Auxotrophic, recombinant bcg strain pasteur and use thereof for combating human infections caused by parasites
A technology of auxotrophic strains, applied in anti-infective drugs, bacterial antigen components, recombinant DNA technology, etc., can solve the problem of not providing plasmid stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
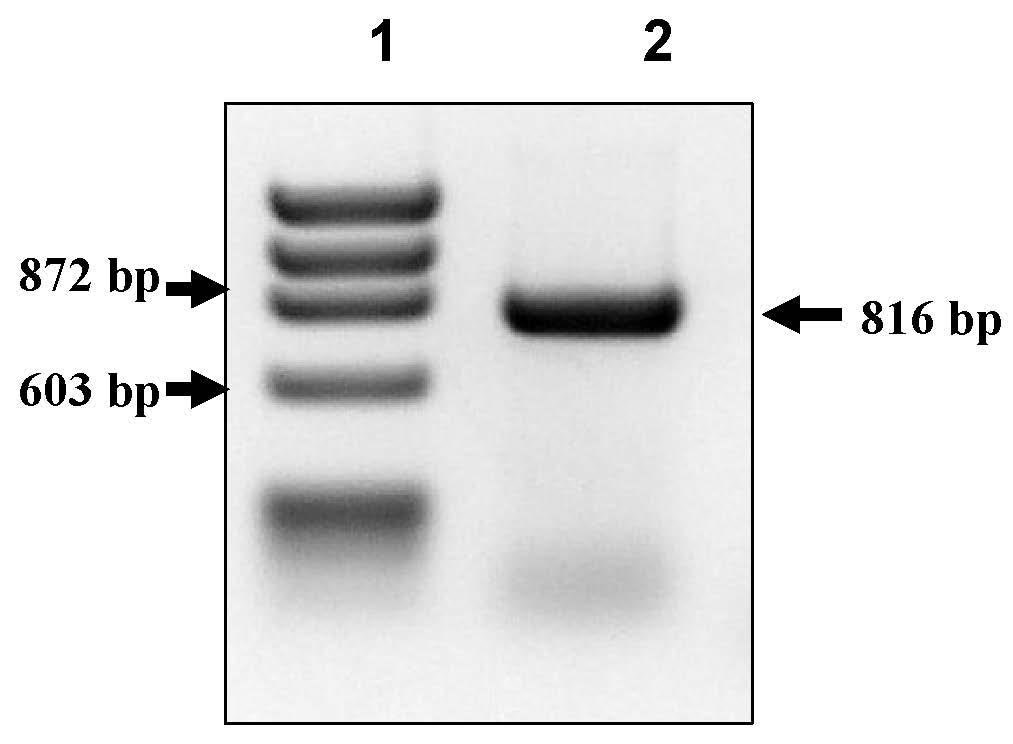
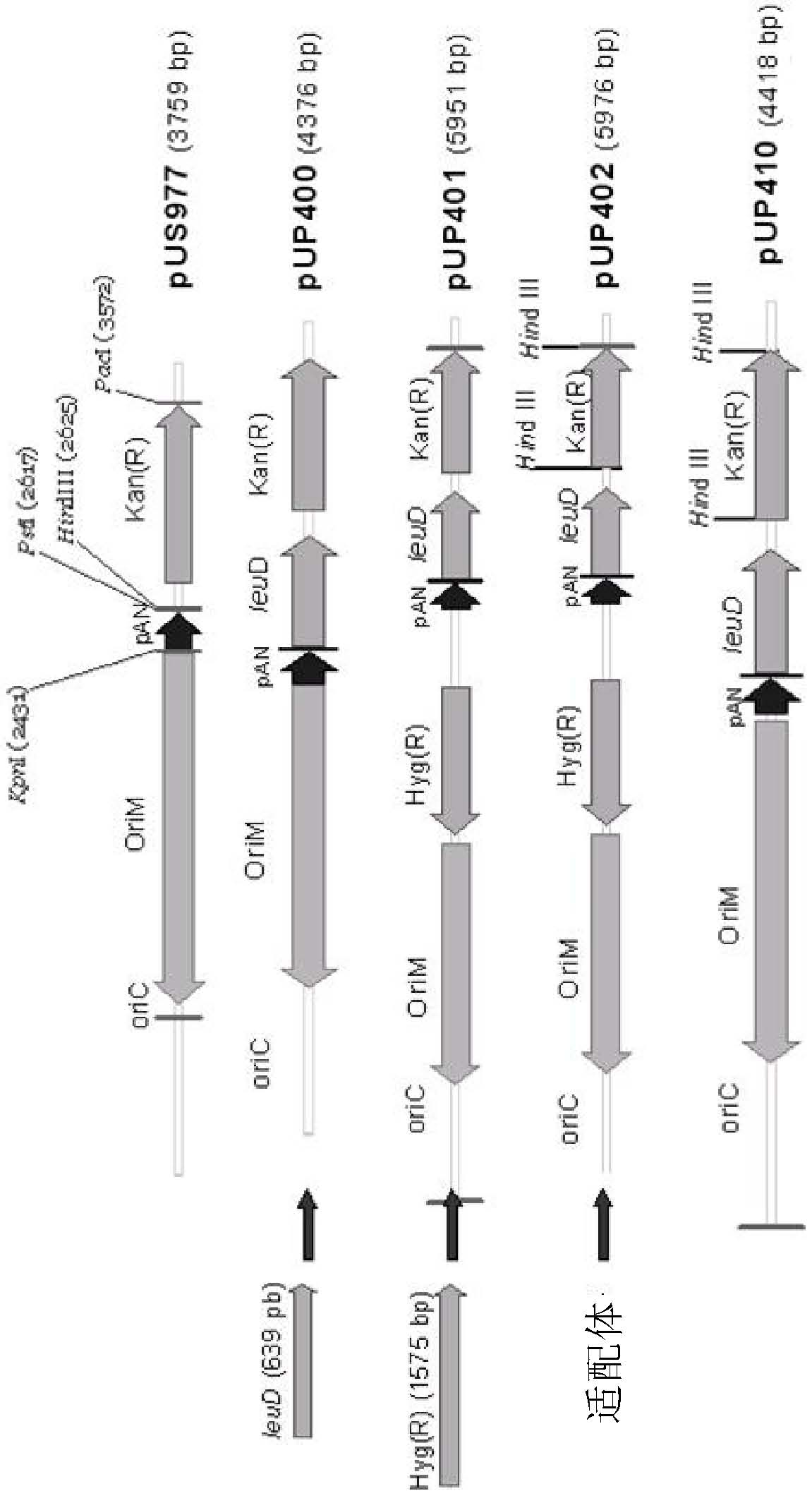
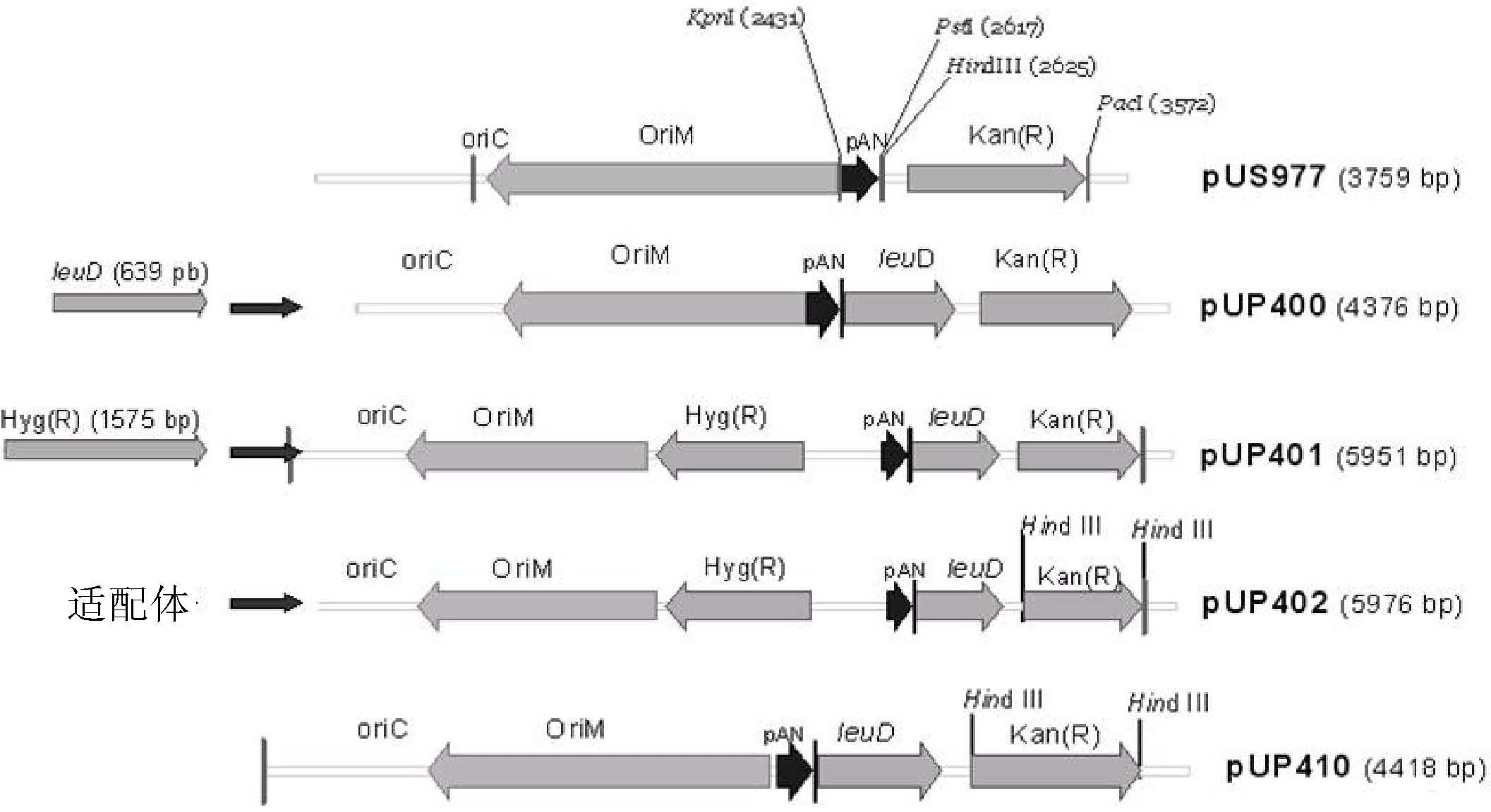
[0017] The present invention relates to the use of the BCGΔleuD strain and the complementary vector pUP410 to obtain a vaccine strain for the control of infections caused by Schistosoma mansoni and related parasites, based on the in vivo expression of Sm14 of Schistosoma mansoni.
[0018] The main purpose of the present invention can be achieved by constructing the expression system of the Sm14 antigen in BCG using auxotrophic complementation as a selectable marker. The expression system used in the present invention was developed by a group consisting of Dr. Johnjoe Mcfadden, Faculty of Biological Sciences, University of Surrey, UK and Dr. Odir Dellagostin, Center for Biotechnology, Pelotas Union University, Brazil, on the subject of the main Developed in a research project for the development of recombinant BCG multiple vaccines and complementary diagnostics for parasitic and epidemic diseases.
[0019] The BCG system using auxotrophic complementation consists and features o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com