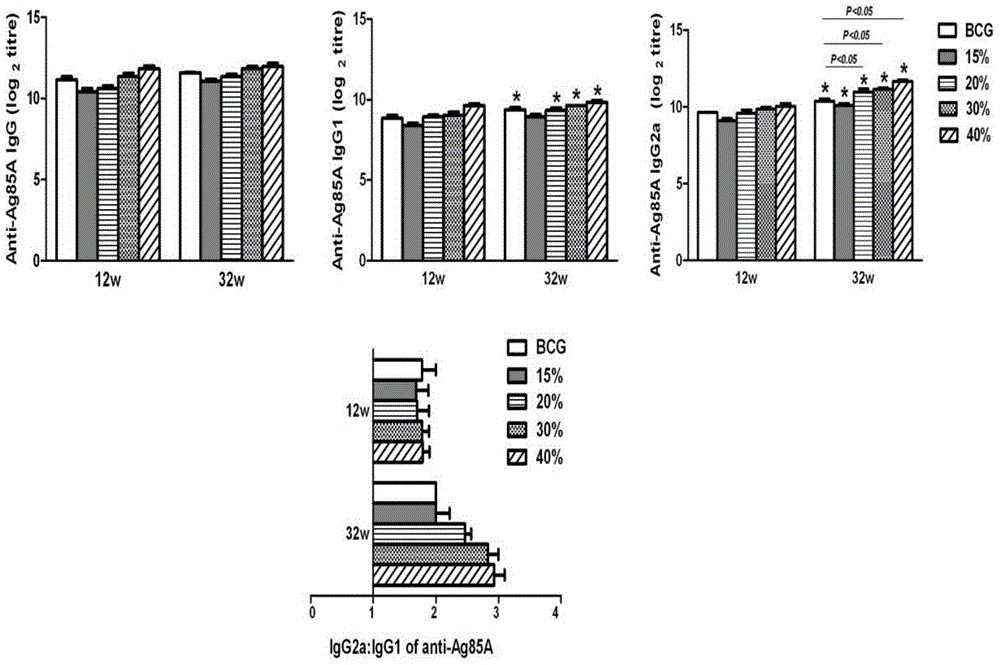
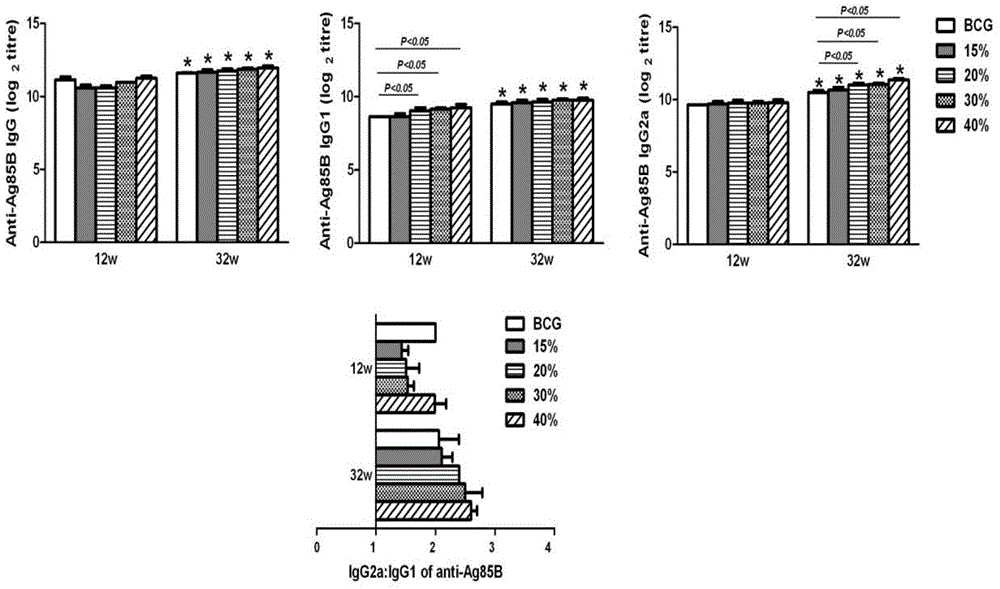
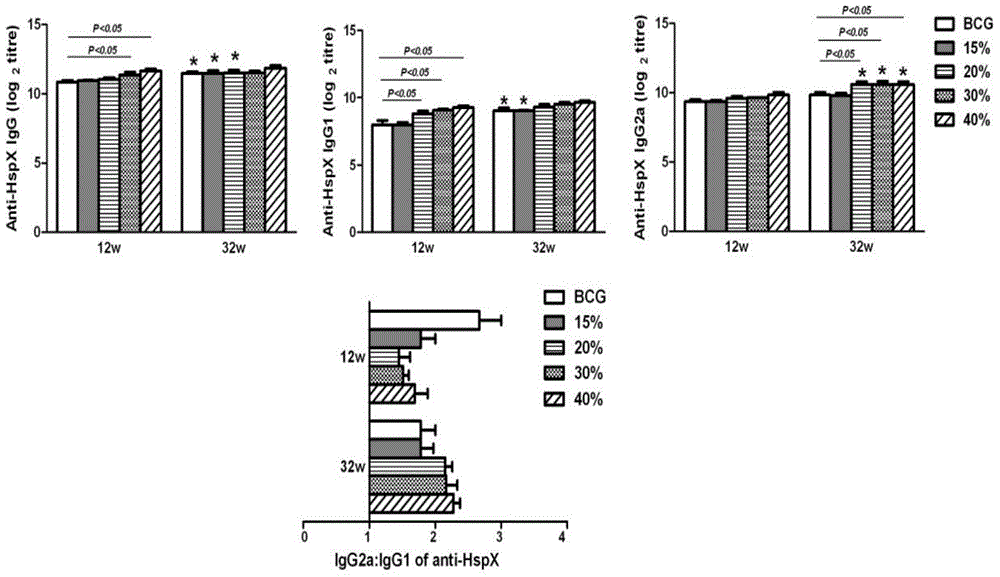
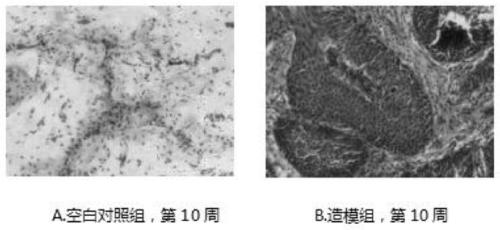
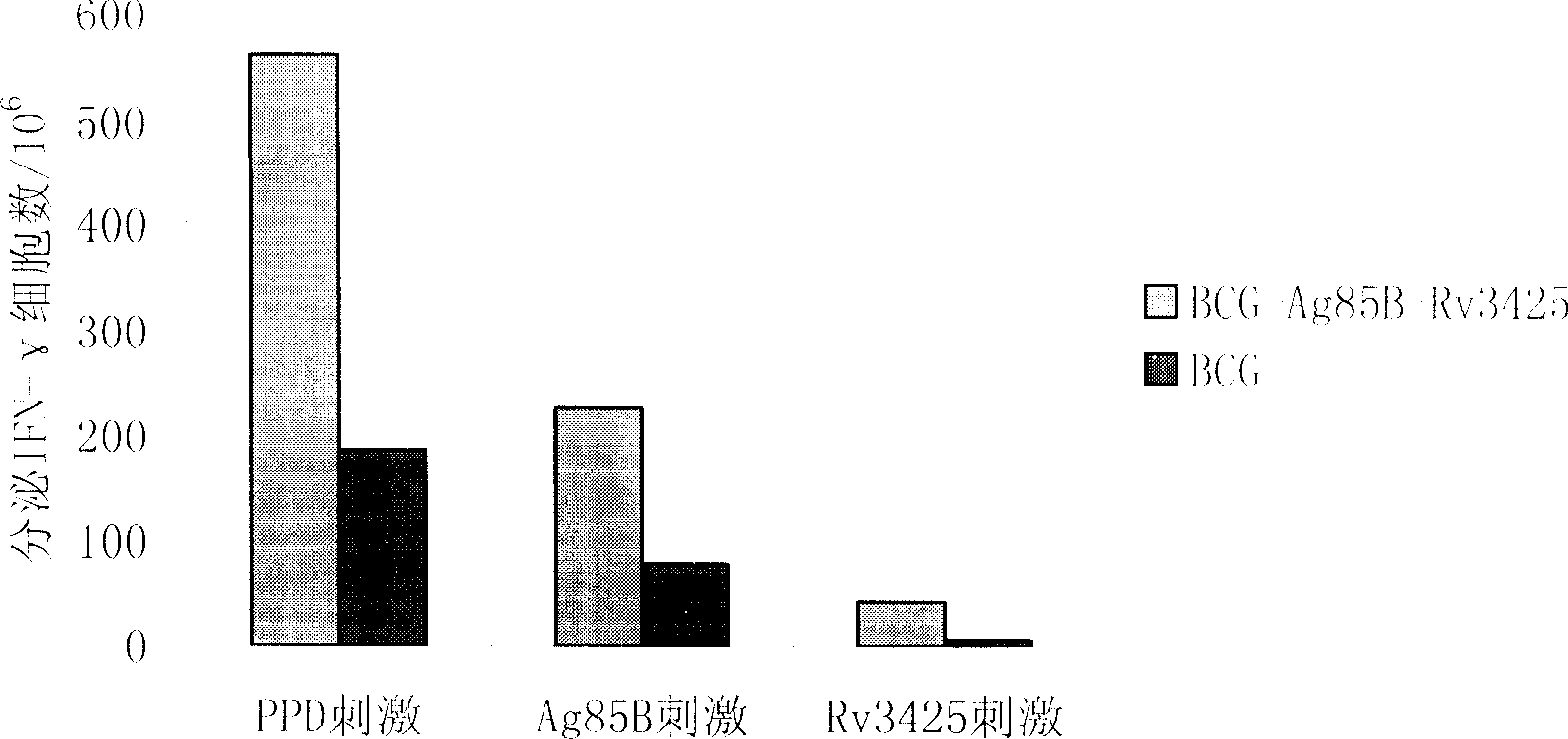Patents
Literature
48 results about "Bacillus Calmette Guerin vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Active immunizing agent and a viable avirulent attenuated strain of Mycobacterium tuberculosis, var. bovis, which confers immunity to mycobacterial infections; used also in immunotherapy of neoplasms due to its stimulation of antibodies and non-specific immunity.
Multiplex polymerase chain reaction (PCR) kit for identifying mycobacterium tuberculosis
InactiveCN102533959ASimple and fast operationEasy to operateMicrobiological testing/measurementSequence designAcid-fast
