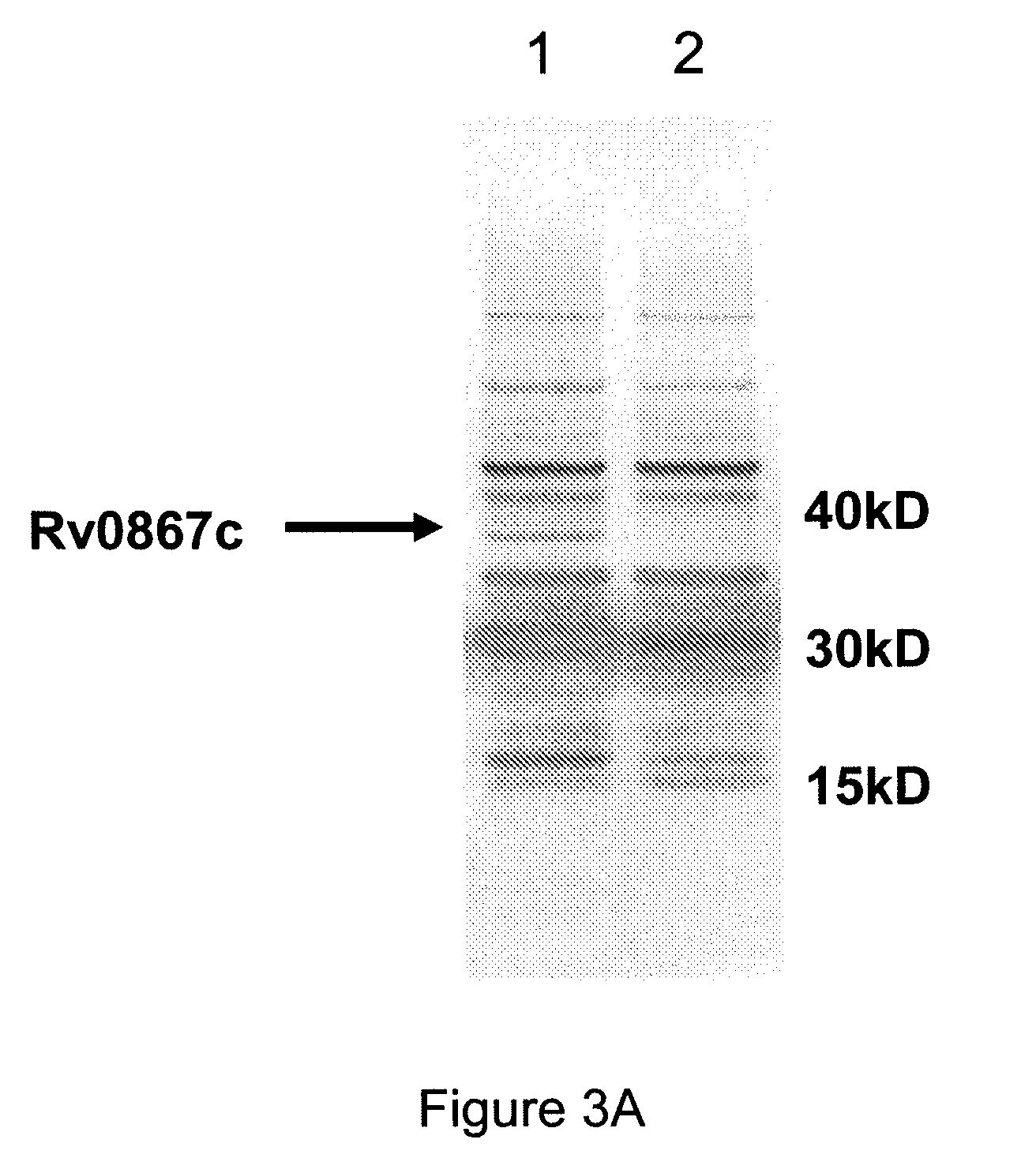
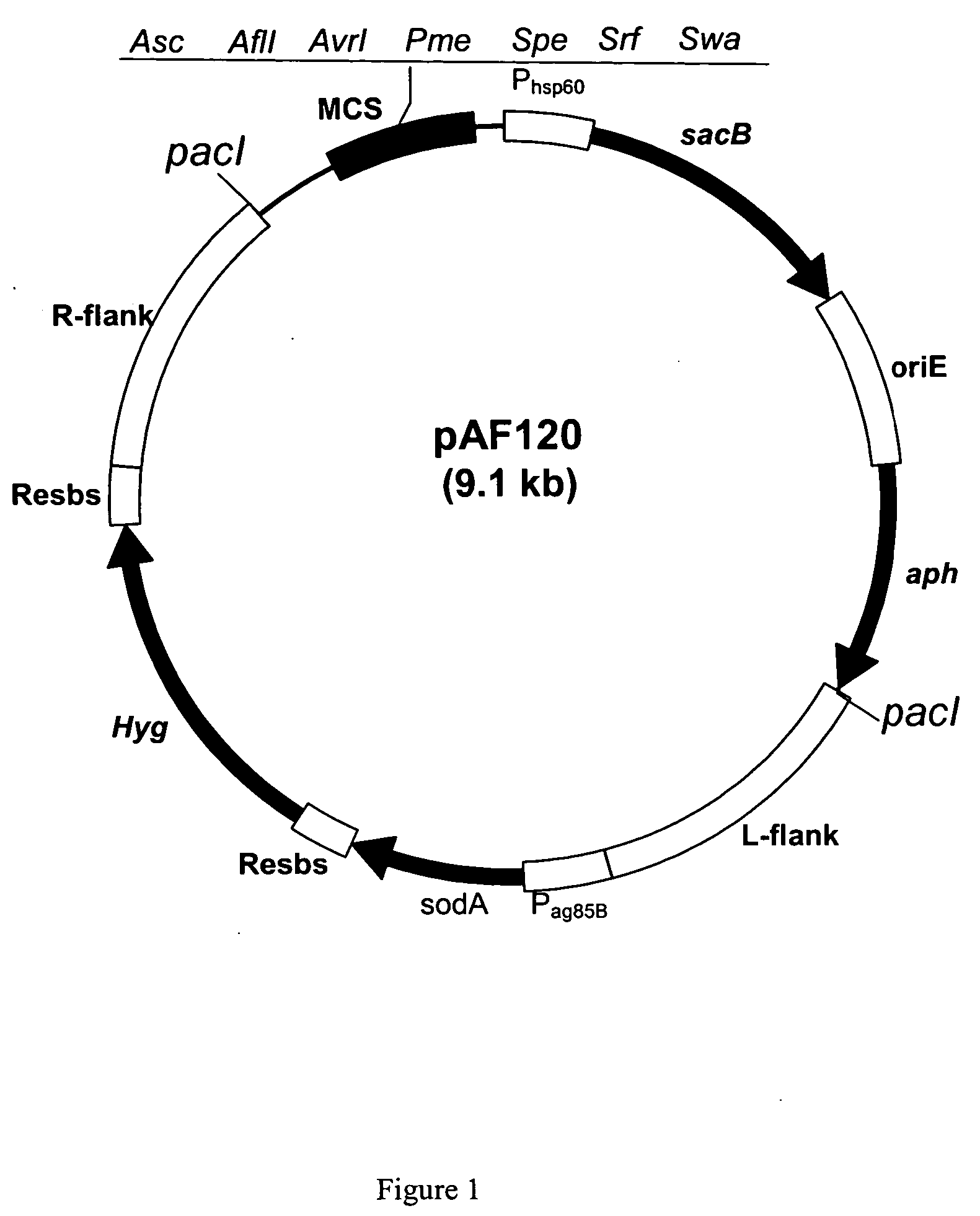
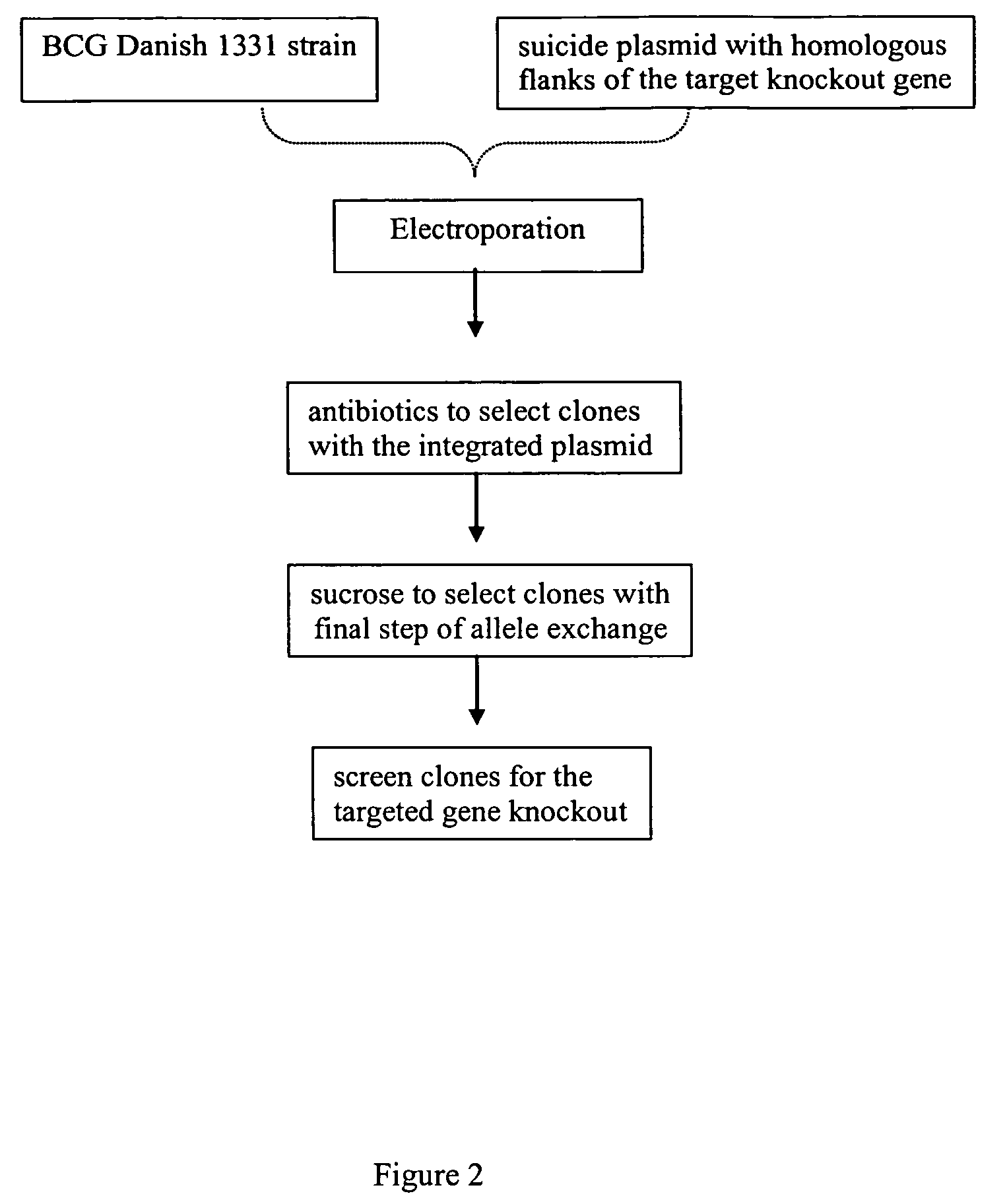
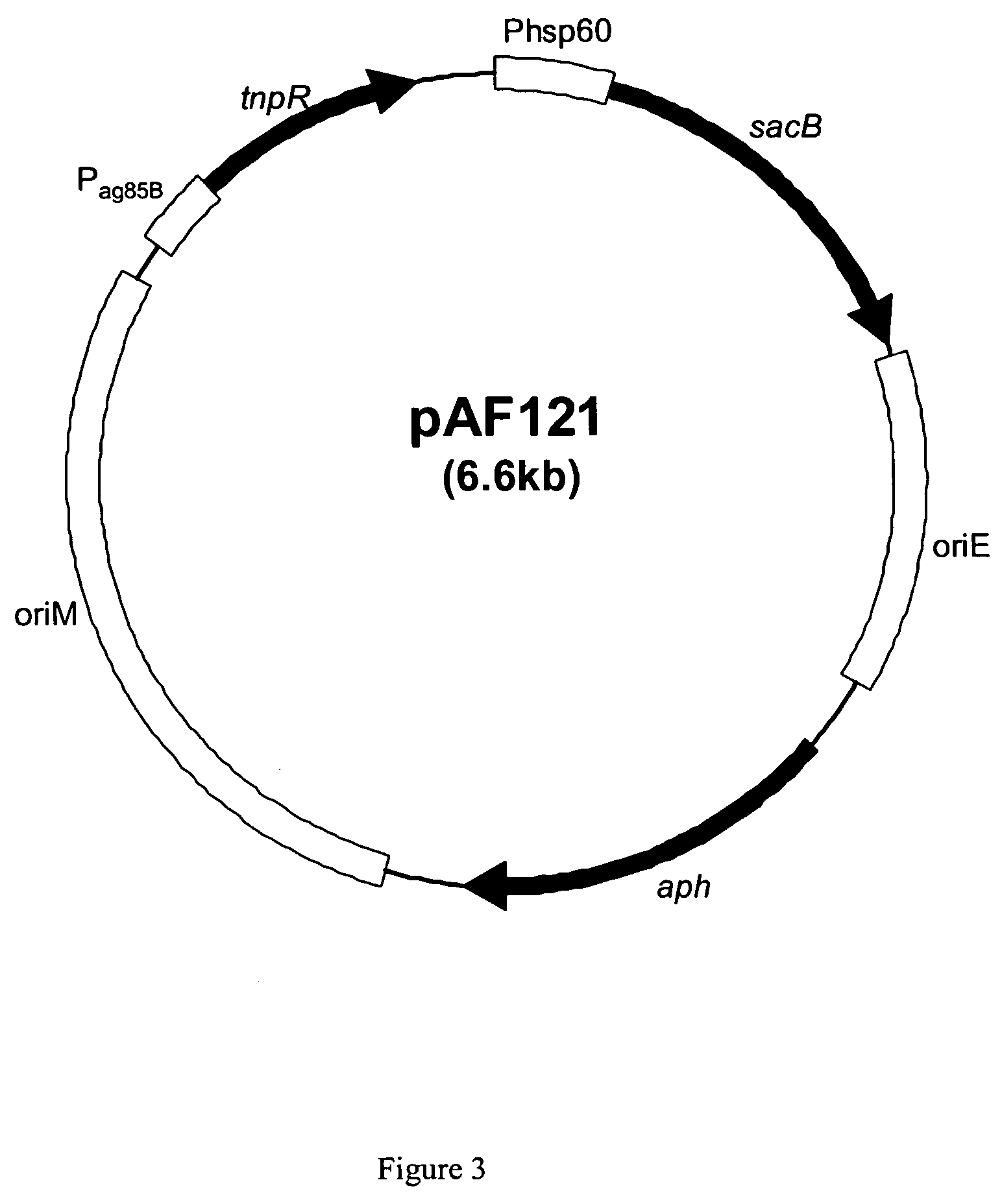
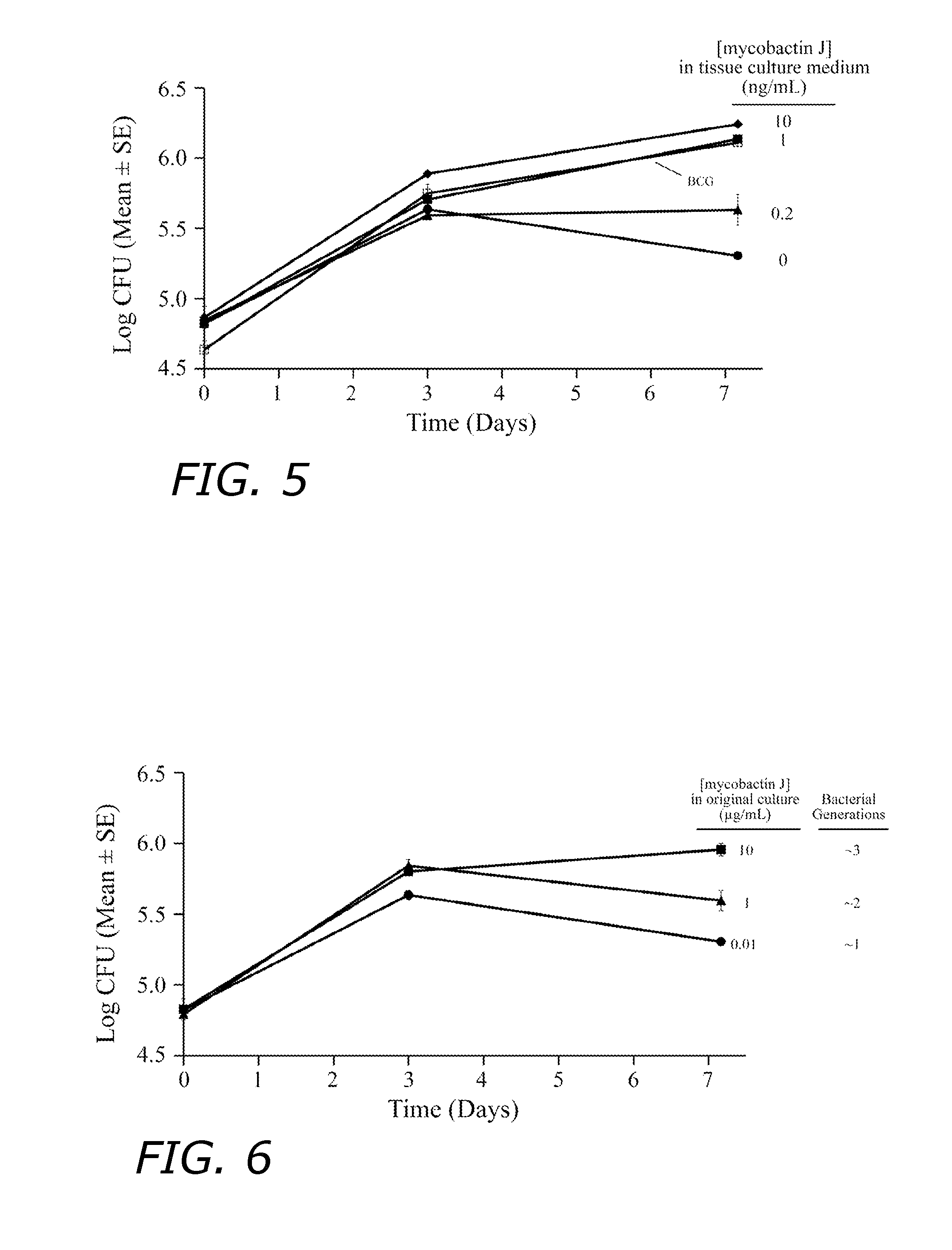
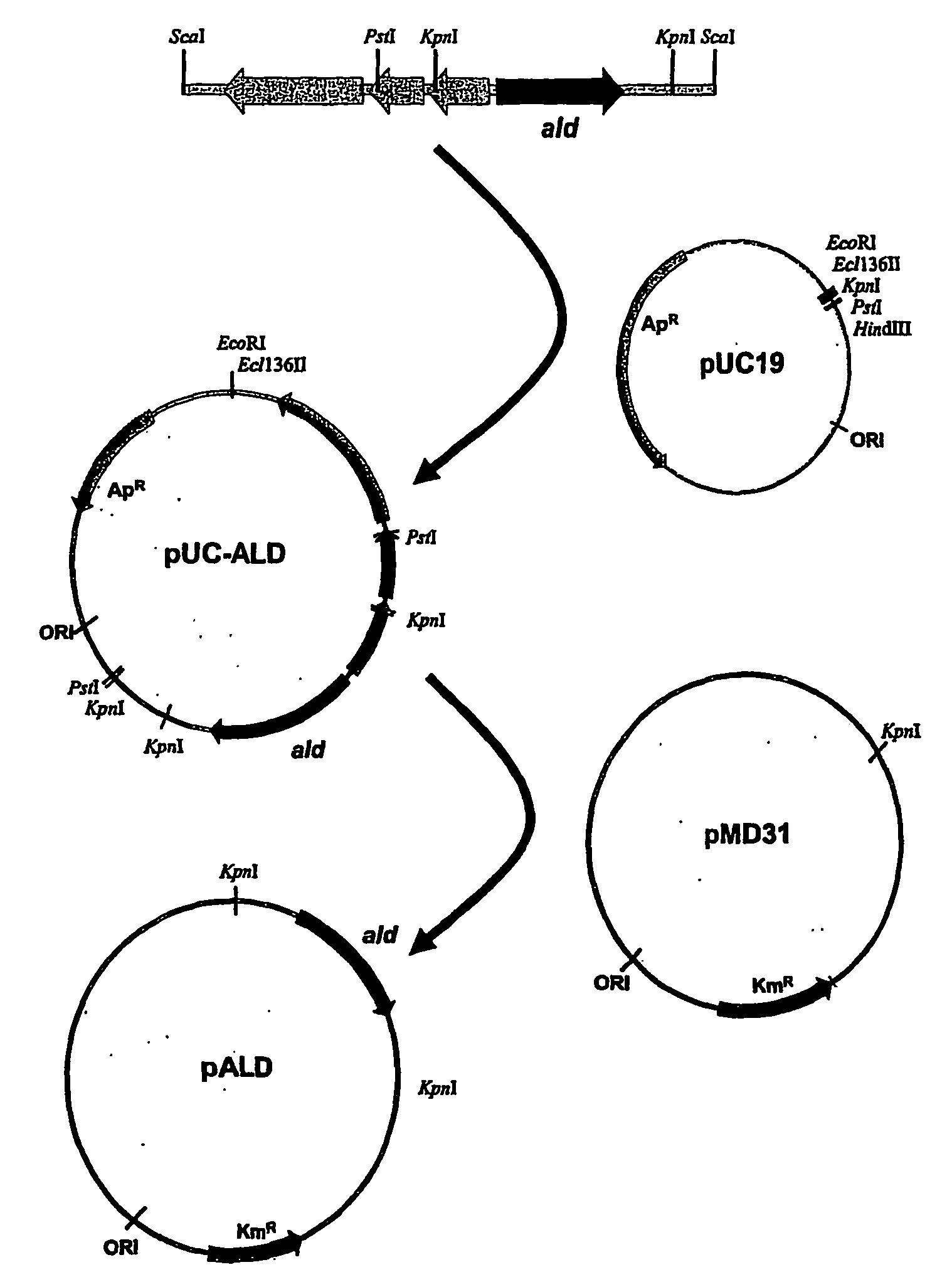
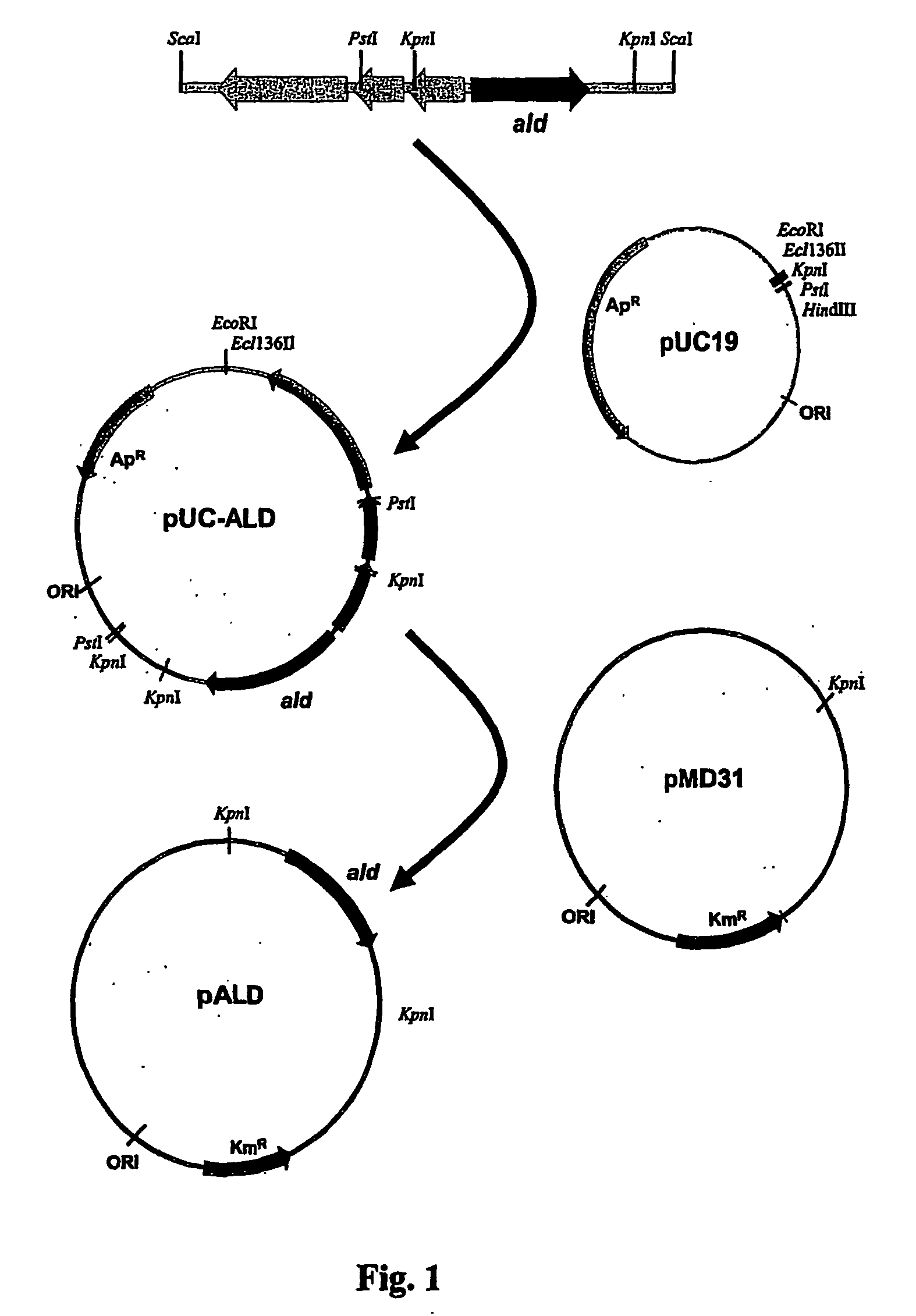
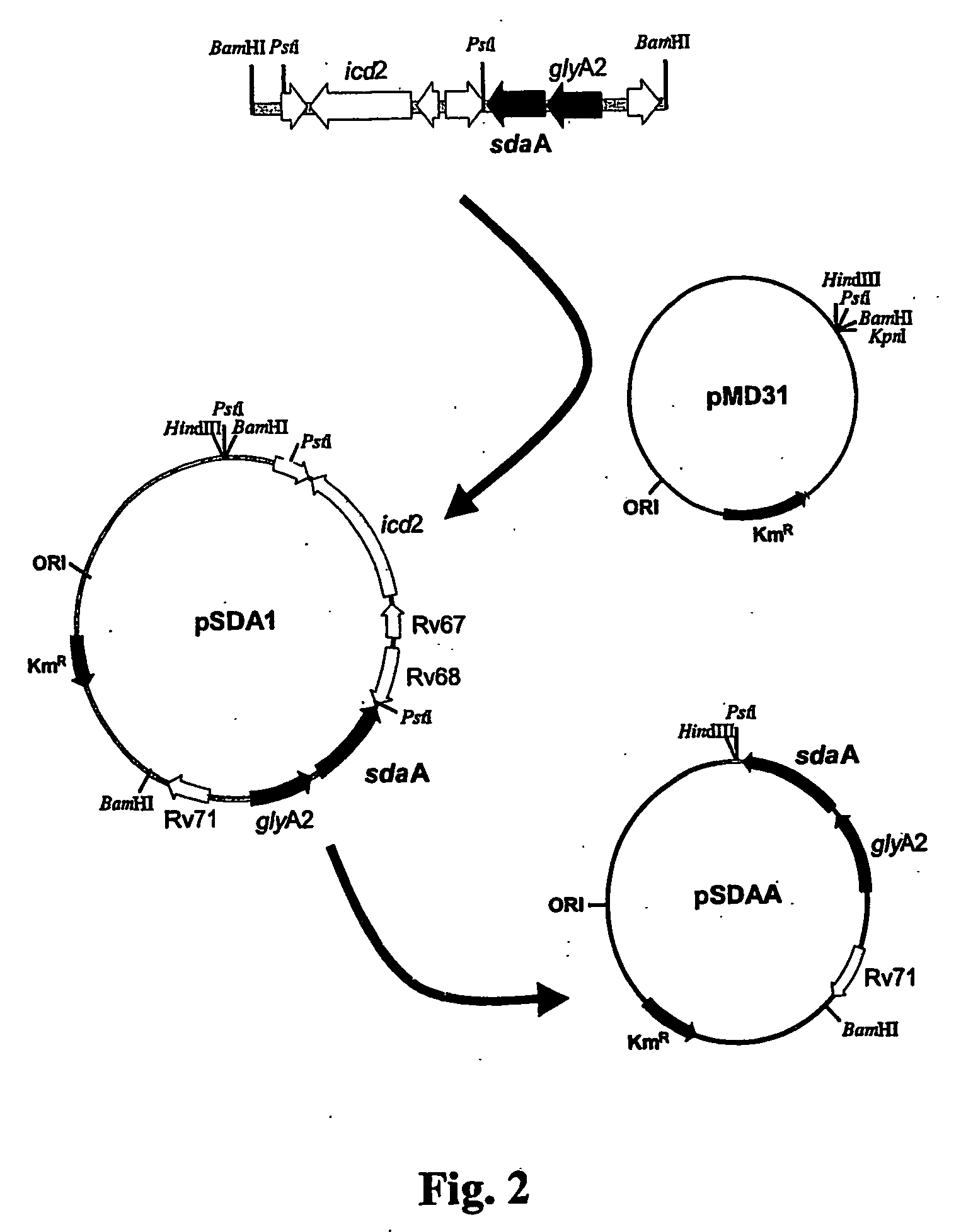
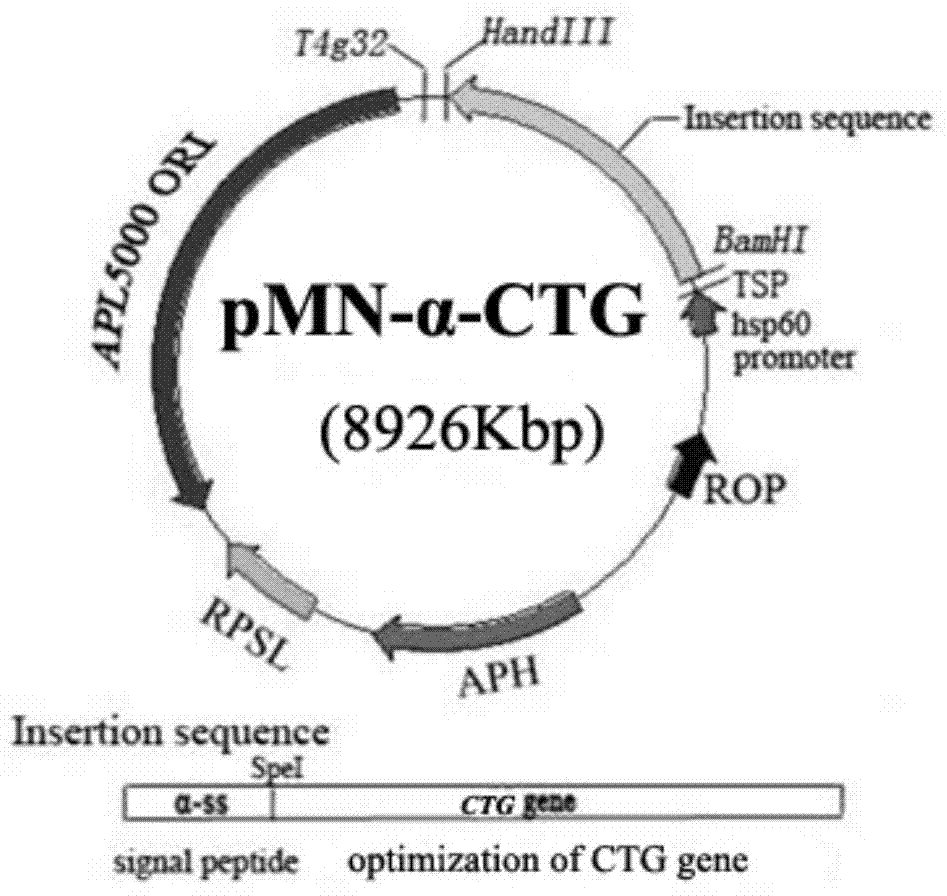
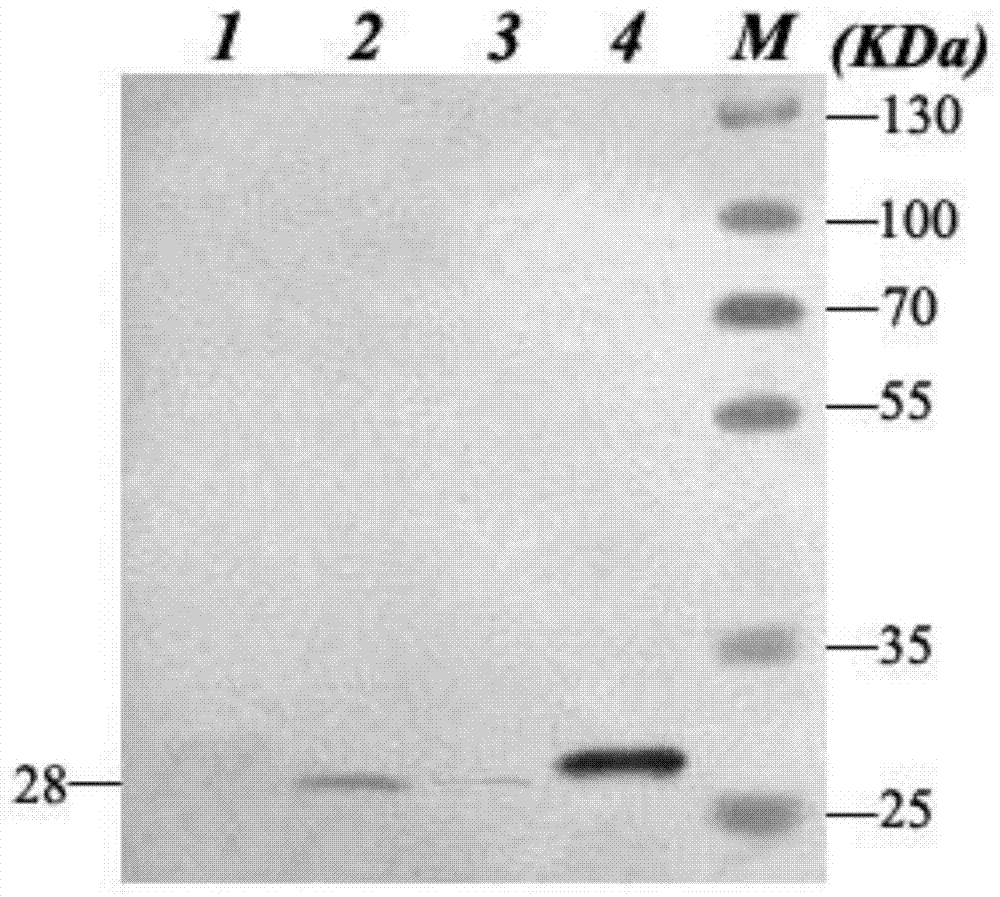
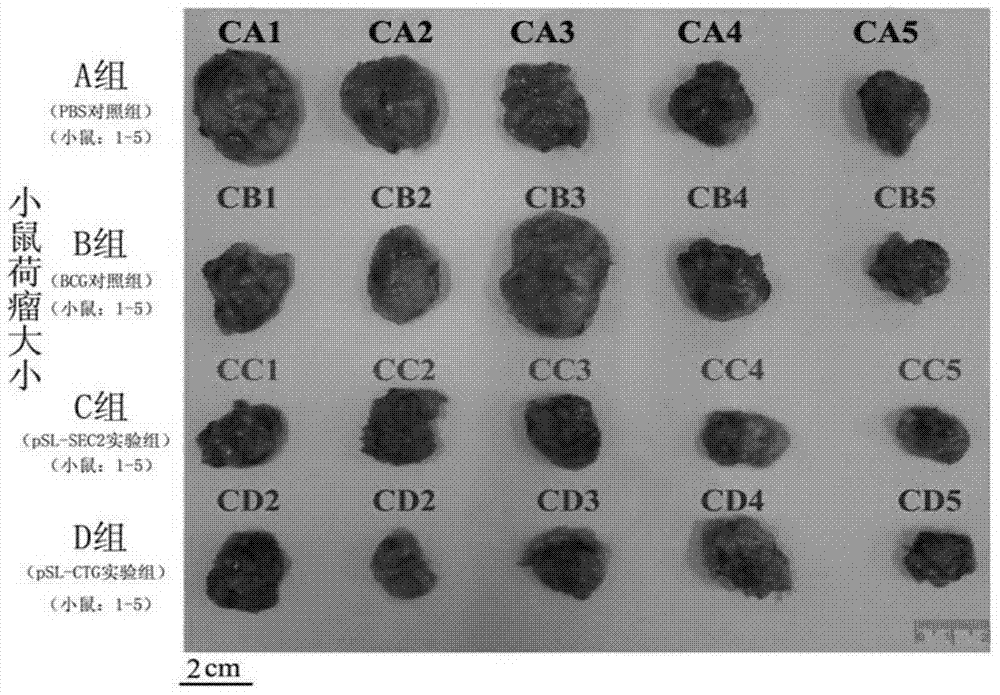
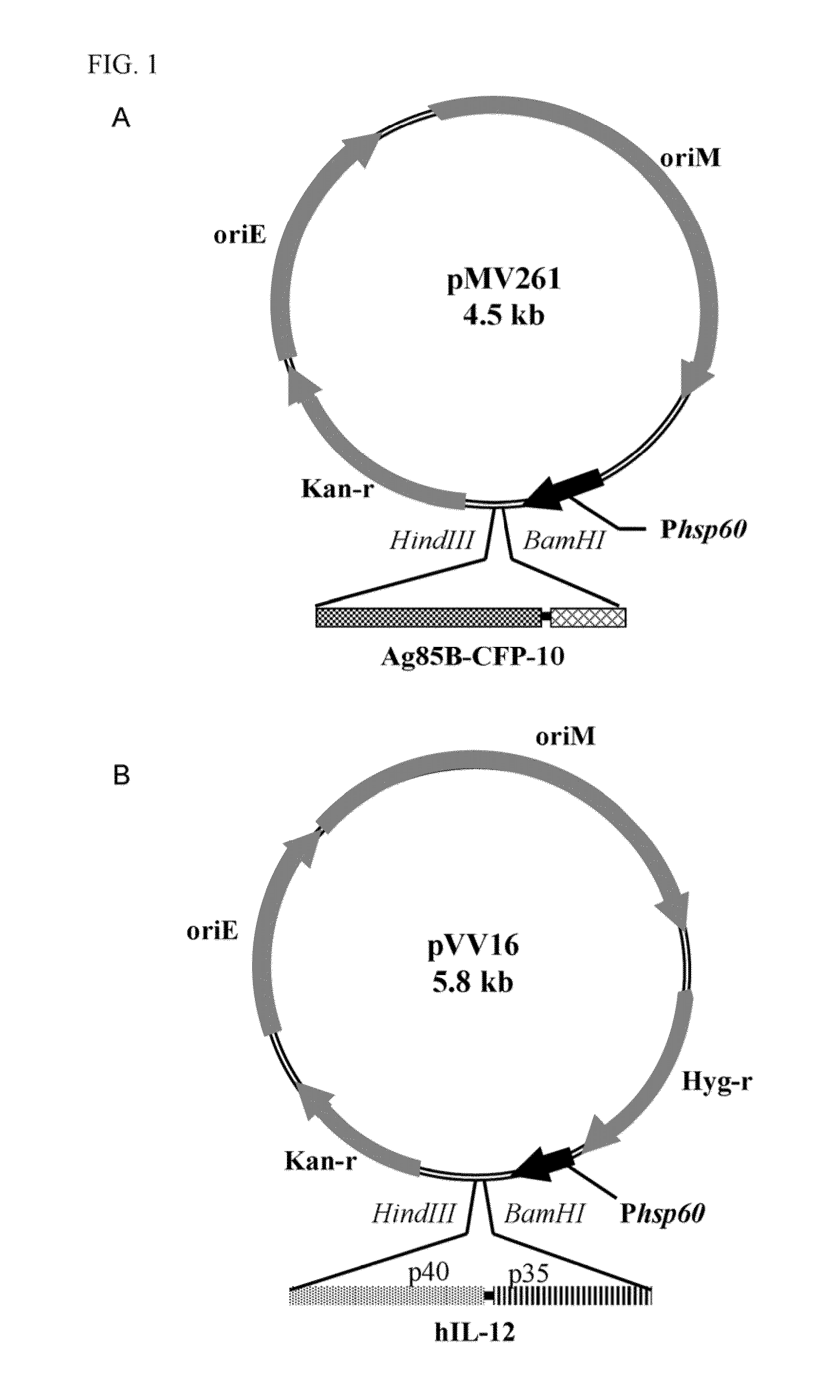
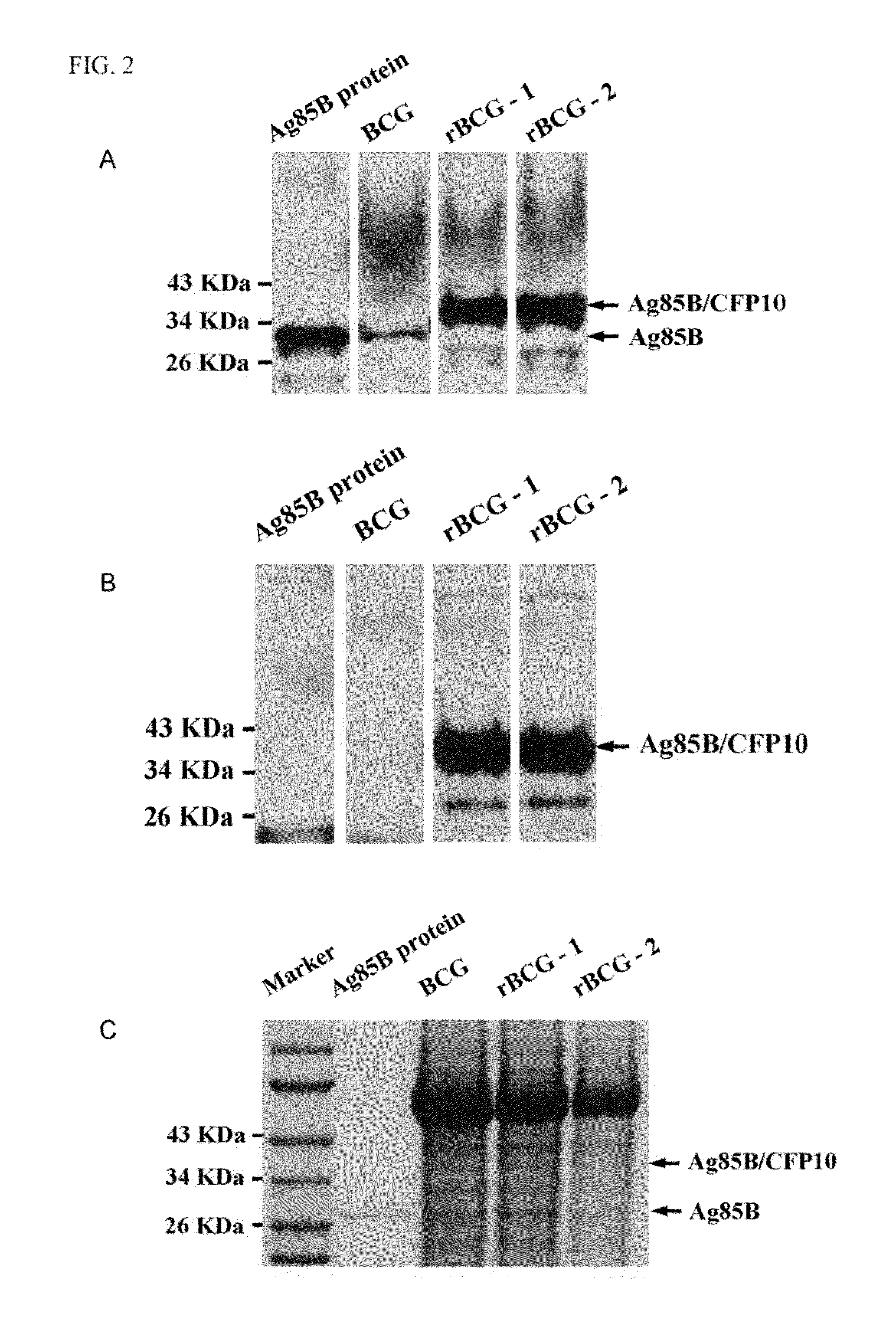
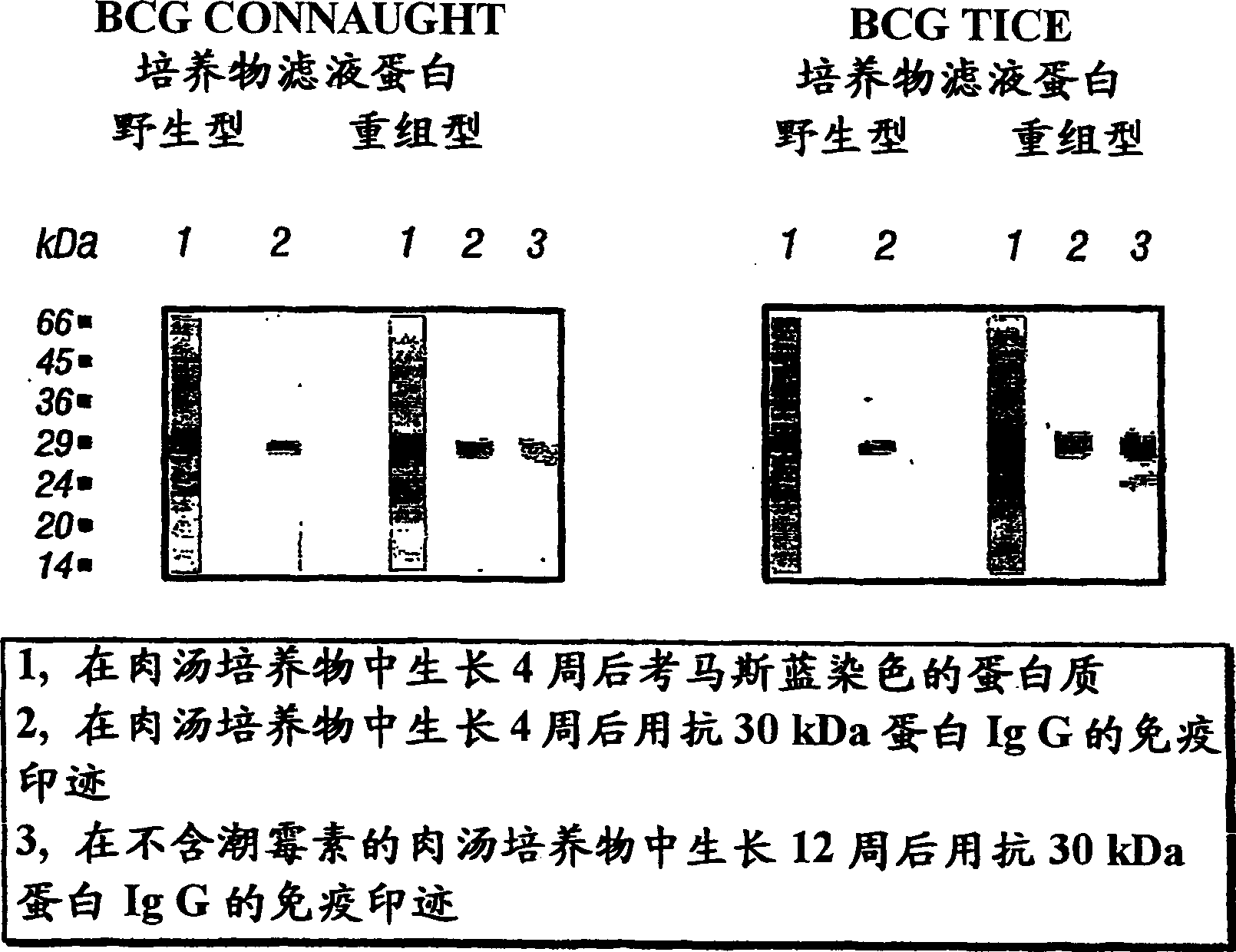
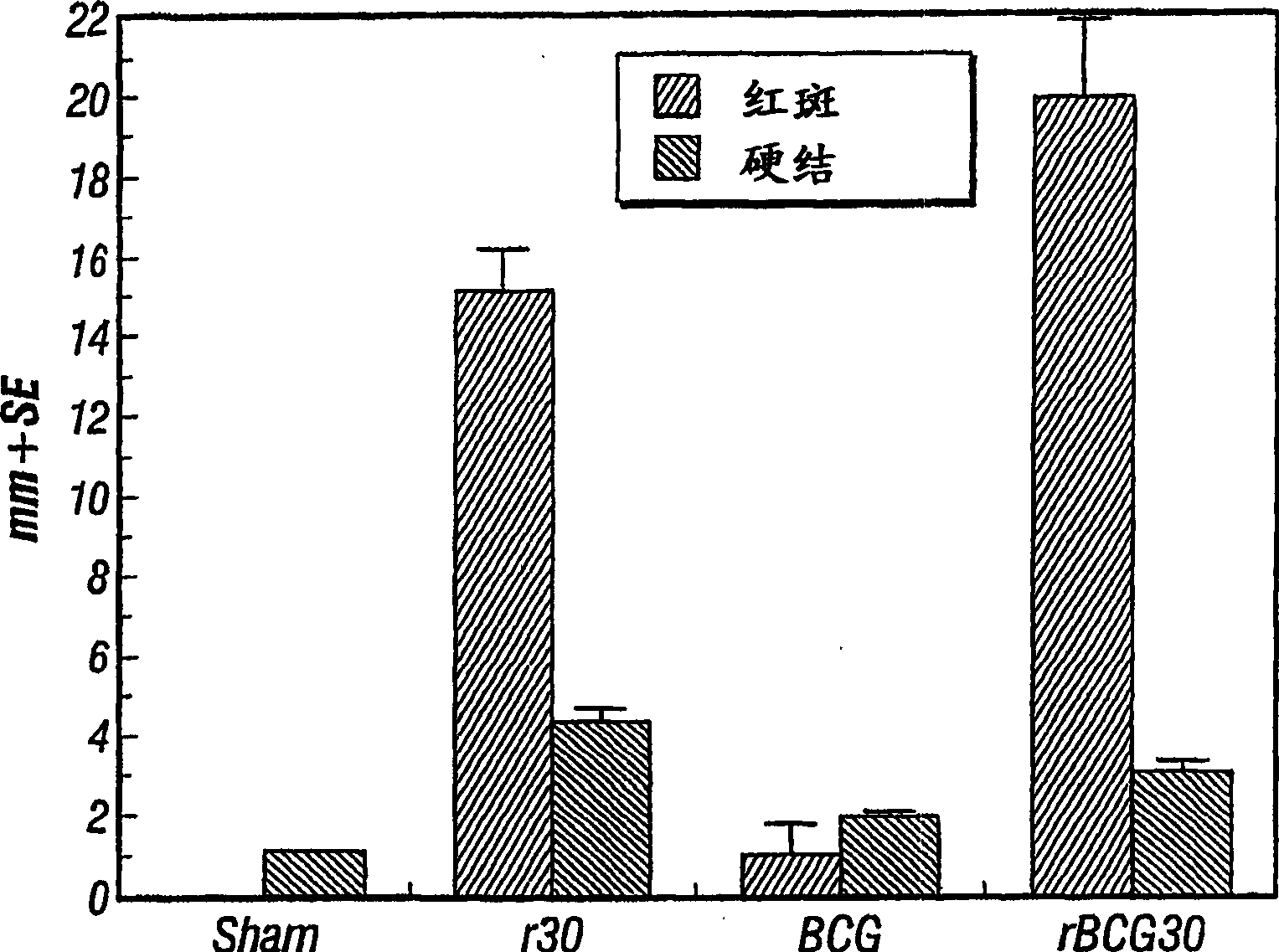
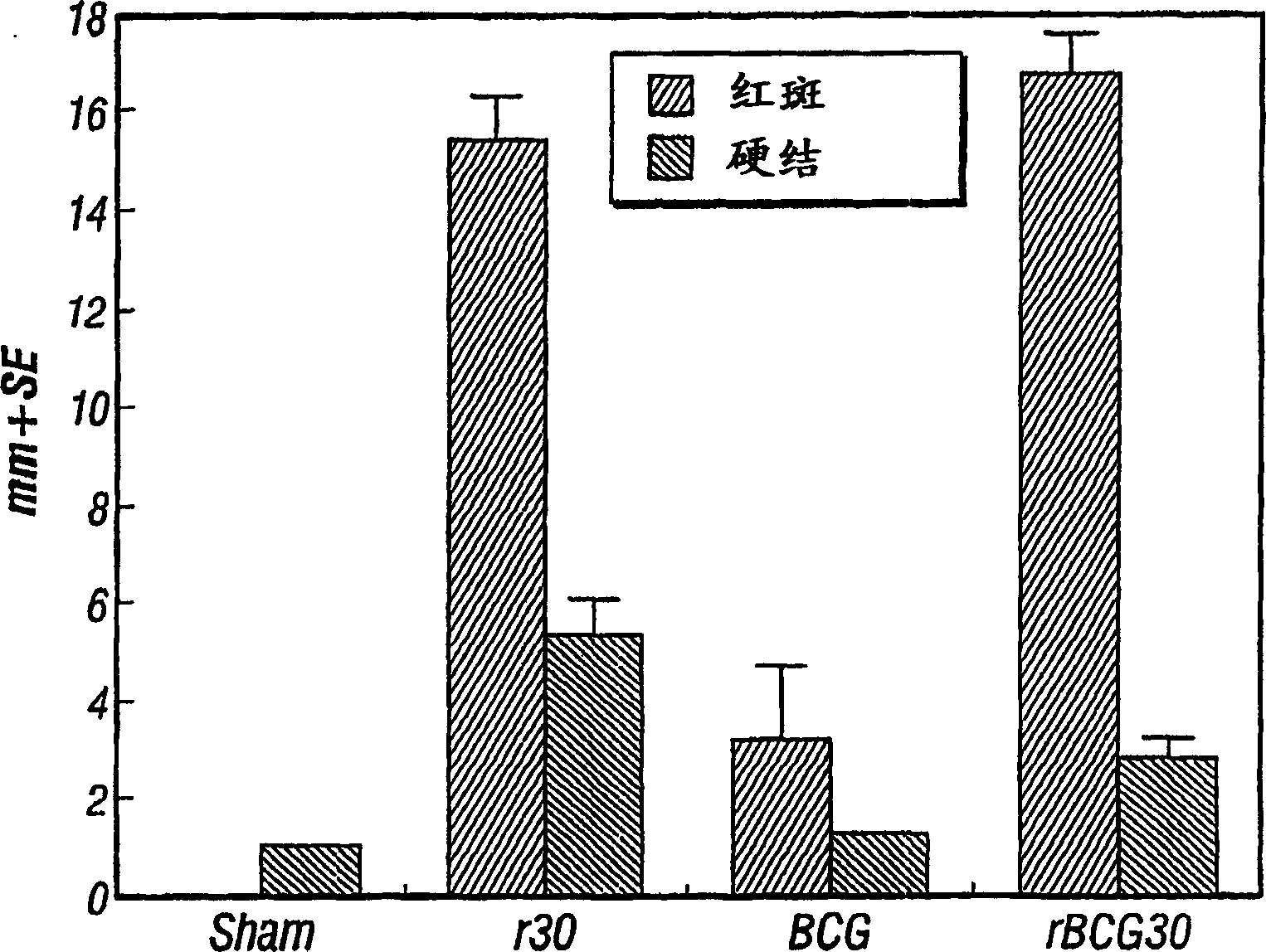
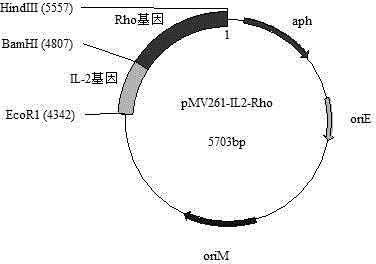
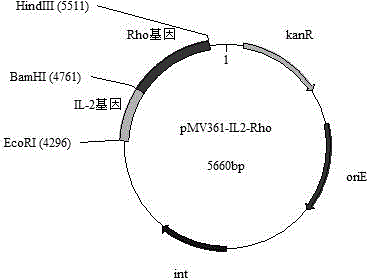
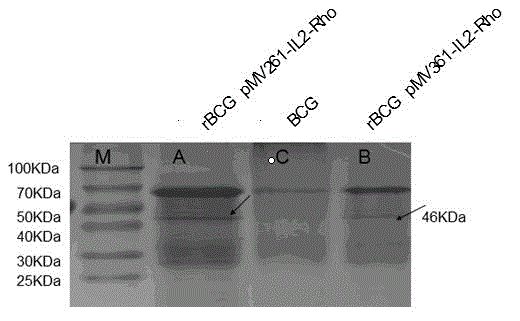
Patents
Literature
80 results about "Recombinant bcg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel recombinant bcg tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Novel Recombinant BCG Tuberculosis Vaccine Designed to Elicit Immune Responses to Mycobacterium Tuberculosis in all Physiological Stages of Infection and Disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to Mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Recombinant BCG strains with attenuated immunosuppressive properties
InactiveUS20060115494A1Reduced capability to manipulate responseStrong immune responseBacterial antigen ingredientsBacteriaCytosolSuper oxide dismutase
Strains of Mycobacterium that have decreased immunosuppressive properties are provided. The Mycobacterium strains are genetically engineered to express but not secrete super-oxide dismutase (Sod). The presence of cytosol bound Sod allows replication and growth of the Mycobacterium, but does not result in attenuation of the host immune response. The Mycobacterium strains provide improved properties for use as vaccines.