Owner:HUAZHONG AGRI UNIV
Immuno-fluorescent staining method for detecting mycobacterium tuberculosis in leukocytes and kit
The invention discloses a method for rapidly detecting mycobacterium tuberculosis in blood (blood plasma and blood cells) through fluorescent staining and a kit.ESAT-6 and CFP-10 antigens of the mycobacterium tuberculosis in the blood are specifically detected by using monoclonal antibodies of RD-1 zone ESAT-6 and CFP-10 antigens of the mycobacterium and are used for etiologic detection and early-stage and rapid clinical diagnosis of tuberculosis diseases.According to the method, the blood is divided into the blood plasma and the blood cells, the mycobacterium tuberculosis enriched in the blood plasma is separated by using a micro-fluid device based on a membrane, meanwhile a slide is coated with hemocytes (leukocytes), and the mycobacterium tuberculosis in the blood plasma and the leukocytes are detected by using the monoclonal antibodies of the ESAT-6 and CFP-10 antigens and adopting direct and indirect fluorescent staining methods.The method is simple in operation, high in sensitivity and strong in specificity and is a definite tuberculosis etiology diagnosis method, and the kit for detecting the mycobacterium tuberculosis in the blood is developed.In addition, by means of the method, pathogenic mycobacterium tuberculosis infection can be detected, and theoretically non-pathogenic mycobacterium tuberculosis produced in blood due to bacillus calmette guerin vaccine inoculation can be also distinguished.