Owner:AERAS GLOBAL TB VACCINE FOUND
Echinococcus granulosus recombinant BCG vaccine and preparation method thereof
InactiveCN103041382AImprove thermal stabilityEasy to transportBacteriaAntiparasitic agentsImmune effectsHeat stability
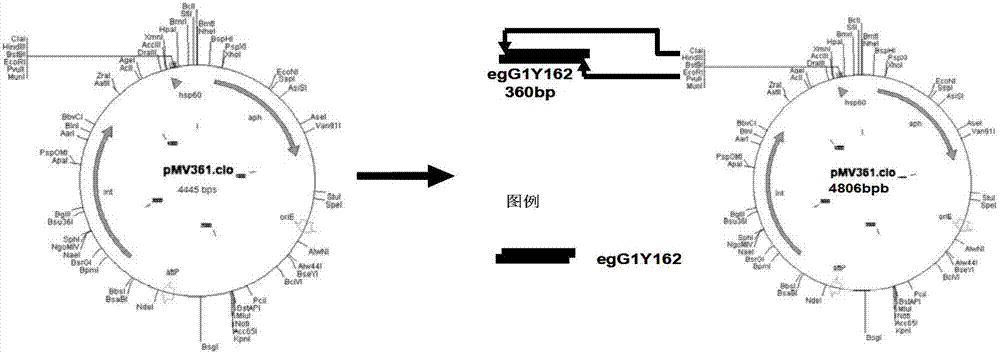
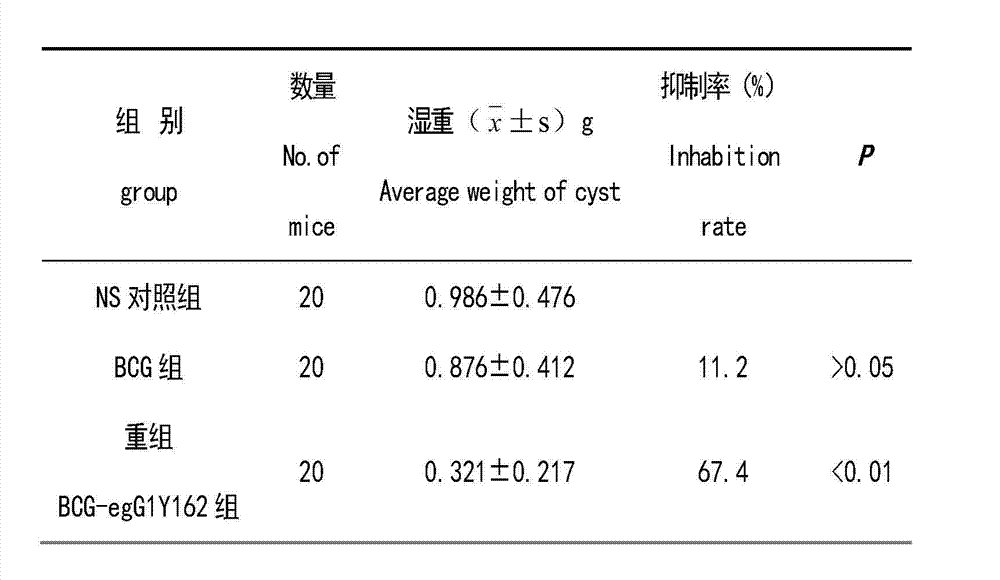
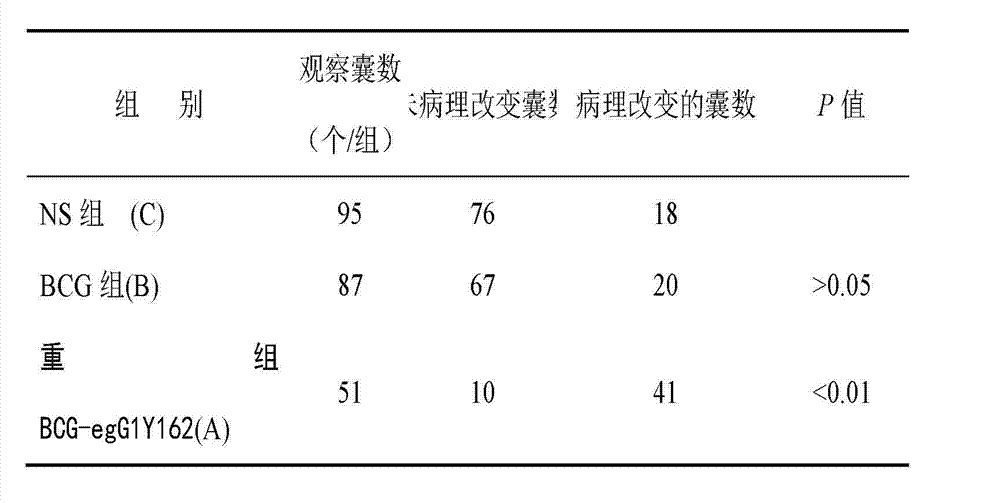
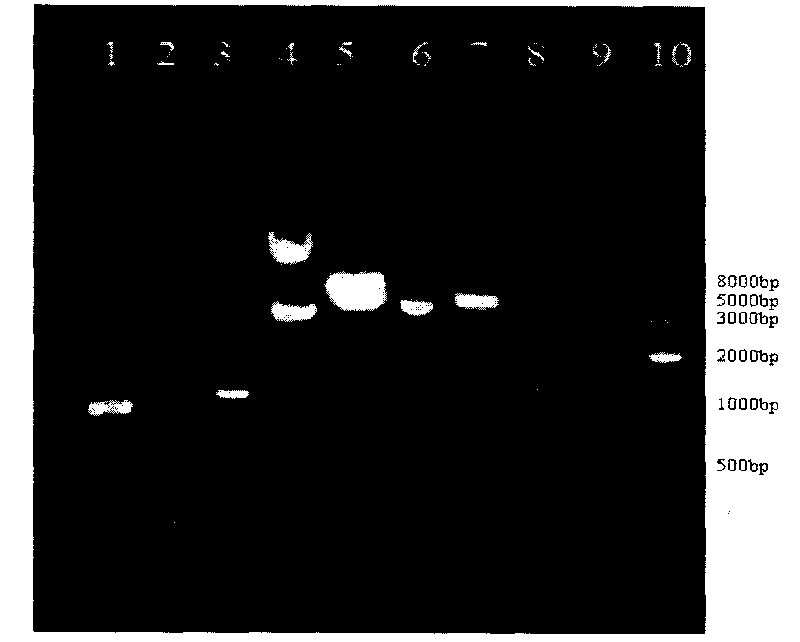
The invention provides the preparation of an echinococcus granulosus recombinant BCG vaccine. The vaccine is good in heat stability and convenient to transport and store; single immunity can induce high potency antibody aiming at target antigen, so that immunity is durable, immune time is few, immune procedure is simplified, and immune effect aiming at echinococcus granulosus is enhanced.
Owner:XINJIANG MEDICAL UNIV
Improved recombinant bacillus calmetter Guerin (BCG)
InactiveCN101721693AHigh expressionSolving the problem of insufficient immune stimulationAntibacterial agentsBacterial antigen ingredientsEscherichia coliShuttle plasmid
The invention discloses an improved recombinant bacillus calmetter Guerin (BCG), belonging to the technical field of new medicaments. The immune protecting effect of a unique anti-tuberculosis vaccine BCG for tuberculosis is not exact and the tuberculosis morbidity gradually rises over the past 10 years. Therefore, the development of a more effective anti-tuberculosis vaccine is important. Gene sequences of mycobacterium tuberculosis ESAT6 and Ag85A are inserted into shuttle plasmids of colon bacillus-mycobacterium tuberculosis for forming recombinant plasmids; and the recombinant plasmids are converted into the BCG to form a recombinant anti-tuberculosis vaccine. Proved by research, the recombinant BCG is used for expressing introduced foreign genes and is a novel anti-tuberculosis vaccine.
Owner:SICHUAN UNIV
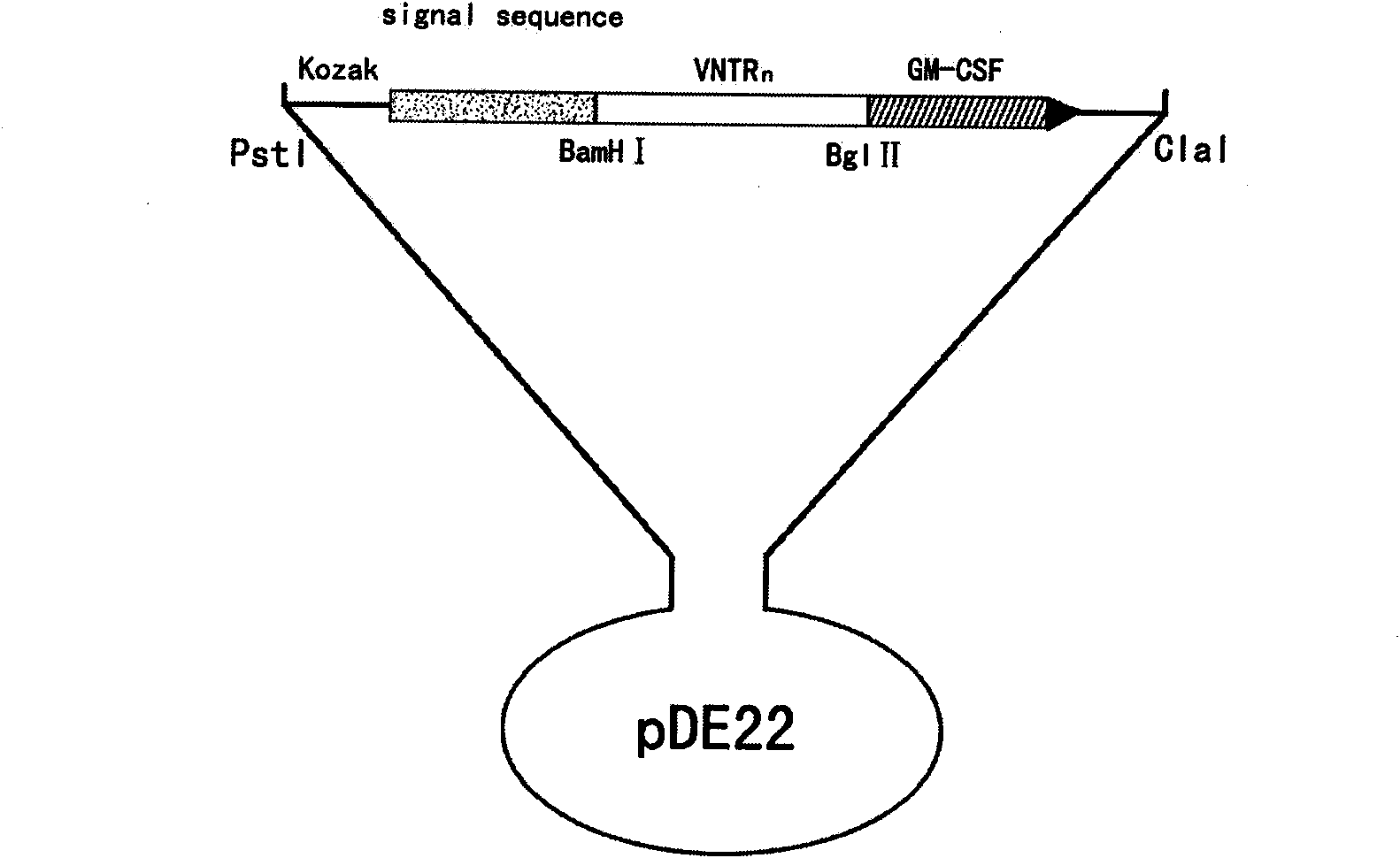
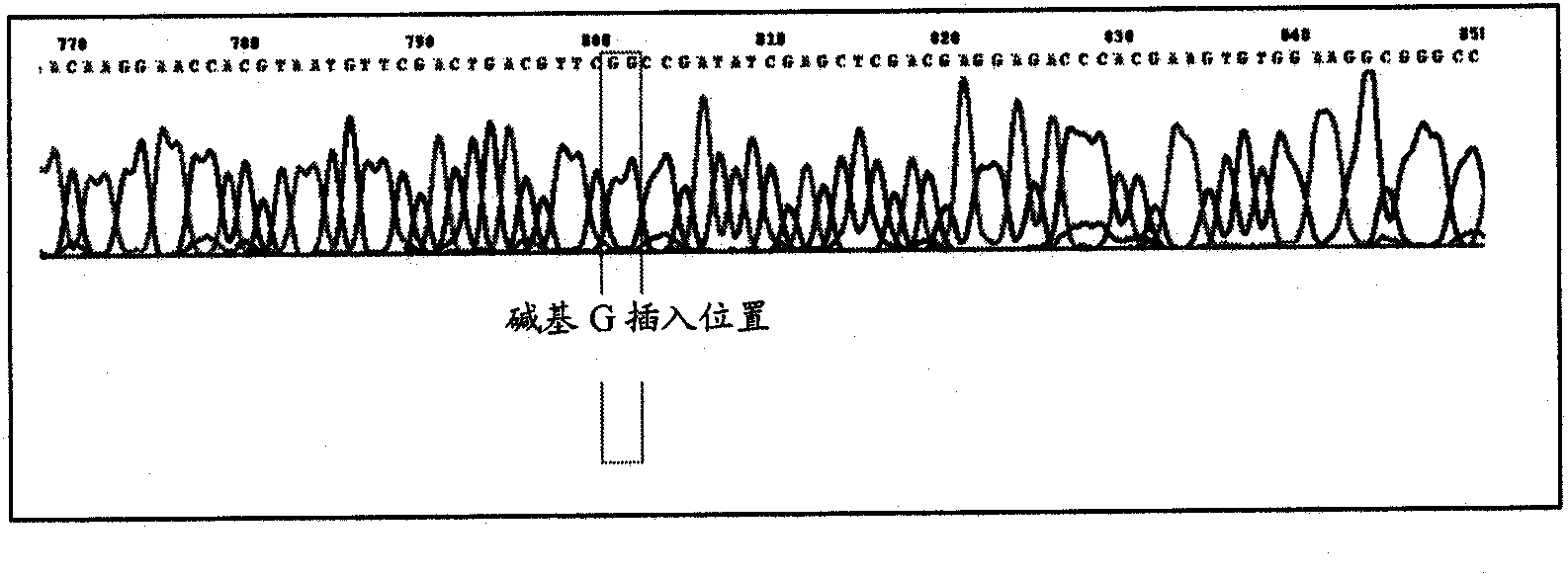
Recombinant BCG vaccine based on human MUC1 repetitive sequence and GM-CSF fusion expression
InactiveCN101575607AGood antigenicityMaintain secretory functionGenetic material ingredientsAntibody medical ingredientsSide effectAdjuvant
The invention discloses a recombinant BCG vaccine based on human MUC1 repetitive sequence and GM-CSF fusion expression. Firstly, MUC1 repetitive sequence multimers and fusion genes connected with GM-CSF genes by connecting DNA are constructed, a signal peptide sequence and a Kozak sequence are further connected, and an expression vector is constructed; and the constructed fusion genes are transfected to BCG vaccines, and applied to the preparation of anti-tumor vaccine or anti-tumor medicament by using MUC1 as a target spot. The recombinant BCG vaccine realizes effective combination of antibody, adjuvant and vaccine vector so that the immunogenic property of the MUC1 tumor vaccine achieves more effective and durable effect; and simultaneously the recombinant BCG vaccine is safe, has no toxic and side effects, and can induce specific T cell immune response and durable secretion expression of the human MUC1 repetitive sequence and GM-CSF fusion protein.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Co-expression system and construction method of polyvalent bacteriophage lyase genes, live vaccine of carrying system and preparation and application of live vaccine
InactiveCN104673821AEnables cheap scalingNo autolysisAntibacterial agentsBacterial antigen ingredientsMycobacterium smegmatisLatent tuberculosis
The invention discloses a co-expression system and construction method of polyvalent bacteriophage lyase genes, a live vaccine of a carrying system and preparation and application of the live vaccine. Eukaryotic expression plasmids are used as an expression vector, and LysinA, LysinB and Holin gene segments are directionally inserted into the plasmids to simultaneously express the co-expression system of the bacteriophage lyase genes of three kinds of targeted mycobacterium tuberculosis. Mycobacterium smegmatis with good targeting property of macrophages or genetically-modified recombinant BCG is used as a live vector, the co-expression system simultaneously carrying three kinds of genes is electrically transformed into the mycobacterium smegmatis or the genetically-modified recombinant BCG, and then the recombinant therapeutic tuberculosis live vaccine is obtained through expansion in vitro. The live vaccine has a good effect on the field of curing active tuberculosis or latent tuberculosis infection caused by proliferative mycobacterium tuberculosis, dormant mycobacterium tuberculosis and drug-resistant mycobacterium tuberculosis.
Owner:伊正君
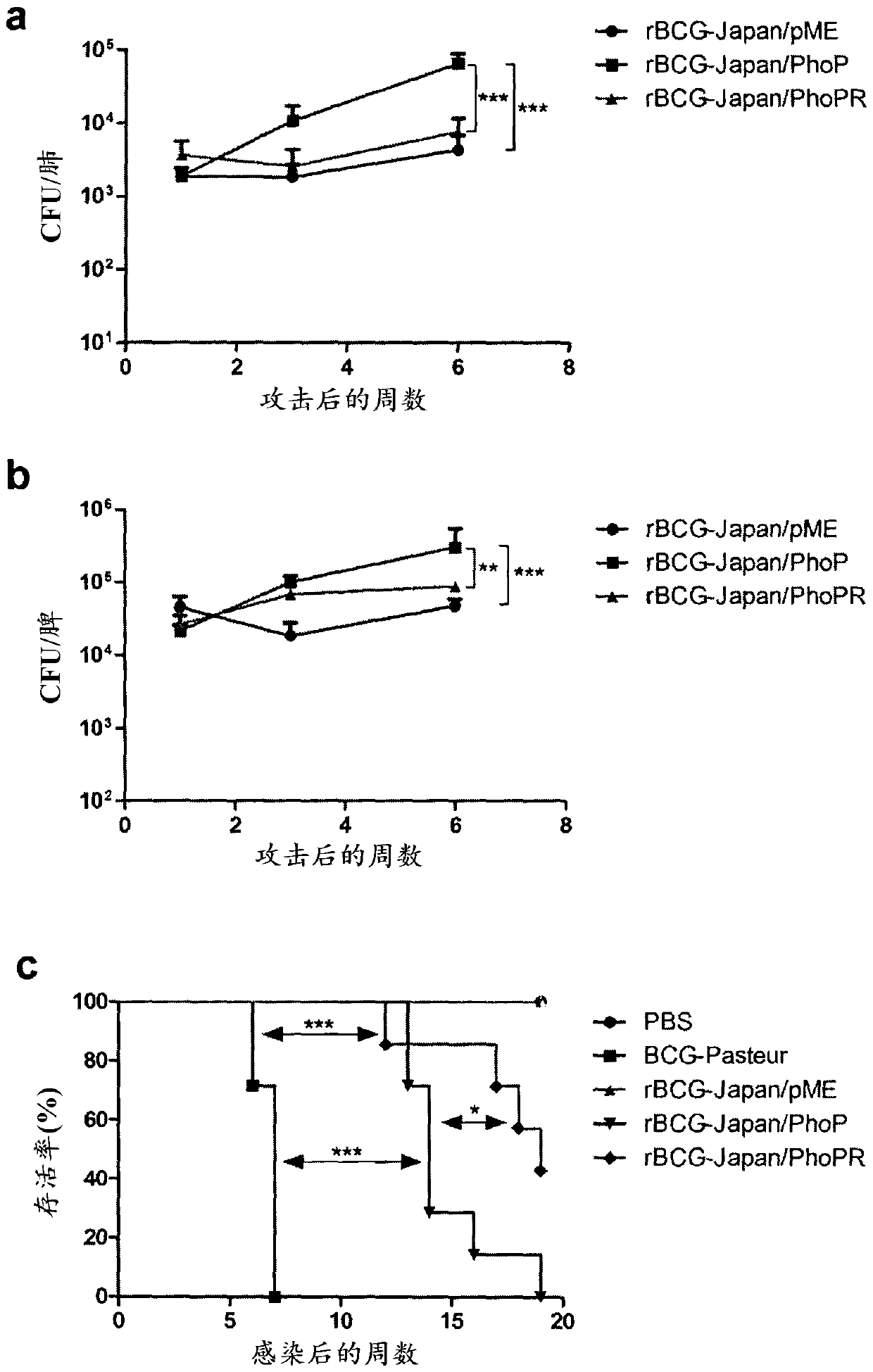
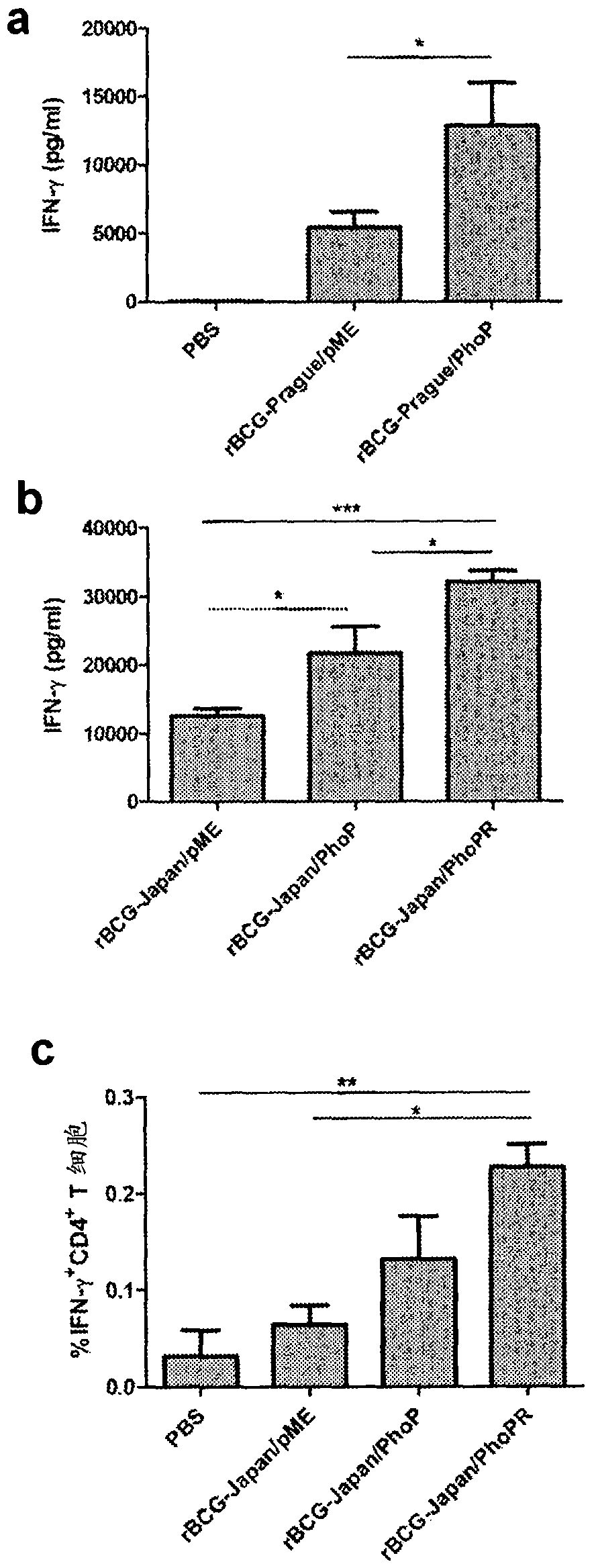
Tuberculosis vaccines including recombinant bcg strains overexpressing phop, and/or phop regulon protein(s)
A live recombinant Mycobacterium bovis-BCG strain comprising a nucleic acid encoding PhoP protein and / or one or more phoP regulon proteins is provided, in which the nucleic acid is capable of being overexpressed. A vaccine or immunogenic composition comprising the live recombinant Mycobacterium bovis-BCG strain and a method for treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis are also provided.
Owner:成都安永鼎业生物技术有限公司
Novel recombinant vaccine used for preventing tuberculosis
InactiveCN101850112AStable expressionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to the construction of a human GM-CSF gene and Mycobacterium tuberculosis ESAT6 gene chimerically expressed GMCSF-ESAT6 protein recombinant Bacillus Calmette Guerin (BCG) vaccine and immunogenicity research thereof, namely the sequence of the human GM-CFS gene and the sequence of the Mycobacterium tuberculosis ESAT6 gene are inserted into the sequence of the same escherichiacoli-Mycobacterium tuberculosis shuttle plasmid pMV361 by gene engineering technology so as to construct a recombinant shuttle plasmid rpMV361GMCSF-ESAT6; and a vector is introduced into a BCG vaccine by an electroporation method so as to construct a recombinant BCG vaccine rBCG:GMCSF-ESAT6. The recombinant BCG vaccine can express the human GM-CSF and Mycobacterium tuberculosis ESAT6 gene chimeric protein GMCSF-ESAT6 stably, and has an immunogenicity superior to that of the conventional BCG vaccine. The invention provides a process for preparing the recombinant BCG vaccine, researches the immunity thereof, and belongs to the field of gene engineering and the field of tuberculosis vaccine. The novel recombinant vaccine prevents the generation and the propagration of tuberculosis more effectively.
Owner:SICHUAN UNIV
Construction and application of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG)
InactiveCN102327604AConnection direction is correctMeet the design requirementsAntibacterial agentsBacterial antigen ingredientsAntigenSide effect
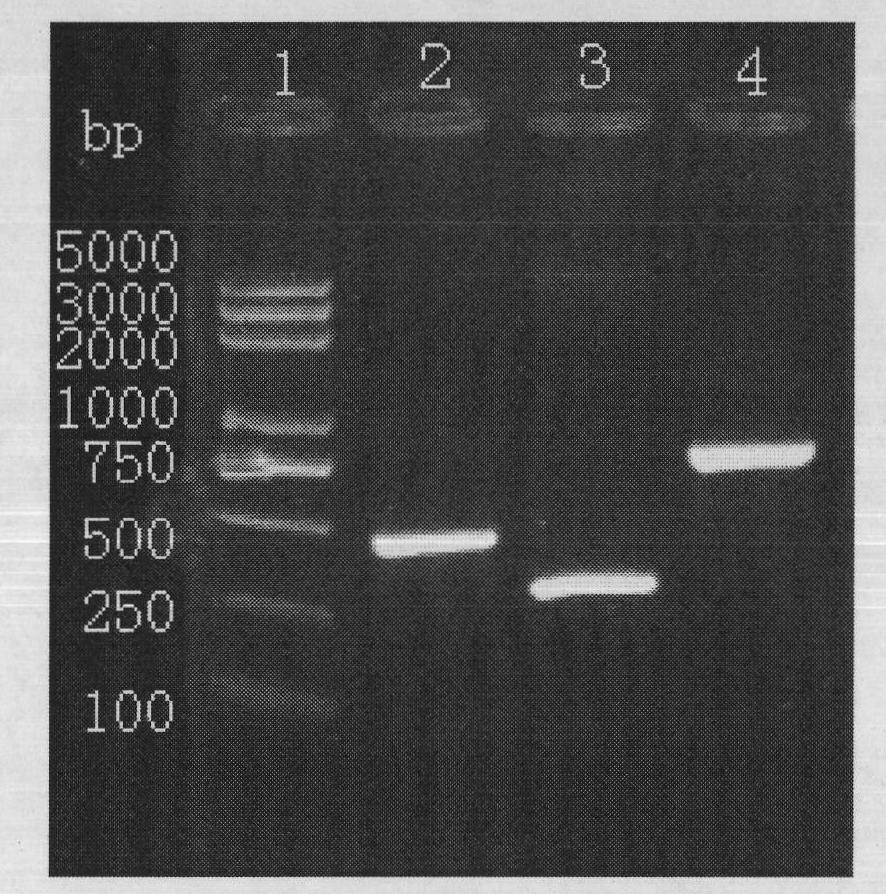
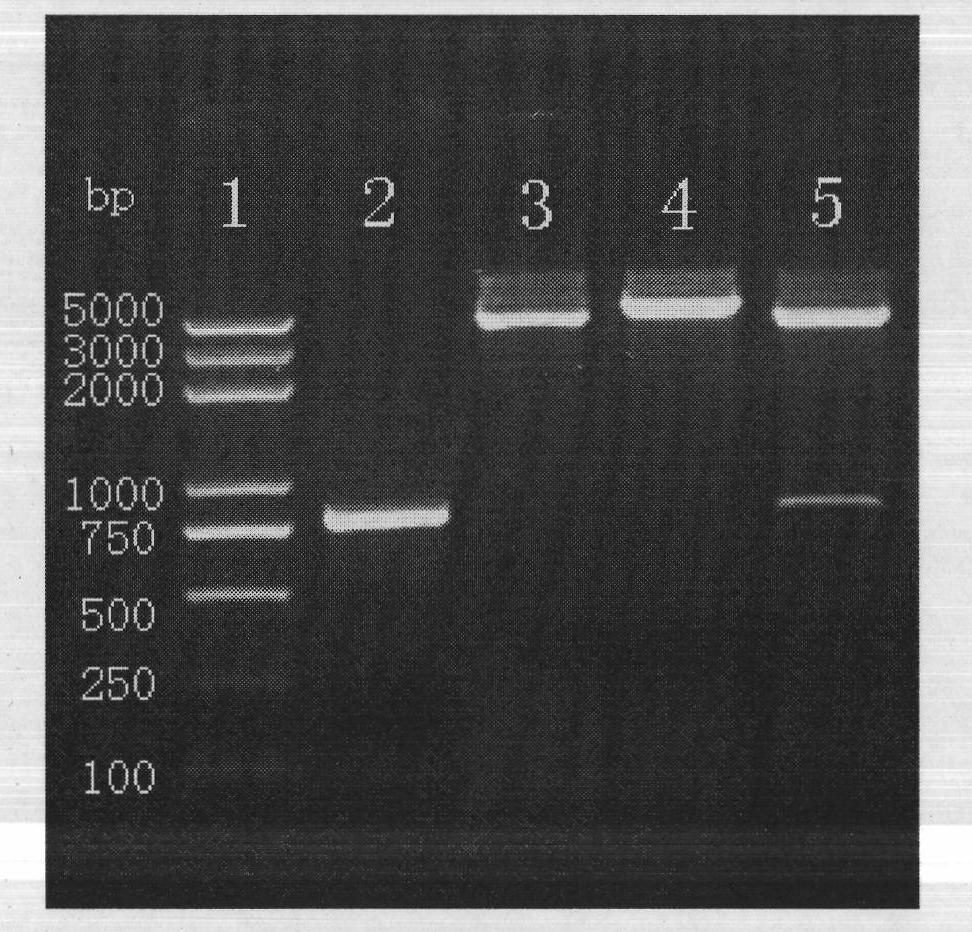
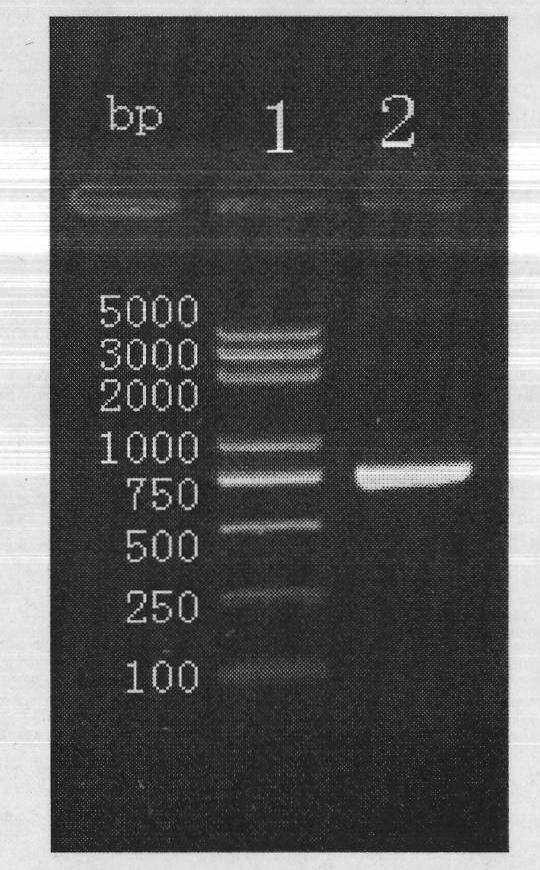
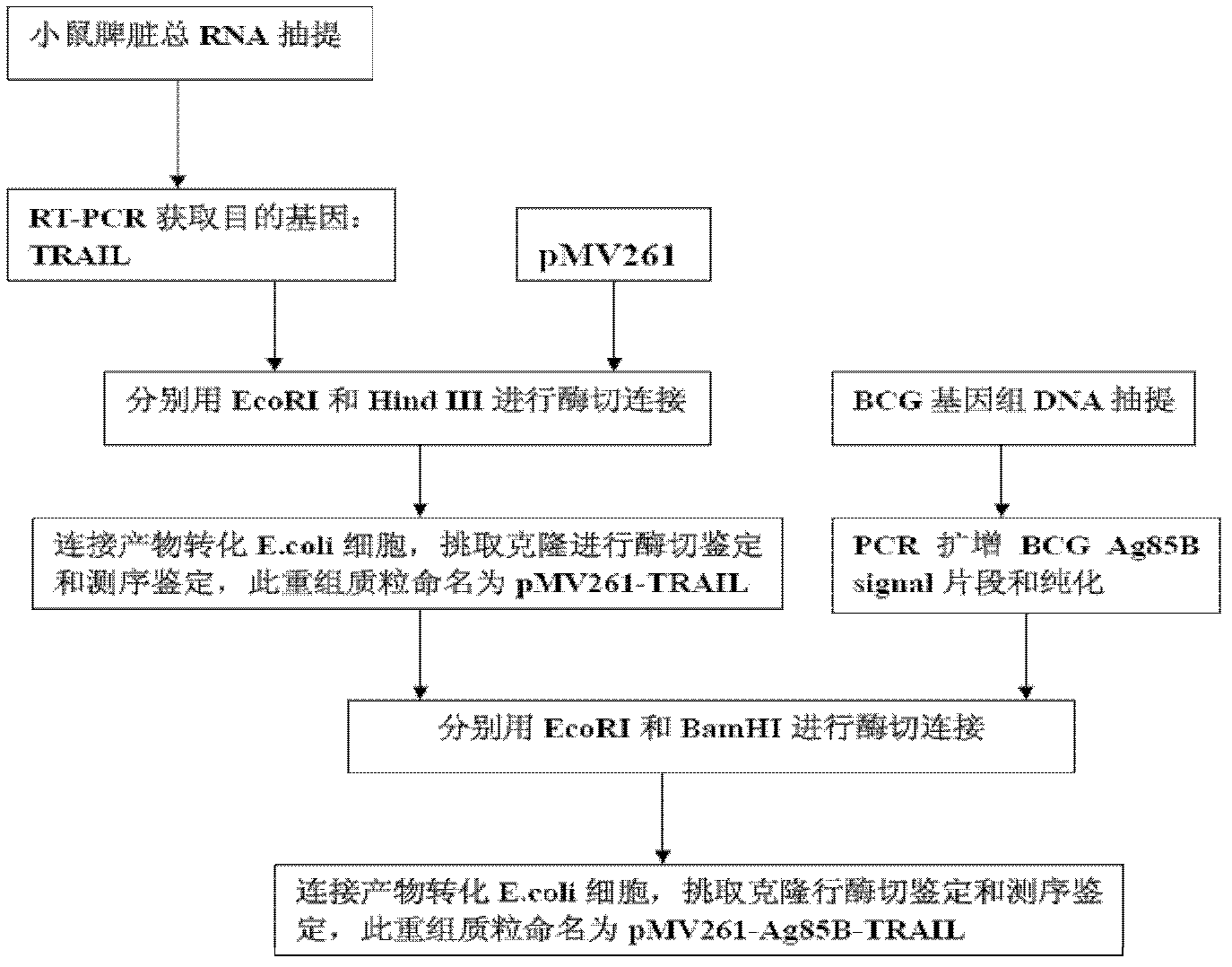
The invention provides construction of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG), and relates to a shuttle expression vector comprising a signal peptide fragment of a major secretory antigen Ag85B of BCG and a gene fragment of a TRAIL and a construction method thereof. The obtained shuttle expression vector pMV261-Ag85B-TRAIL is used for constructing rBCGTRAIL, and can be applied to preparation of TRAIL rBCG for treating superficial bladder tumors, preventing postoperative recurrence thereof and preventing tuberculosis. The rBCG has dual functions of TRAIL and BCG, so that cooperative and synergistic actions of the TRAIL and BCG can be better brought into play; rBCG-TRAIL can secrete TRAIL, and the using amount of the rBCG-TRAIL can be lower than that of the BCG under the condition that the same or better immune effect is achieved, so that the toxic or side effect is reduced; and the rBCG-TRAIL can directly secrete TRAIL efficiently on a certain part, so that tumor cells can be killed in cooperation with the rBCG-TRAIL, and high cost caused by the use of a foreign cell factor is avoided.
Owner:沈周俊
RECOMBINANT BCG OVEREXPRESSING phoP-phoR
The present invention provides a live recombinant Mycobacterium bovis-BCG strain and a tuberculosis (TB) vaccine or immunogenic composition comprising a nucleic acid capable of overexpression, the nucleic acid encoding PhoP and PhoR proteins. A method for treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis using the strain is also provided.
Owner:成都安永鼎业生物技术有限公司
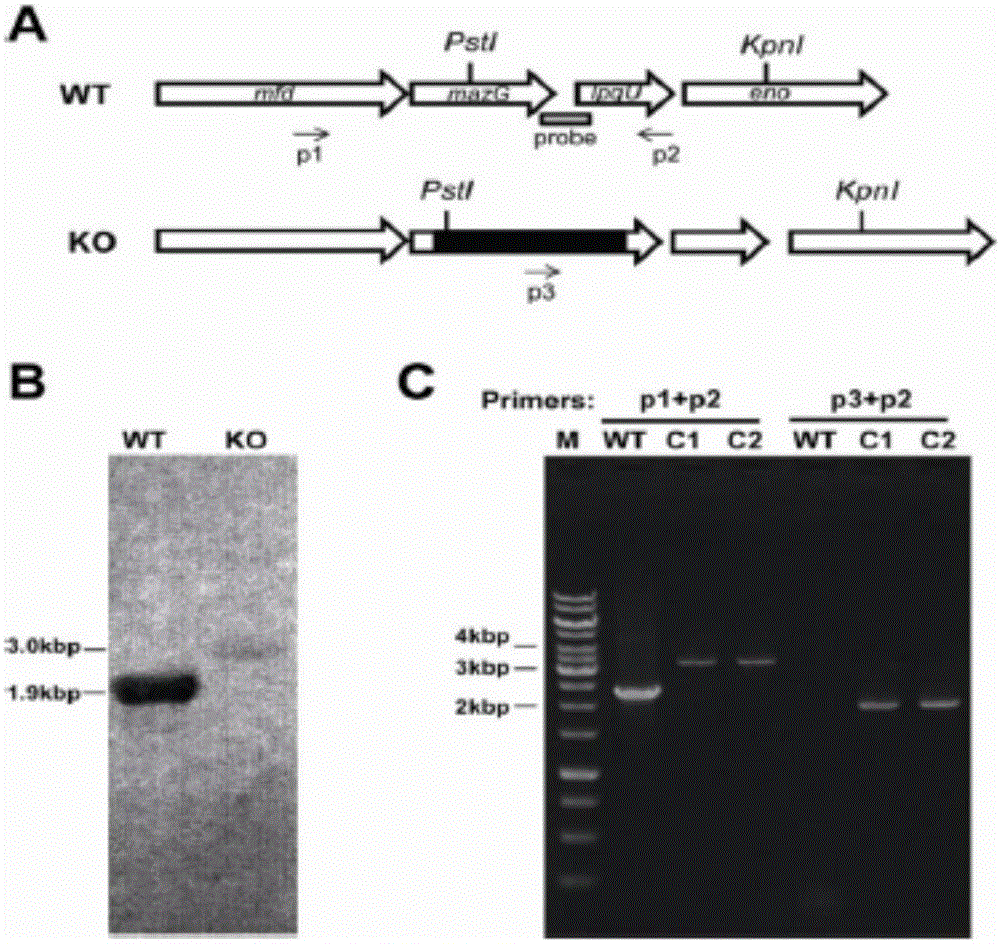
Antituberculous recombinant bacillus calmette-guerin with mazG gene deleted