Owner:肖乐义
Recombinant bacillus Calmette-Guerin vaccine and its preparation method
InactiveCN1737153AIncrease secretionGood effectBacterial antigen ingredientsVector-based foreign material introductionEscherichia coliTuberculosis mycobacterium
The invention relates to a recombinant bacillus Calmette-Guerin vaccine and its preparation method, wherein tuberculosis mycobacterium ag85b, esat-6 and IFN-gamma gene sequences are inserted into colibacillus-tuberculosis mycobacterium shuttle plasmids to form recombinant plasmid, and are transformed into BCG to form recombinant tuberculosis vaccine. The recombinant vaccine can be used for prevention and treatment of tuberculosis with better immunological effects than BCG.
Owner:FUDAN UNIV
Recombinant Bacillus Calmette Guerin vaccine
InactiveCN104474538AImprove protectionAvoid negative effectsAntibacterial agentsBacterial antigen ingredientsAntigenBacterial strain
The invention discloses a recombinant Bacillus Calmette Guerin vaccine (BCG), total bacteria amount of which is between 105CFU and 106CFU. The recombinant Bacillus Calmette Guerin vaccine comprises recombinant BCG for overexpression of 85 A antigen, recombinant BCG for overexpression of 85 B antigen and recombinant BCG for overexpression of HspX antigen. By synergism of multiple bacterial strains of the recombinant BCG vaccine, the recombinant BCG vaccine has an immunoprotection function in different development phases of tuberculosis, and negative effects of mechanisms or factors such as simultaneous overexpression of multi-antigen or fusion expression, etc. on preventive effect of the BCG itself can be avoided.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for treating bladder cancer through promoting pharmorubicin by bacillus calmette guerin vaccine
PendingCN109675023ASmall toxicityImprove treatment efficiencyCompound screeningOrganic active ingredientsSide effectRetention time
The invention discloses a method for treating bladder cancer through promoting pharmorubicin by a bacillus calmette guerin vaccine (BCG), and specifically relates to the field of combined treatment ofthe bladder cancer in clinical medicine. The method comprises a method for researching synergism of the BCG to the pharmorubicin in a cellular level and a method for synergism of the in-vivo BCG to the pharmorubicin. According to the invention, through using the method for researching the synergism of the BCG to the pharmorubicin in the cellular level and the method for the synergism of the in-vivo BCG to the pharmorubicin, a combined medication scheme is provided to improve treatment efficiency and reduce toxic and side effects during drug perfusion. The method disclosed by the invention isnot high in prefused drug concentration, but long in intra-bladder retention time, thereby being better in the anti-tumor effect without generating an adverse reaction.
Owner:GUANGDONG PHARMA UNIV
Recombinant Ag85B-Rv3425 bacillus Calmette-Guerin vaccine
This invention is associated with the gene engineering field and tuberculosis vaccines field. In recent decades, the increase of tuberculosis drug resistant strain and the coinfection of mycobacterium tuberculosis and HIV lead the increase of incidence of tuberculosis, and made it the second killer of human after HIV. Recently, the only tuberculosis vaccines used globally is BCG. This invention inserts the most important protective antigen gene of mycobacterium tuberculosis and missed mycobacterium tuberculosis gene in BCG to the multi-cloning coliform - mycobacterium tuberculosis shuttle-plasmid multiple cloning sites to form recombined plasmid and transforms it to the BCG to form recombined tuberculosis vaccines. The result of experiment shows that, compared with BCG vaccine, the protective antigen can express more efficient, the original missing Rv3425 antigen also get expressed, and the induced IFN- gamma expression also increased significently. This indicates that the recombined tuberculosis vaccines can induce higher cell immunity level than BCG.