The invention provides antituberculous recombinant bacillus calmette-guerin (BCG delta mazG) with a mazG gene deleted. The mazG gene in a genome of the antituberculous recombinant bacillus calmette-guerin with the mazG gene deleted is replaced with a hygromycin resistant gene. The invention further provides a preparation method of the BCG delta mazG and application thereof in preparing a recombinant vaccine for preventing tuberculosis. After the BCG delta mazG is inoculated subcutaneously into a mouse, a T cell immune response is obviously improved, after the immunized mouse is infected with a mycobacterium tuberculosis standard strain H37Rv, the bacterial load of the infected animal lung and spleen is significantly decreased, the pathological degree is relieved, and after the immunized mouse is infected for five weeks, a T cell secondary immune response is significantly improved. It is found through bacterial in-vivo survival tests that after high-dose intravenous inoculation is conducted, the BCG delta mazG can survive on viscera of the spleen, the lung, the liver and the kidney of the mouse continuously for 20 w or above, and the amount of bacteria of a BCG delta mazG recombinant strain is obviously increased compared with a wild strain. It is proved through experiments that the BCG delta mazG recombinant strain has antituberculous immunogenicity, protective efficacy and in-vivo persistence activity which are significantly improved, and the antituberculous recombinant bacillus calmette-guerin (BCG delta mazG) with the mazG gene deleted is expected to become one of the novel candidate vaccines for preventing tuberculosis infection.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
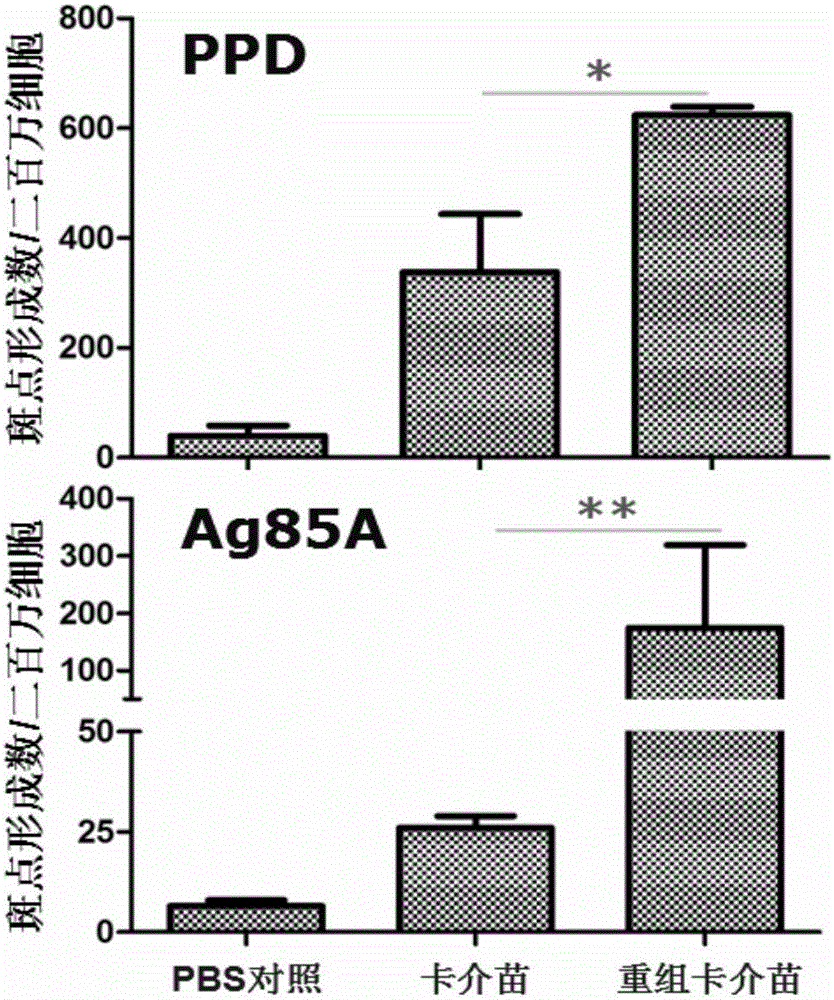
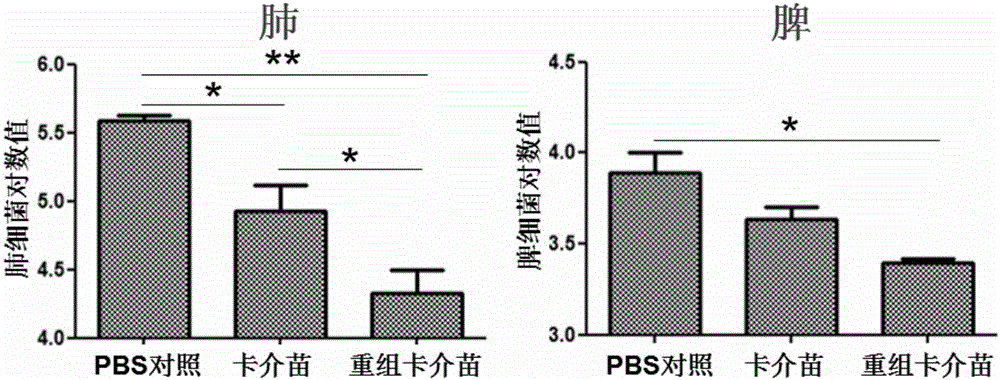
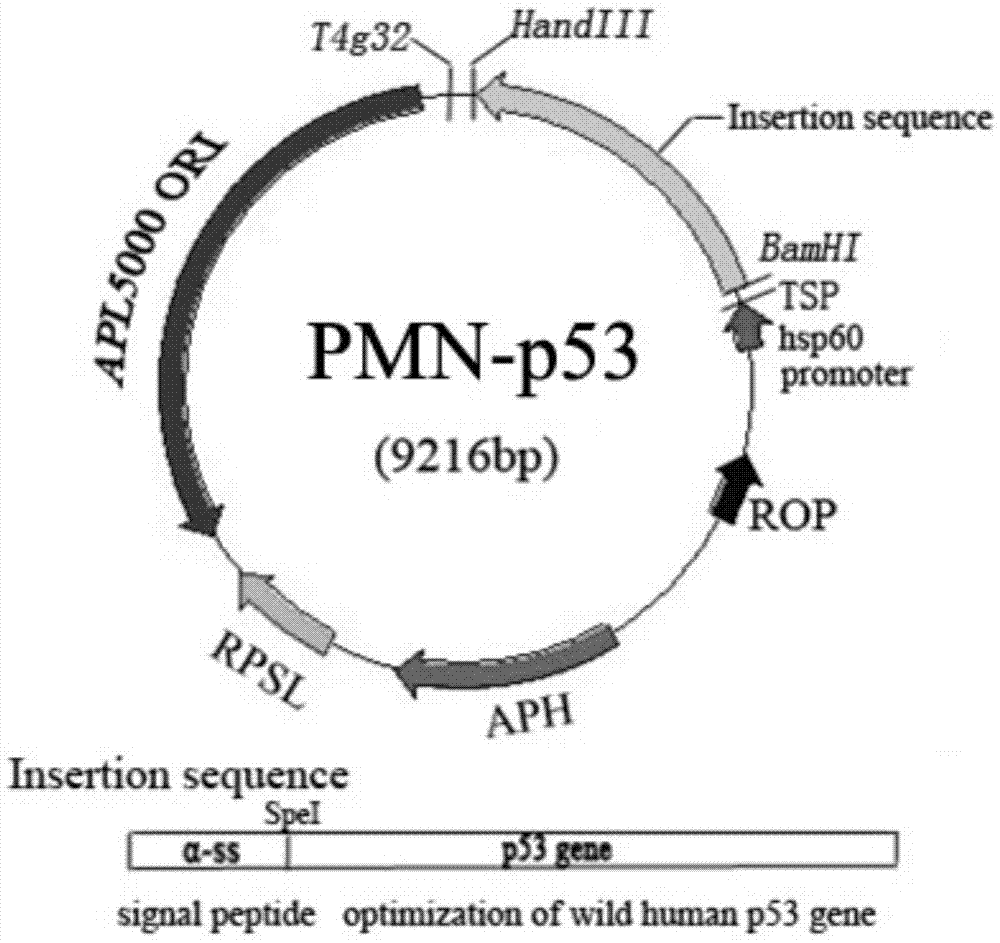
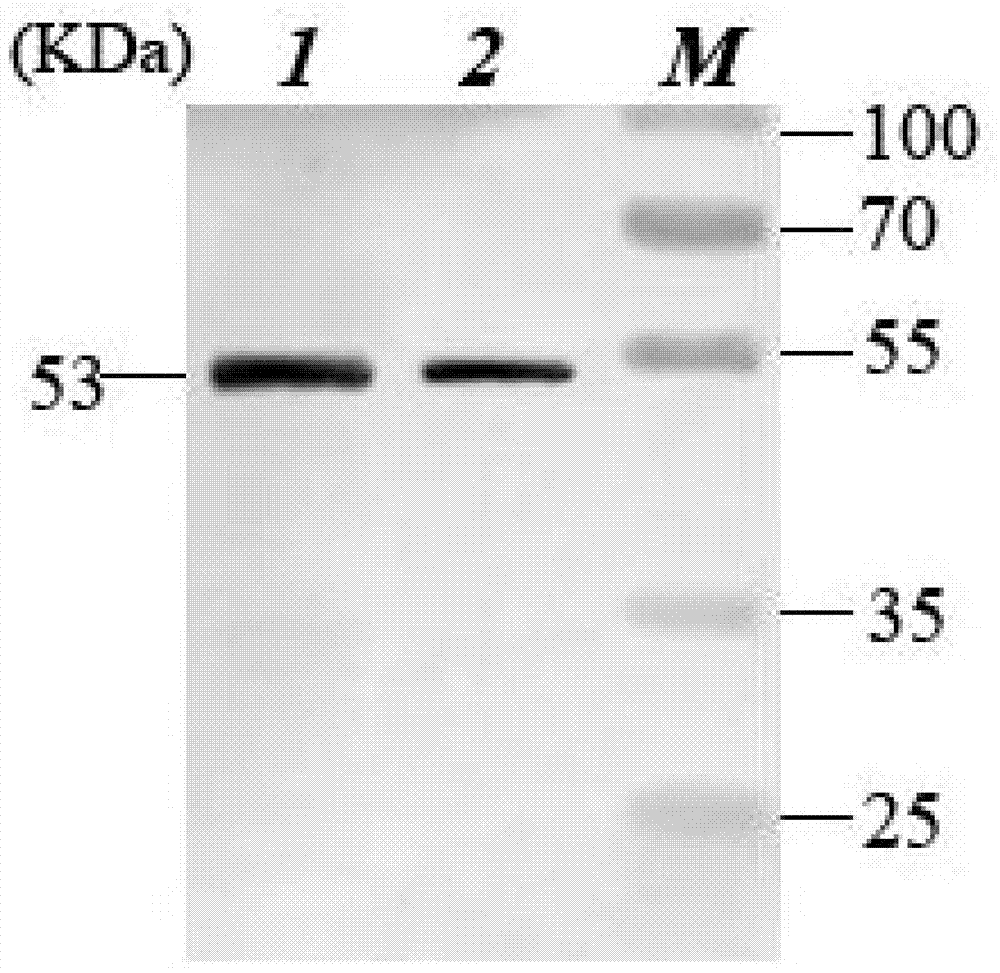
Recombination BCG viable bacterium strain capable of expressing and secreting human p53 protein, viable bacterium vaccine and construction method and application thereof
ActiveCN103497926APlay a role in preventioPlay a therapeutic roleBacteriaGenetic material ingredientsVaccinationHuman tumor
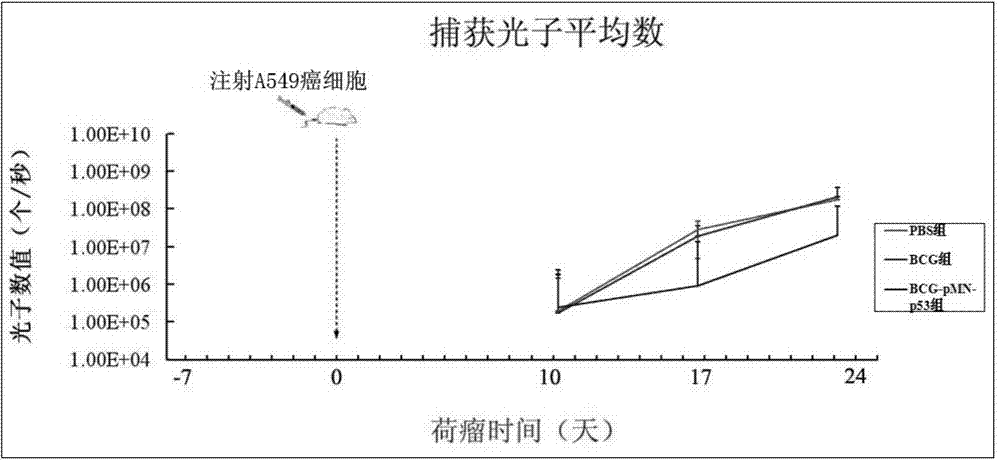
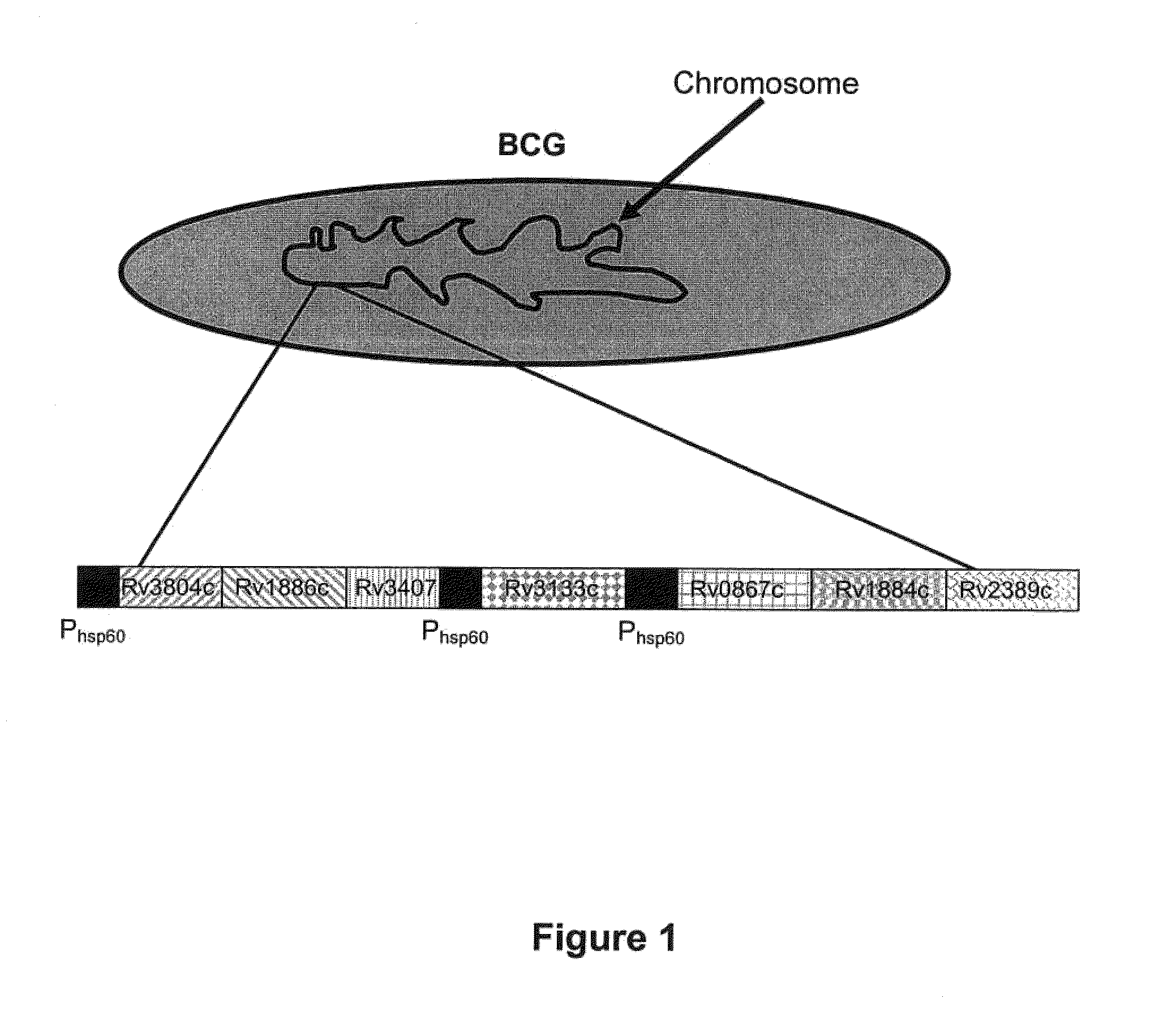
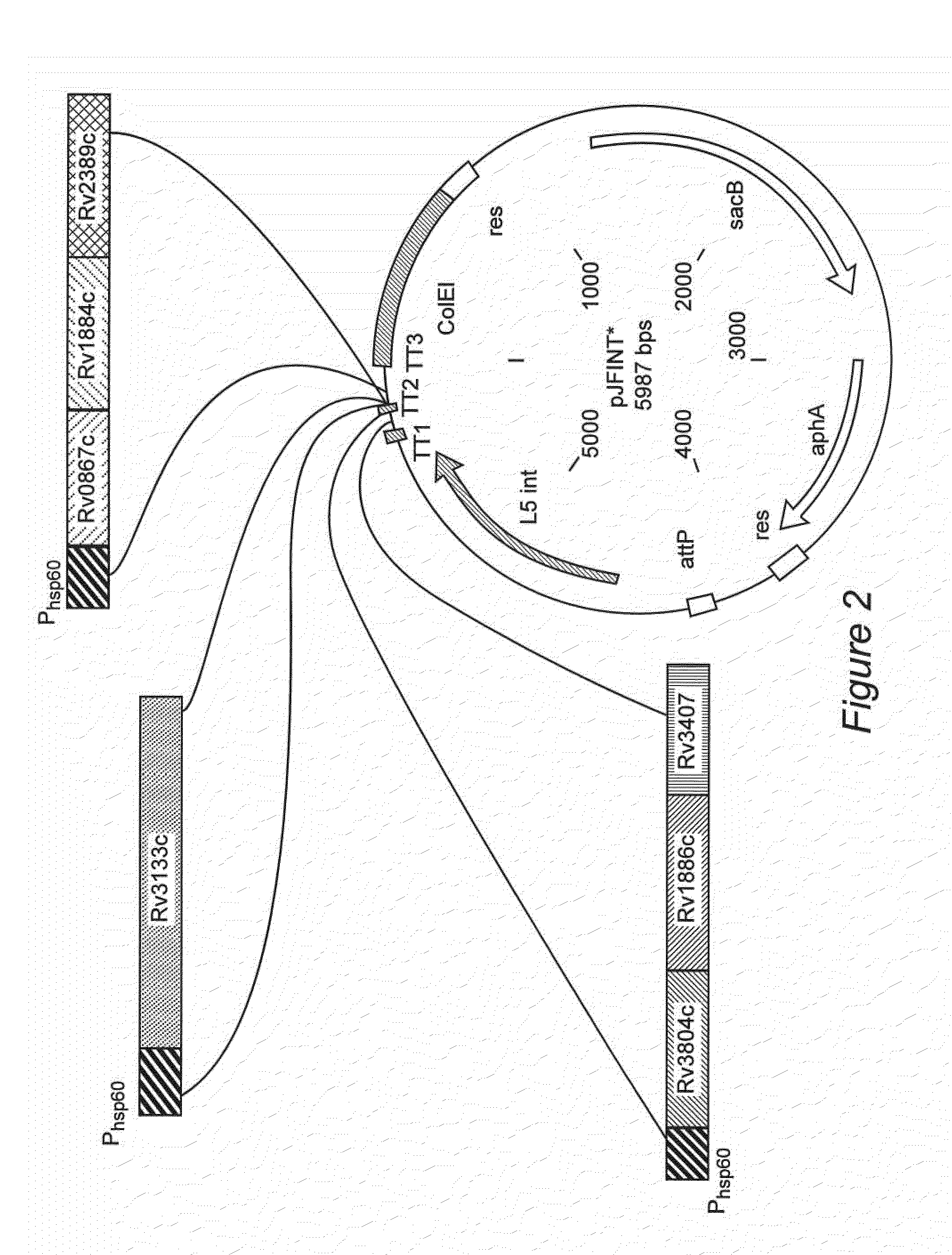
The invention provides a recombination BCG viable bacterium strain capable of expressing and secreting human p53 protein, a viable bacterium vaccine and a construction method and application thereof, and relates to the technical field of biology, in particular to the technical field of genetic engineering. The novel viable bacterium vaccine capable of being used for prevention and treatment of human tumor diseases is provided. The recombination BCG viable bacterium strain is provided first, and the recombination BCG viable bacterium strain is a recombination strain which is obtained after a human p53 optimization gene sequence obtained after the preference of a codon is modified by a human p53 gene sequence for coding the human p53 protein referring to a BCG genome is led into the BCG viable bacterium strain. The invention further provides the construction method of the recombination strain and the viable bacterium vaccine obtained through the construction method. The viable bacterium strain can express and secrete the human p53 protein in cells, and zoology experiments show that the function for prevention and treatment of cancers can be achieved after the viable bacterium strain is used as a vaccination organism.