Owner:FUDAN UNIV
Recombinant bacillus calmette guerin vaccine for toxoplamasis and preparation method thereof
InactiveCN102198267AImprove thermal stabilityEasy transportationProtozoa antigen ingredientsMicroorganism based processesEscherichia coliGondii toxoplasma
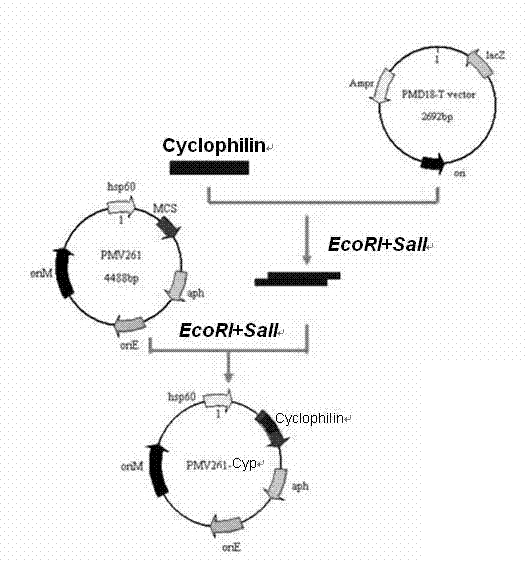
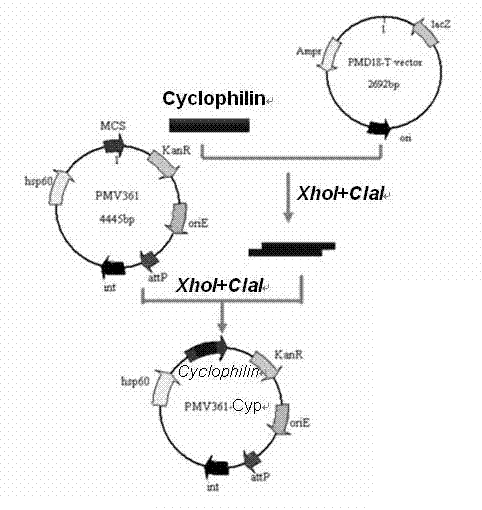
The invention provides a recombinant bacillus calmette guerin (BCG) vaccine for toxoplamasis and a preparation method thereof. The preparation method for the recombinant bacillus calmette guerin vaccine comprises the following steps of: performing target (TA) cloning on an obtained toxoplasma gondii cyclophilin gene; sequencing and identifying correctly, performing enzyme cutting and recovering target fragments; connecting the target fragments with an Escherichia coli-mycobacterium shuttle expression vector pMV261 and an integrated expression vector pMV361 which are subjected to enzyme cutting reaction respectively; and converting recombinant plasmids to BCG, and screening by resistance and polymerase chain reaction (PCR) to obtain the recombinant bacillus calmette guerin vaccine for the positive toxoplasma gondii. The vaccine is high in heat stability and easy to transport, store and produce, is not needed to be purified and can be directly used for immune protection tests, and a complex process of protein aftertreatment is avoided, so the cost is reduced greatly, and the recombinant bacillus calmette guerin vaccine is suitable for vast rural areas.
Owner:JILIN UNIV
Recombinant human co-stimulatory molecule bacilli-calmette-guerin strain and process for making same
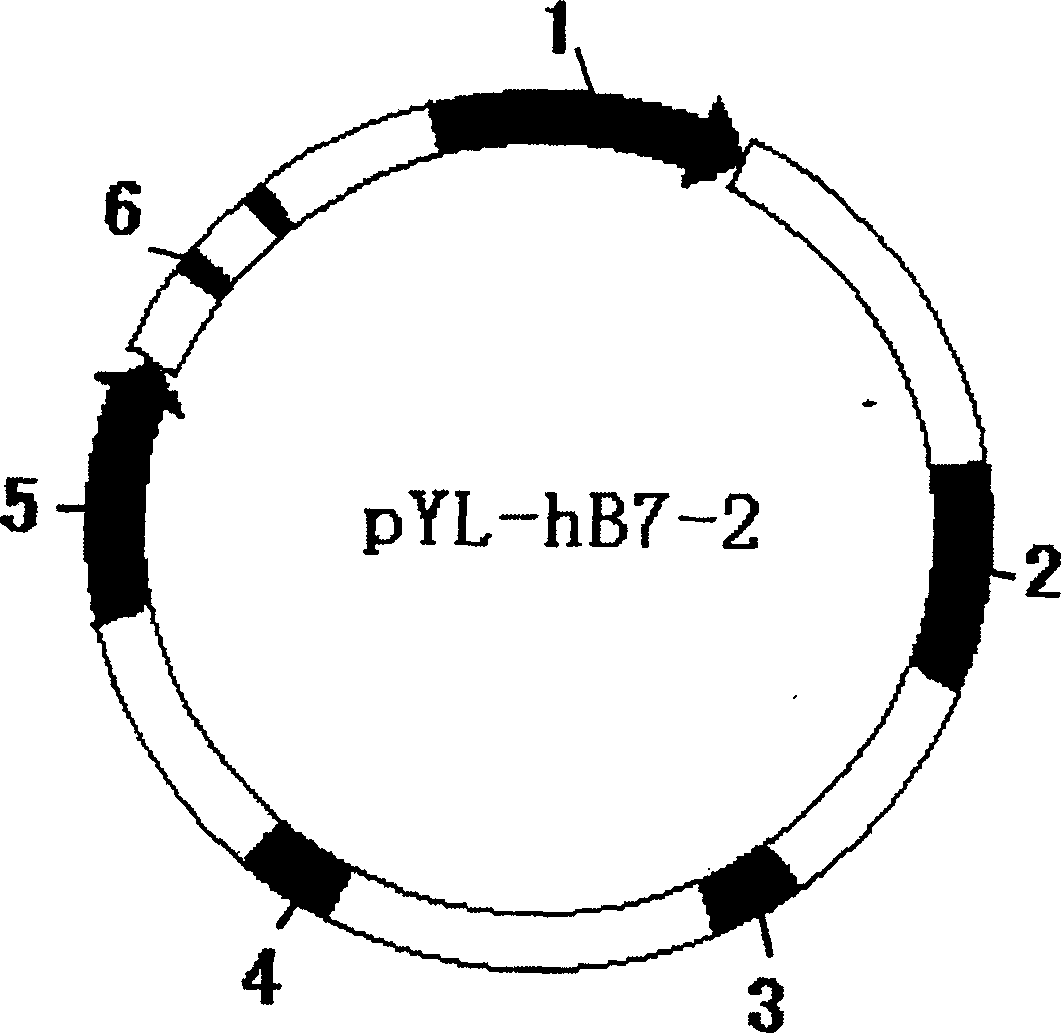
The invention discloses a recombinant human co-stimulatory molecule bacilli-calmette-guerin strain and process for preparation, wherein the cultrue preservation number is CGMCC No.1120. The preparing process consists of carrying out hB7-2(IgC+IgV) fragment polymerase chain reaction, establishing plasmid pYL-hB7-2 and transforming, carrying out E.Coli-pYL-hB7-2 monoclonal bacterial colony expansion, plasmid extraction and purification, electrophoresis, enzyme cutting and determination of pYL-hB7-2 plasmid through PCR reaction, bacillus Calmette-Guerin vaccine electrical transformation, rBCG-hB7-2 monoclonal bacterial colony selection, expansion, and determination.
Owner:天津市泌尿外科研究所 +4
Modified new coronavirus S gene, recombinant plasmid and recombinant bacillus calmette guerin vaccine constructed by same and application of recombinant bacillus calmette guerin vaccine
PendingCN113403330AEvoke an immune responsePrevent intrusionAntibacterial agentsSsRNA viruses positive-senseGene terminatorVirus Protein
The invention discloses a modified new coronavirus SARS-CoV-2S protein, a recombinant plasmid and a recombinant bacillus calmette guerin vaccine constructed by the modified new coronavirus SARS-CoV-2S protein, and application of the recombinant bacillus calmette guerin vaccine, and belongs to the technical field of biological agents. Through modifying a partial sequence of gene promoter region and a partial sequence of gene terminator region, the modified new coronavirus S gene complete sequence can be smoothly expressed in an escherichia coli-mycobacterium tuberculosis shuttle plasmid pMV261 to obtain a recombinant plasmid pMVS; the recombinant plasmid pMVS is introduced into BCG through electric transformation, and recombinant BCG (rBCG) is obtained; the rBCG can successfully express S protein, arouse human immune response and induce antibody generation to prevent virus invasion, and the constructed rBCG is a subunit vaccine, has a better protection effect than parent BCG, can prevent or treat new coronavirus and mycobacterium tuberculosis, and has huge social benefits.