Owner:深圳市微宇生物科技有限公司
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
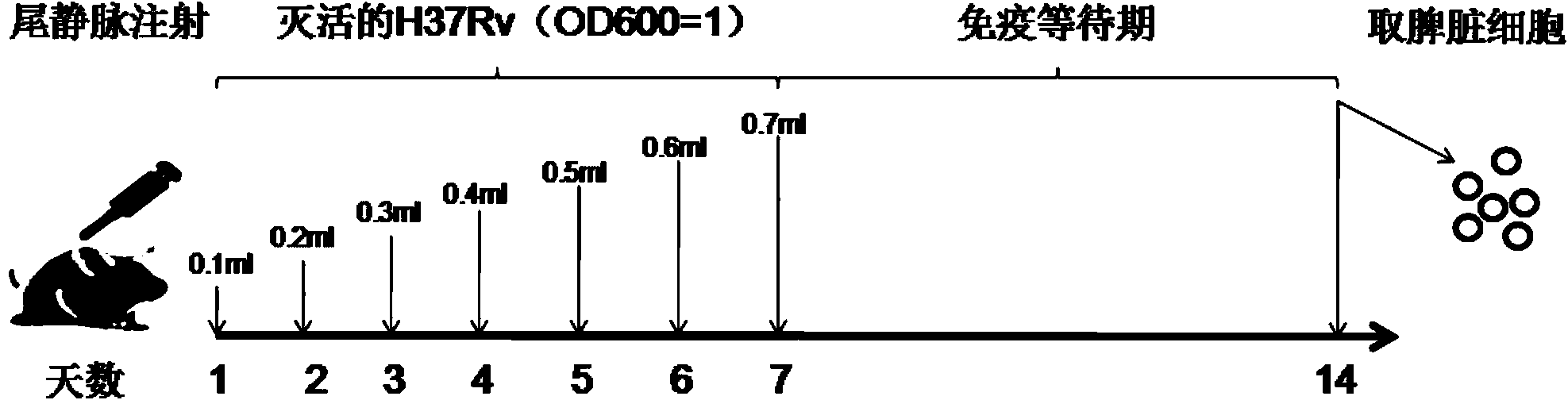
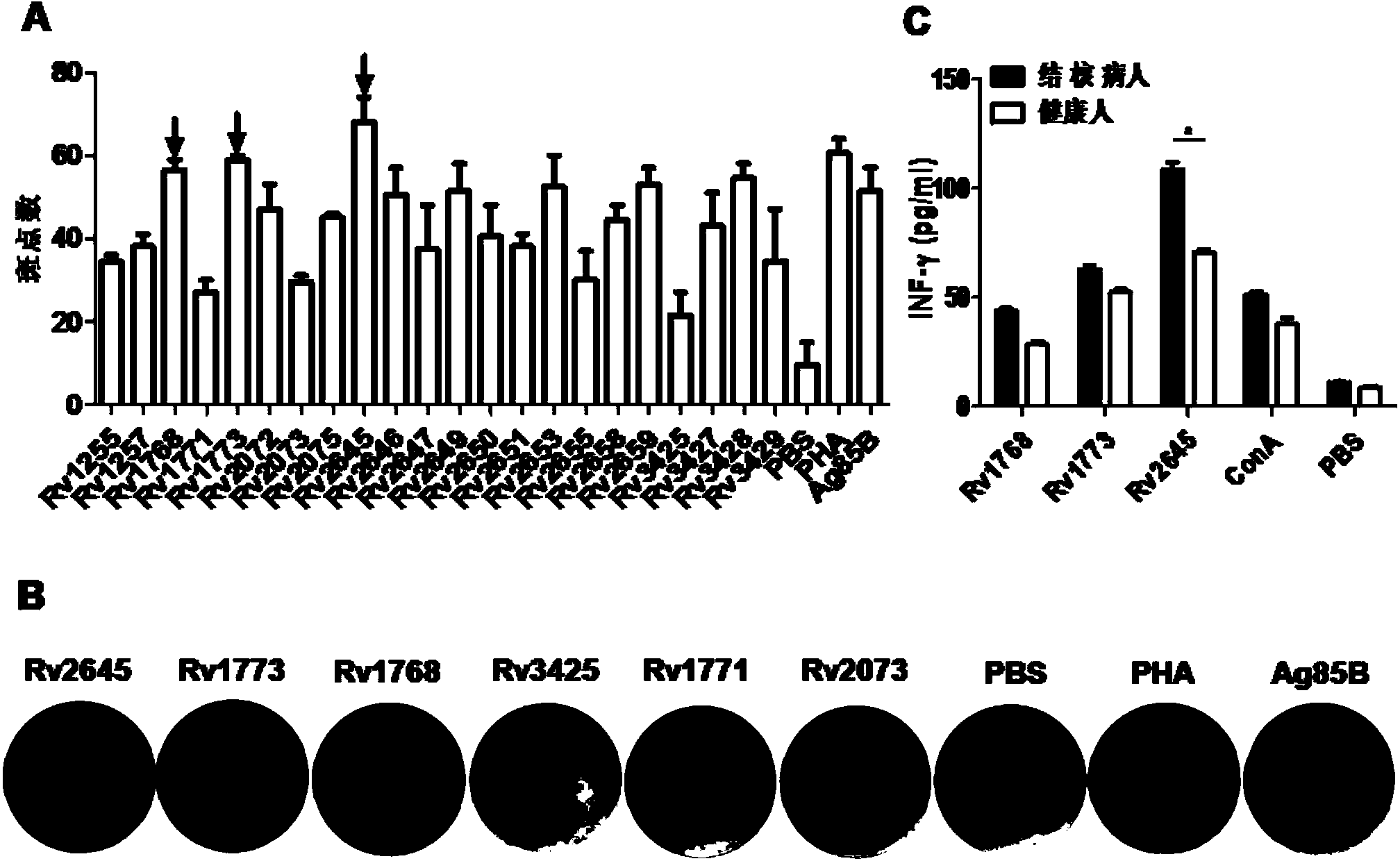
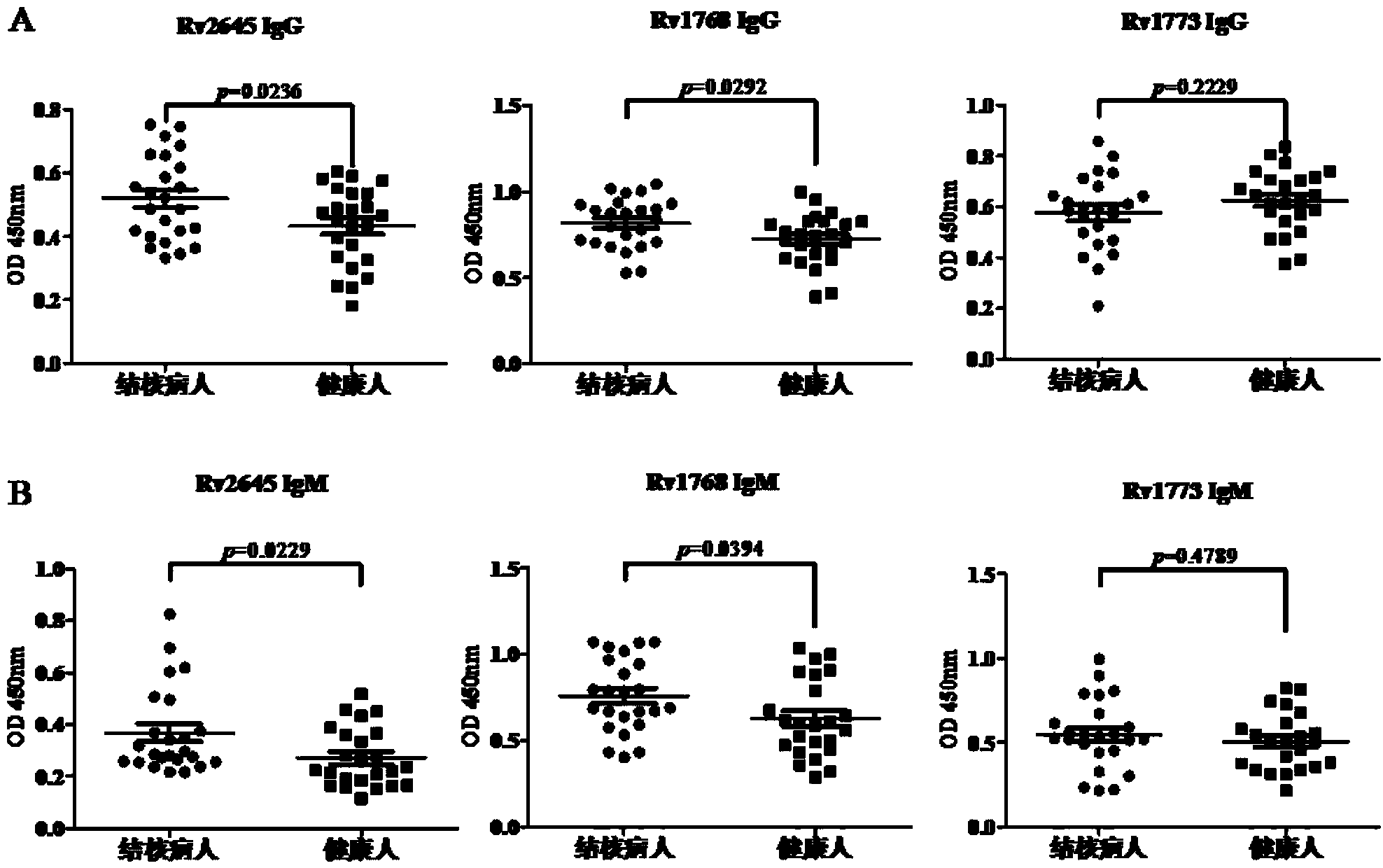
Preparation for Rv2645 protein, and application thereof on aspects of tuberculosis diagnosis and reorganized BCG vaccines
InactiveCN104292315AImproving immunogenicitySimple methodAntibacterial agentsBacterial antigen ingredientsSerum igeGamma interferon
The invention discloses preparation for Rv2645 protein, and the application thereof on aspects of tuberculosis diagnosis and recombination BCG vaccines. According to the preparation for Rv2645 protein and the application thereof, the Rv2645 protein in a tuberculosis gene RD10-14 zone is found to have the ability of inducing high-level gamma interferon, and when used for the diagnosis of the serum antibodies, especially IgG4 subtype antibodies, of a tuberculosis patient, has prominent statistics difference compared with the serum antibodies of healthy people; as the dominant antigen of the mycobacterium tuberculosis, the Rv2645 protein has the function of inducing high-level cellular immunity and humoral immunity, and can be used as a novel diagnostic reagent for tuberculosis; the Rv2645 protein can further be used for novel recombination BCG vaccines or protein vaccines for tuberculosis, the constructed recombination BCG capable of expressing the Rv2645 protein has the function of antituberculous immunity protection. According to the preparation for the Rv2645 protein and the application thereof provided by the invention, the Rv2645 protein is proved to have potential application value when used for tuberculosis diagnosis and reorganized BCG vaccines, so that the preparation and the application thereof has significant economic benefits.
Owner:WUHAN UNIV
Modified new coronavirus S gene, recombinant plasmid and recombinant bacillus calmette guerin vaccine constructed by same and application of recombinant bacillus calmette guerin vaccine
PendingCN113403330AEvoke an immune responsePrevent intrusionAntibacterial agentsSsRNA viruses positive-senseGene terminatorVirus Protein
The invention discloses a modified new coronavirus SARS-CoV-2S protein, a recombinant plasmid and a recombinant bacillus calmette guerin vaccine constructed by the modified new coronavirus SARS-CoV-2S protein, and application of the recombinant bacillus calmette guerin vaccine, and belongs to the technical field of biological agents. Through modifying a partial sequence of gene promoter region and a partial sequence of gene terminator region, the modified new coronavirus S gene complete sequence can be smoothly expressed in an escherichia coli-mycobacterium tuberculosis shuttle plasmid pMV261 to obtain a recombinant plasmid pMVS; the recombinant plasmid pMVS is introduced into BCG through electric transformation, and recombinant BCG (rBCG) is obtained; the rBCG can successfully express S protein, arouse human immune response and induce antibody generation to prevent virus invasion, and the constructed rBCG is a subunit vaccine, has a better protection effect than parent BCG, can prevent or treat new coronavirus and mycobacterium tuberculosis, and has huge social benefits.
Owner:JINING MEDICAL UNIV
Growth regulatable recombinant BCG compositions
InactiveUS8163294B2Antibacterial agentsBacterial antigen ingredientsImmunogenicityIntracellular pathogen
Immunogenic compositions comprising growth regulatable recombinant attenuated intracellular pathogens that have been transformed to express recombinant immunogenic antigens of the same or other intracellular pathogens are provided. Exemplary immunogenic compositions include, growth regulatable and growth limited recombinant attenuated intracellular pathogen immunogenic compositions.
Owner:RGT UNIV OF CALIFORNIA
Human interferon alpha-2b recombinant bacillus calmette-guerin, construction method and identification method thereof
InactiveCN101381728AImprove anti-bladder cancer effectReduce the applied doseBacterial antigen ingredientsMicrobiological testing/measurementSide effectImmunocompetence
The invention relates to a recombinant BCG vaccine rBCG-IFN alpha-2b for secreting human IFN alpha-2b and a construction method and an identification method thereof, wherein a BCG vaccine Ag85B signal peptide fragment which has the function of secretion and genes of human IFN alpha-2b are cloned to pMV261 by the genetic engineering technology, and a BCG vaccine shuttle expression vector pMV261-Ag85B-IFN alpha-2b is obtained; and the vector is induced into BCG by the electrotransformation technology, and the recombinant BCG vaccine rBCG-IFN alpha-2b is established; and the human IFN alpha-2b can be highly efficiently secreted in virtue of the secretion function of the pMV261-Ag85B-IFN alpha-2b on BCG replication and signal peptide. The recombinant BCG vaccine rBCG-IFN alpha-2b obtained not only keeps the immunogenicity of the prior BCG but also can continuously secrete the cell factor-the IFN alpha-2b, thereby improving the immunocompetence of the BCG; the IFN alpha-2b can be directly acted on tumor cells to inhibit proliferation and induced differentiation of the tumor cells, has good antitumor action, and can reduce the application dosage and reduce the toxic and side effect caused by the BCG; and the IFN alpha-2b solves the problems of large application dosage, high incidence rate of side effects, short response time and expensive cost caused by exogenous IFN alpha-2b.
Owner:丁国庆
Recombinant calmette-Guerin bacillus vaccine for secretion of human interferon-alphaza and its constructing method
InactiveCN1710071AImprove drug efficacyReduce the risk of tumor recurrenceRecombinant DNA-technologySignal peptideInterferon
This invention involves reorganization BCG vaccine for secretion of people interferon- alpha2a, and structuring method. Utilize gene engineering to clone BCG vaccine Ag85B signal peptide part and the genes of people IFN - alpha 2a playing a secreting role to pMV261 to receive the BCG vaccine shuttling expression carrier pMSIFN - alpha 2a. Adopt electric boring technology to lead pMSIFN - alpha 2a into BCG to recombinate rBGGIFN - alpha2a. Depending on rBGGIFN - alpha 2a duplicating in BCG and the secretion function of the signal peptide, can secrete IFN - alpha2a efficiently.
Owner:SHANGHAI JIAO TONG UNIV
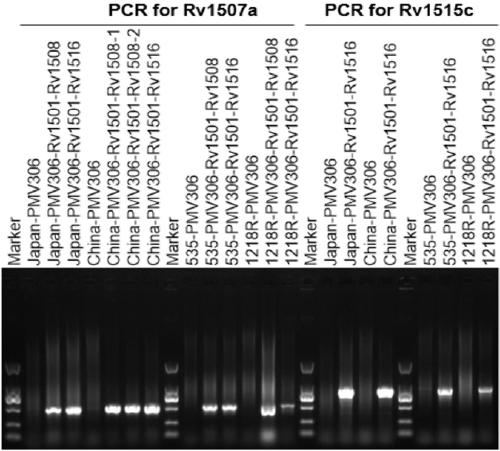
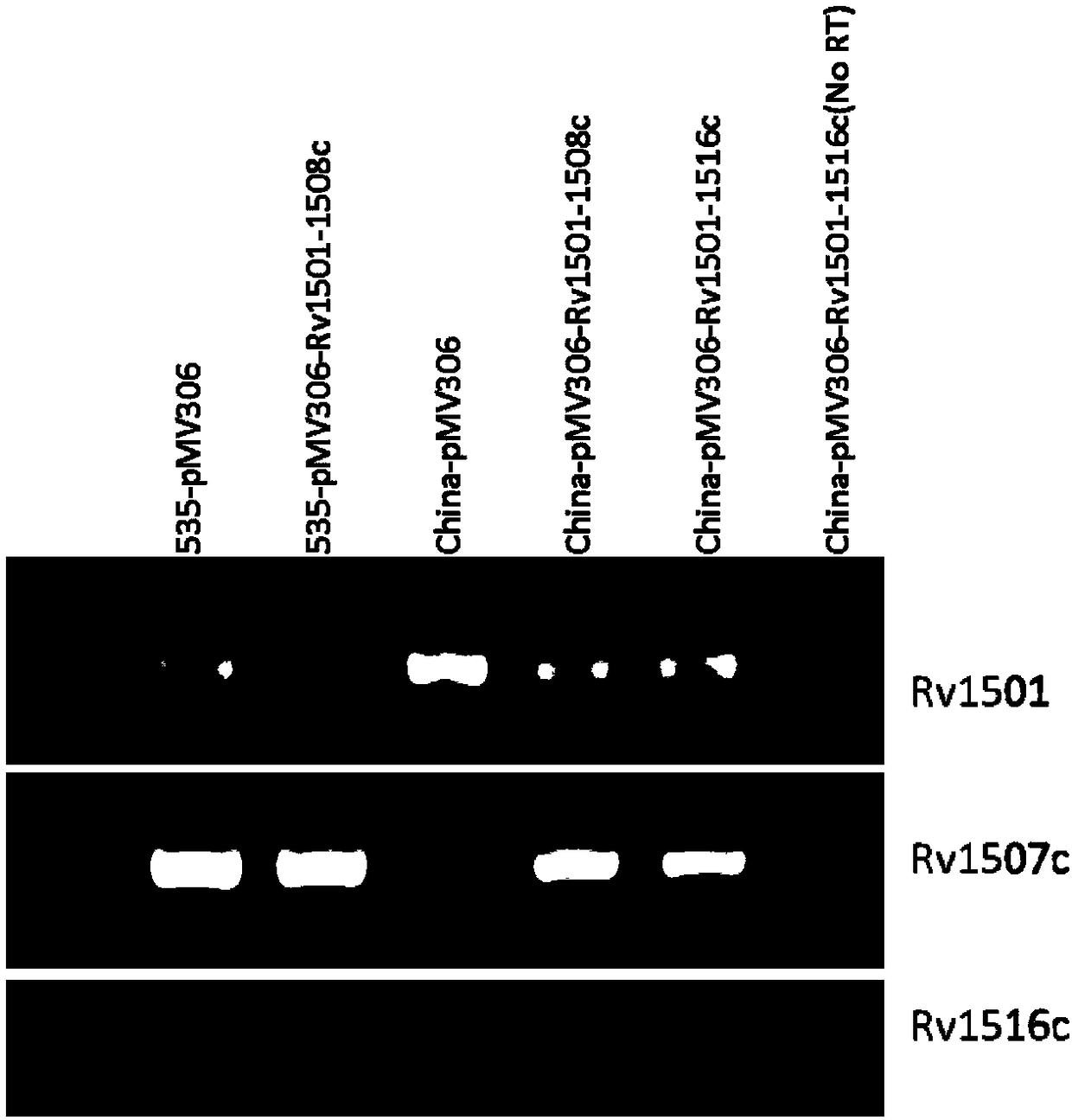
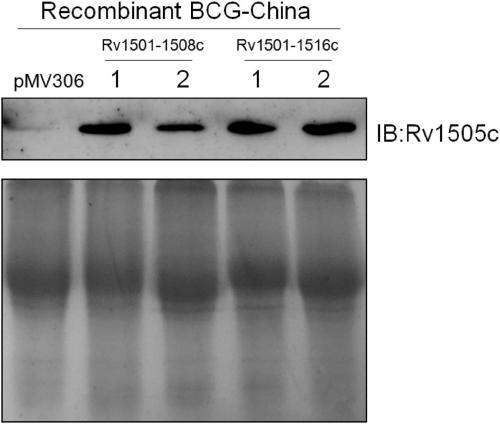
A kind of recombinant BCG and its application
ActiveCN108949783BImprove defectsConsistent structureAntibacterial agentsBacterial antigen ingredientsEscherichia coliBacterial strain
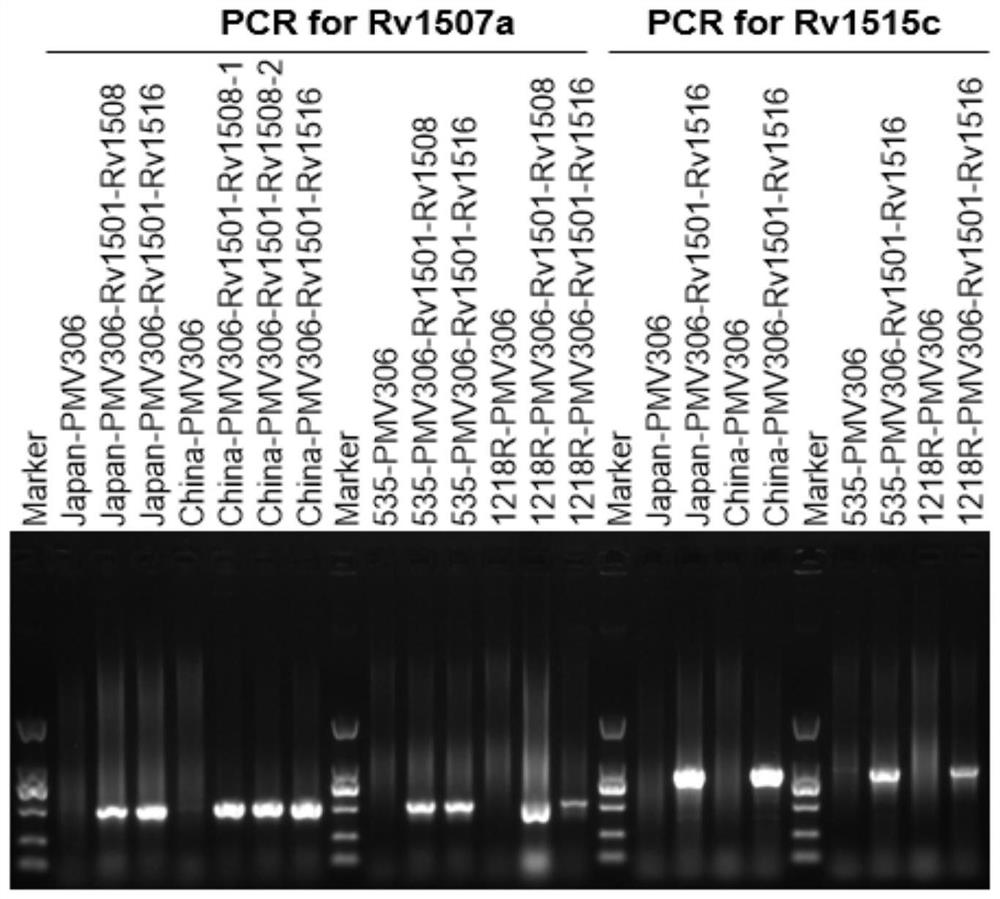
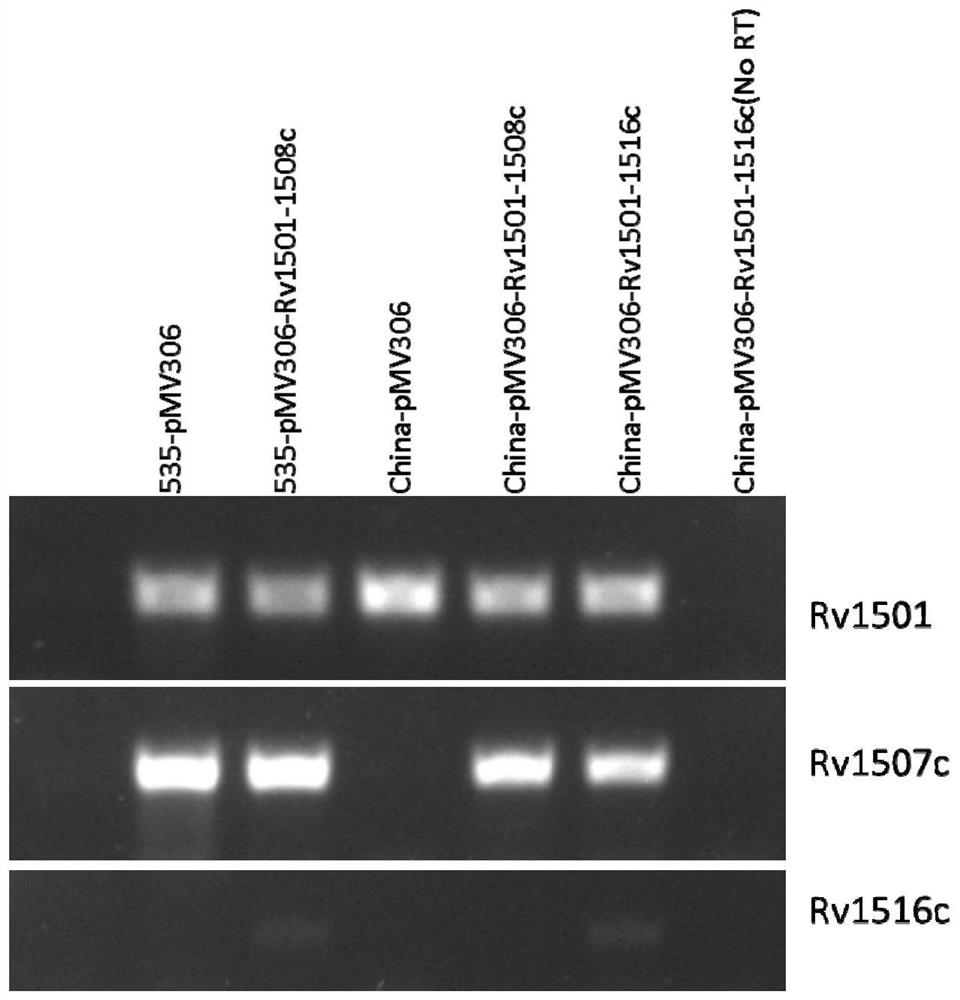
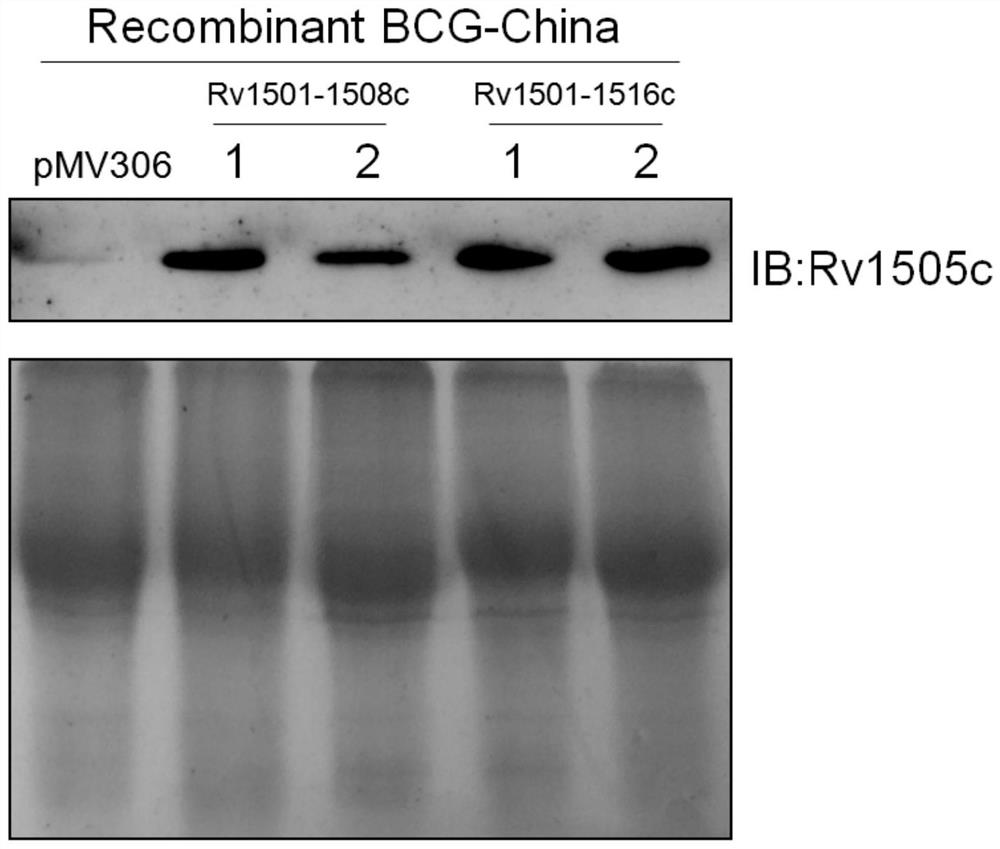
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
Tuberculosis Vaccines Including Recombinant BCG Strains Expressing Alanine Dehydrogenase, Serine Dehydratase and/or Glutamine Synthetase
InactiveUS20070264286A1Release growth inhibitionInduce long-term protective immunityAntibacterial agentsBacteriaSerine dehydrataseTGE VACCINE
The invention relates to a live recombinant Mycobacterium bovis-BCG strain comprising a nucleic acid capable of expression, the nucleic acid encoding at least one protein or polypeptide that exhibits alanine dehydrogenase activity, glutamine synthetase activity, or serine dehydratase activity.
Owner:LIU JUN
Recombination BCG viable bacterium strain capable of expressing and secreting staphylococcus aureus enterotoxin protein, viable bacterium vaccine and construction method and application thereof