Owner:JINING MEDICAL UNIV
Animal immune adjuvant as well as preparation method and application method thereof
InactiveCN104667272AKeep aliveGuaranteed stabilityAntibacterial agentsBacterial antigen ingredientsSide effectLevamisole
The invention discloses an animal immune adjuvant as well as a preparation method and an application method thereof, relates to a medical preparation characterized in an immunostimulation additive, in particular to an immunostimulation additive for animals, and aims at providing an animal immune adjuvant which is free of a toxic or side effect and is used for directly modifying the antigen-reinforced immune effect, and a preparation method and an application method of the animal immune adjuvant. The animal immune adjuvant comprises an oil agent and an immunoenhancer; the oil agent comprises liquid paraffin and a surfactant; the surfactant comprises lanolin and span-80; and the immunoenhancer comprises a bacillus calmette guerin vaccine, muramyl dipeptide, levomisole and nanoscale hydroxyapatite. The animal immune adjuvant prepared by the method is capable of effectively inducing cellular immunity and humoral immune response of antigenic specificity; and the antibody with high valence and high sensitivity is obtained by kinds of modifications. The animal immune adjuvant is applied to the fields of immunology and in vitro diagnosis.
Owner:SUQIAN HENGRUI BIOTECH
Recombinant bacillus calmette-guerin vaccine strain with over-expression mycobacterium tuberculosis Rv3586 and application of recombinant bacillus calmette-guerin vaccine strain
ActiveCN106479946AGood immune protectionStrong cellular immune responseAntibacterial agentsBacterial antigen ingredientsMicrobiologyBcg bladder instillation
The invention relates to a recombinant bacillus calmette-guerin vaccine strain with over-expression mycobacterium tuberculosis Rv3586 and application of the recombinant bacillus calmette-guerin vaccine strain. Plasmids with encoding genes of the mycobacterium tuberculosis Rv3586 are transformed into bacillus calmette-guerin vaccine, and the recombinant bacillus calmette-guerin vaccine strain can be obtained by means of screening, is called as Bacillus calmette-Guerin rBCG-DisA and is called as rBCG-DisA for short, and