ActiveCN103497927APowerful ImmunotherapyFor the purpose of treating cancerBacteriaGenetic material ingredientsVaccinationWild type
The invention provides a recombination BCG viable bacterium strain capable of expressing and secreting staphylococcus aureus enterotoxin protein, a viable bacterium vaccine and a construction method and application thereof, and relates to the technical field of biology, in particular to the technical field of genetic engineering. The novel viable bacterium vaccine capable of preventing and treating mankind tumor diseases is provided. The recombination BCG viable bacterium strain is provided first, and the recombination BCG viable bacterium strain is a recombination strain which is obtained after a wild type staphylococcus aureus enterotoxin gene sequence for coding wild type staphylococcus aureus enterotoxin protein or a mutant type staphylococcus aureus enterotoxin gene sequence for coding mutant type staphylococcus aureus enterotoxin protein is led into the BCG viable bacterium strain. The invention further provides the construction method of the recombination strain and the viable bacterium vaccine obtained through the construction method. The viable bacterium strain can express and secrete the staphylococcus aureus enterotoxin protein in cells, and zoology experiments show that the function for prevention and treatment of cancers can be achieved after the viable bacterium strain is used as a vaccination organism.
Owner:SHENZHEN UNIV
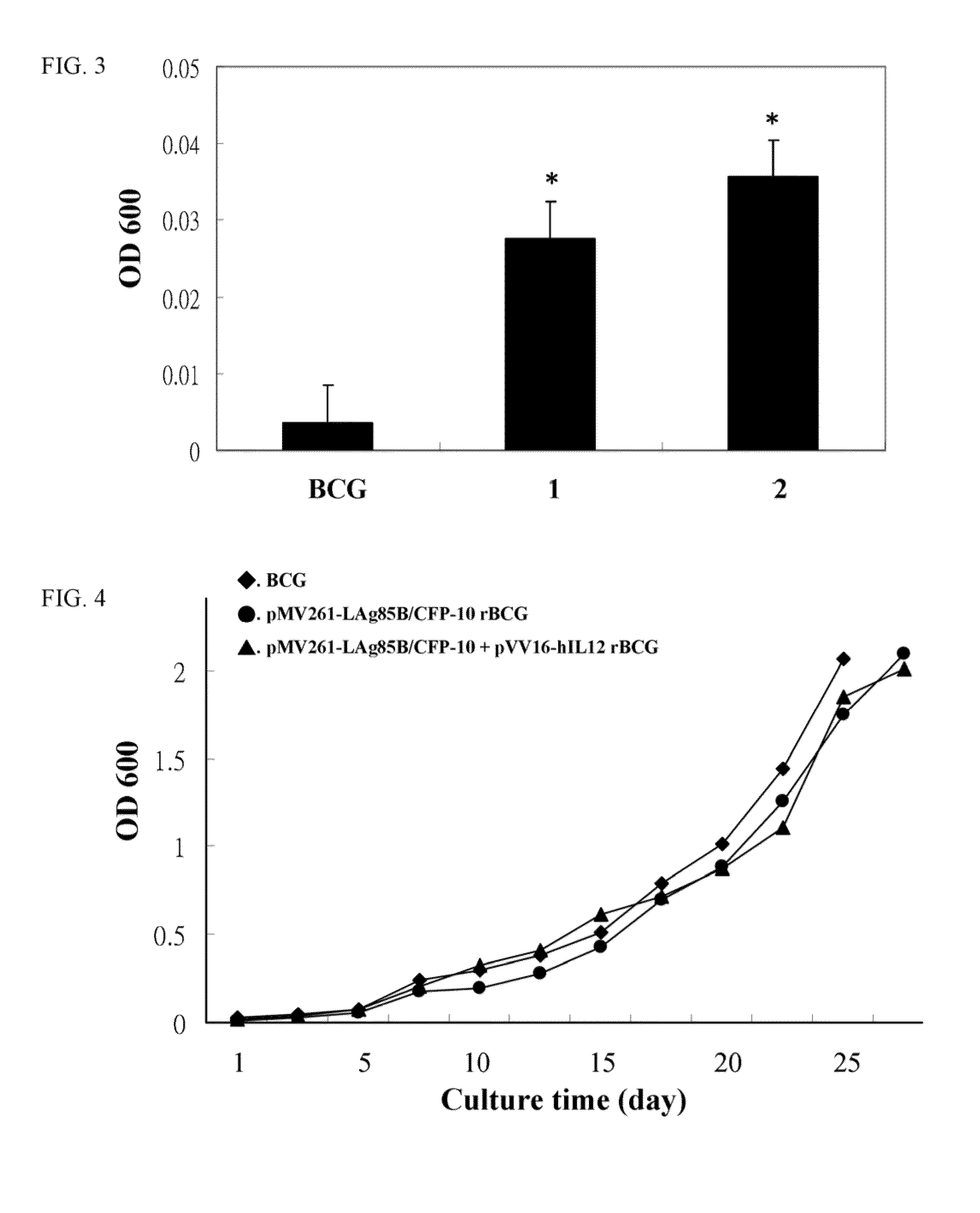
Recombinant bcg strains with enhanced ability to inhibit intracellular mycobacterial growth
A recombinant bacterial cell strain is disclosed. It comprises: a) a first vector comprising a fusion transgene encoding Ag85B-CFP10 fusion protein, the fusion transgene being operably linked to a promoter effective for expression of the Ag85B-CFP10 fusion protein; and b) a second vector comprising a transgene encoding interleukin-12 (IL-12), the transgene being operably linked to a promoter effective for expression of the IL-12 protein. A method of inhibiting intracellular growth of Mycobacterium in a subject is also disclosed.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Recombinant bacillus calmette guerin vaccine, and applications thereof
ActiveCN108949783ADoes not compromise or enhances securityImprove securityAntibacterial agentsBacterial antigen ingredientsBacterial strainTGE VACCINE
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
Recombinant calmette-Guerin bacillus vaccine for secretion of tumour necrosis factor-alpha and its constructing method
InactiveCN100360668CImprove drug efficacyReduce the risk of tumor recurrenceAntibacterial agentsPeptide/protein ingredientsGenetic engineeringTechniques of genetic engineering
The invention relates to a reorganization BCG vaccine for secreting tumor necrosis factor-alpha and its construction, which uses the genetic engineering technology to clone the secretion BCG vaccine Ag85b signal peptide and the TNF-alpha gene piece to pcMv261, therefore obtain the BCG vaccine shuttle express carrier pcMstnf-alpha, then use the electricity transformation technology to induce pMSTNF-alpha to the BCG to reconstruct BCG vaccine rBCGTNF-alpha. Depend upon pcMstnf-alpha duplication in the BCG and the signal peptide secretion, it can secrete TNF-alpha efficiently.
Owner:SHANGHAI JIAO TONG UNIV
Immunostimulatory Recombinant Intracellular Pathogen Immunogenic Compositions and Methods of Use
ActiveUS20110129492A1Prevent and treat diseaseAntibacterial agentsBiocideIrritationExtracellular proteins
Immunogenic compositions comprising recombinant intracellular pathogens that have been transformed to express recombinant immunogenic antigens of the same or other intracellular pathogens and immunostimulatory molecules are provided. Exemplary immunogenic compositions include, but are not limited to, recombinant BCG expressing Mycobacteria major extracellular proteins and immunostimulatory molecules.
Owner:RGT UNIV OF CALIFORNIA
Auxotrophic, recombinant bcg strain pasteur and use thereof for combating human infections caused by parasites
The present invention relates to a recombinant BCG vaccine strain of Mycobacterium bovis that expresses the Sm14 antigen of Schistosoma mansoni (BCGr Pasteur delta LeuD / p delta K410-hsp60*-Sm14). The vaccine strain according to the present invention is used for combating infection by parasites, particularly Schistosoma mansoni. The vaccine strain is a leucine-auxotrophic strain derived from the BCG substrain Pasteur, supplemented for leucine after genetic transformation with the construct p delta K410-hsp60*-SM14. The effectiveness of the BCGr Pasteur delta LeuD / p delta K410-hsp60*-Sm14 strain for combating Schistosoma mansoni infection by expressing the recombinant Sm14 antigen in vivo is demonstrated in the present invention.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
Recombinant intracellular pathogen vaccines and methods for use
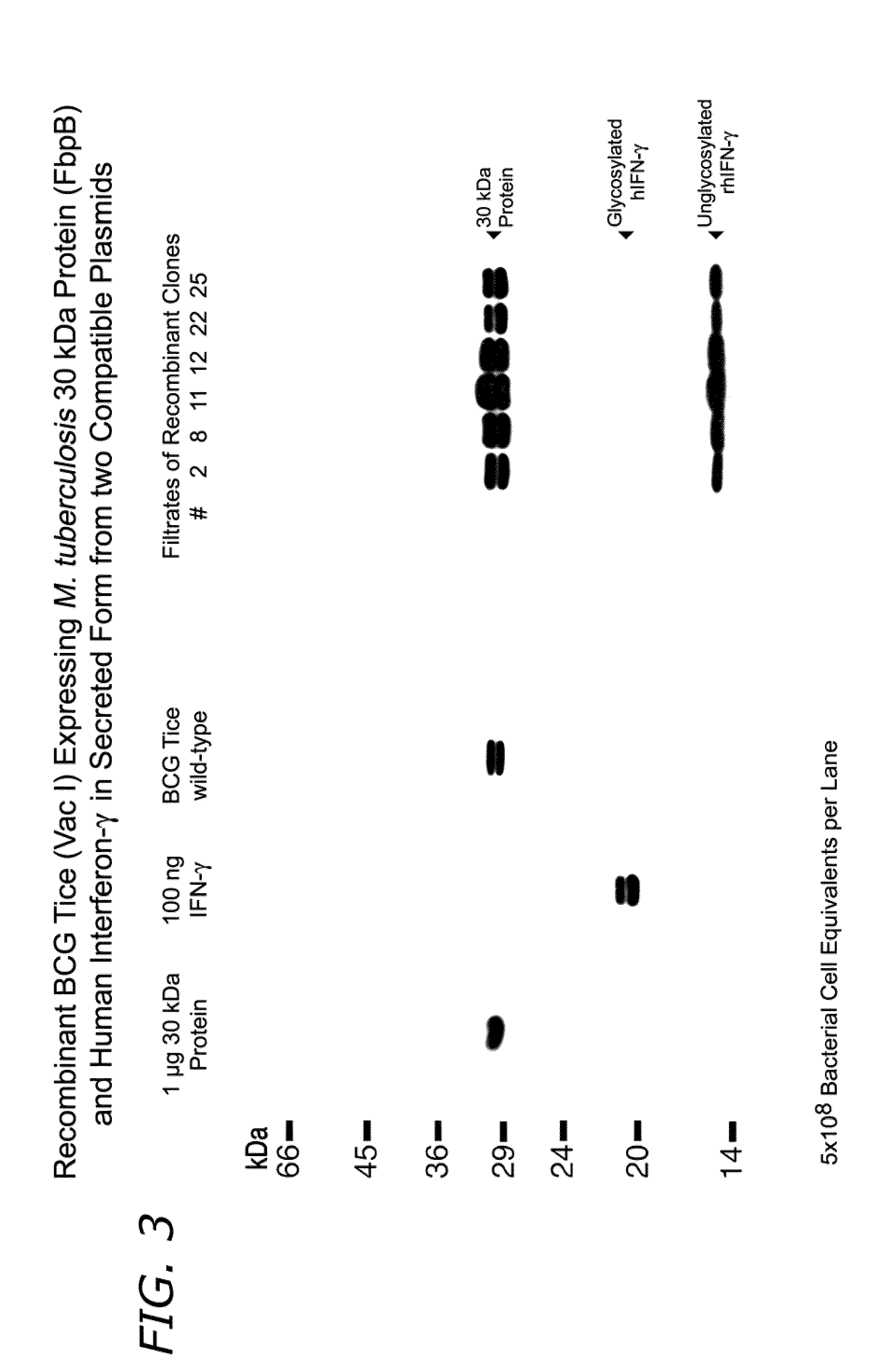
Vaccines and immunotherapeutics for preventing intracellular pathogen diseases in mammals are provided that consist of recombinant attenuated intracellular pathogens that have been transformed to express recombinant immunogenic antigens of the same r other intracellular pathogens. Exemplary vaccines and immunotherapeutics include attenuated recombinant Mycobacteria expressing the major extracellular non-fusion proteins of Mycobacterial and / or other intracellular pathogens. These exemplary vaccines are shown to produce surprisingly potent protective immune response in mammals that surpass those of any previously known anti-mycobacterium vaccine. More specifically, a recombinant BCG expressing the 30 kDa major extracellular non-fusion protein of Mycobacterium tubercolosis is provided. Additionally, methods for preventing and treating diseases caused by intracellular pathogens are provided. The methods of treating and preventing intracellular pathogen diseases utilize the described surprisingly efficacious vaccines and immunotherapeutics.
Owner:RGT UNIV OF CALIFORNIA
Recombinant bacillus calmette-guerin for preventing animal toxoplasmosis and preparation method
InactiveCN104958758AGood immune protectionEasy to solveBacterial antigen ingredientsBacteriaAdjuvantCytokine
The invention provides recombinant bacillus calmette-guerin for preventing animal toxoplasmosis and a preparation method of the recombinant bacillus calmette-guerin. The recombinant bacillus calmette-guerin (rBCG) can serve as an adjuvant and a carrier and has exogenous genes and living vaccine; after a user is inoculated with the recombinant bacillus calmette-guerin, the body of the user has specific humoral immunity and cellular immunity, cell factors and toxoplasma antigen protein can be expressed, by the expressed cell factors, the expressed protein has a high immunoprotection effect, advantages of the expressed cell factors and advantages of the expressed protein are combined, and the purpose of preventing the toxoplasmosis well is achieved. The recombinant bacillus calmette-guerin is high in thermostability, easy to convey and store and easy to produce, does not need to be purified and can be directly used for a immunoprotection test, and complex working procedures of aftertreatment of the protein are omitted, so that the cost is greatly reduced. The recombinant bacillus calmette-guerin is suitable for vast rural areas.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com