a preservation number of the bacillus calmette-guerin vaccine strain is CCTCC M 2016335. The recombinant bacillus calmette-guerin vaccine strain and the application have the advantages that the recombinant bacillus calmette-guerin vaccine strain with the over-expression mycobacterium tuberculosis Rv3586 has merits of target antigens and bacillus calmette-guerin vaccine and can be used for individual immunization, or booster immunization can be carried out by the recombinant bacillus calmette-guerin vaccine strain and mycobacterium tuberculosis Ag85B-ESAT6 subunit vaccine, high immune response of normal and affected mice can be induced, the mycobacterium-resistant protective ability of organisms can be improved, and accordingly the recombinant bacillus calmette-guerin vaccine strain has an excellent application prospect.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Recombinant bacillus calmette-guerin vaccine secreting bacteria resuscitation somatomedin and application thereof
InactiveCN101862450ASecretion persistsStable growth curveAntibacterial agentsBacterial antigen ingredientsBacteroidesGrowth Factor Gene
The invention discloses a recombinant bacillus calmette-guerin vaccine secreting bacteria resuscitation somatomedin and application thereof. The recombinant bacillus calmette-guerin vaccine uses the bacillus calmette-guerin vaccine as host cell. An exogenous expression vector carrying out transfection on the host cell is an expression vector comprising a bacteria resuscitation somatomedin genetic fragment. The recombinant bacillus calmette-guerin vaccine can continuously secrete Rpf factors with bioactivity and can induce a humoral immunity response level and a cellular immune response level. An antibody which is generated by aiming at the exogenous Rpf can interdict the stimulating effect of the Rpf to MTB. The level of inducing IFN-gamma of the recombinant bacillus calmette-guerin vaccine is superior to that of BCG. The recombinant bacillus calmette-guerin vaccine is applied to preparation of a preventive immunity vaccine for MTB infection and / or MTB inapparent infection.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
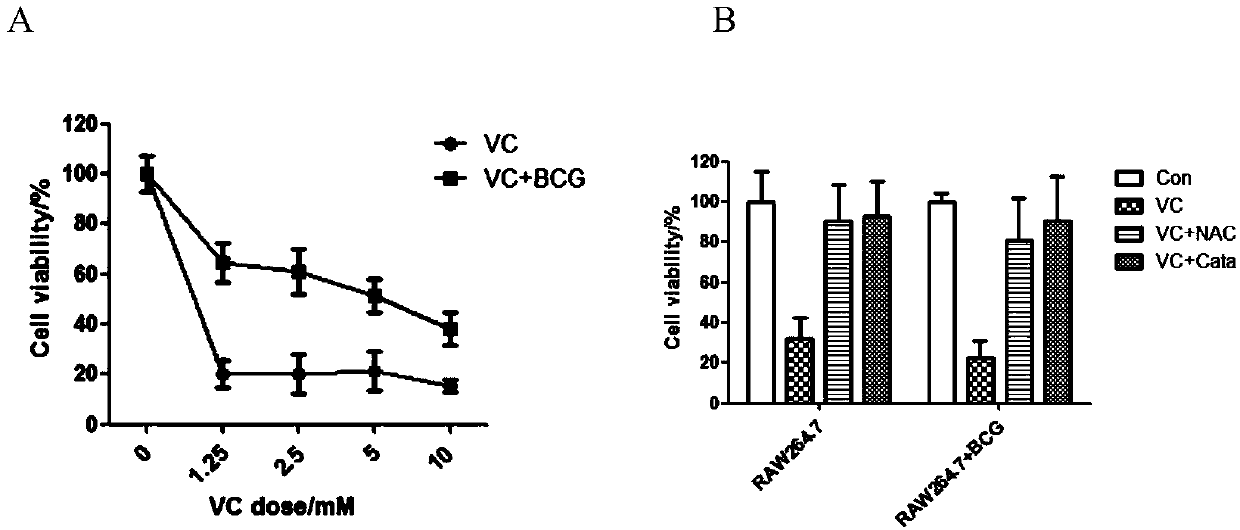
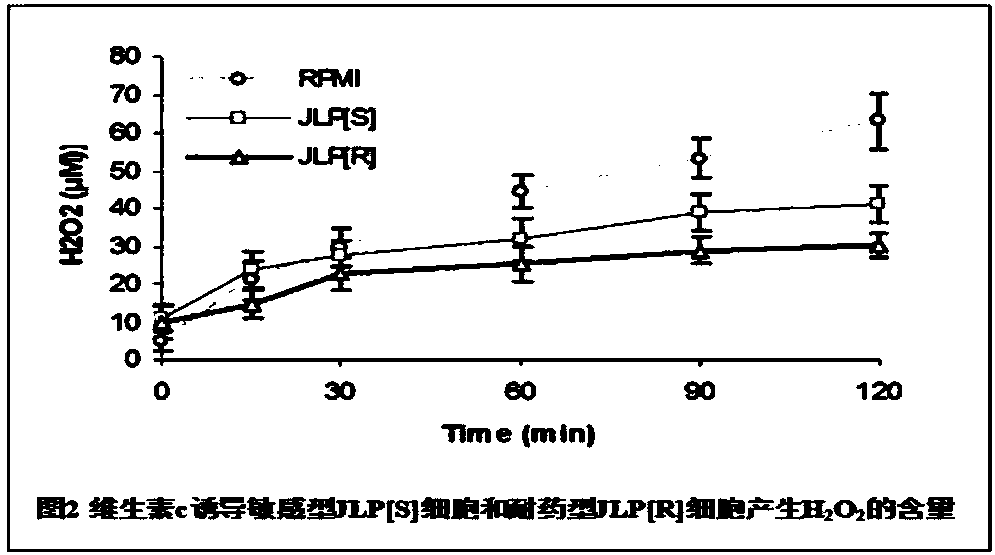
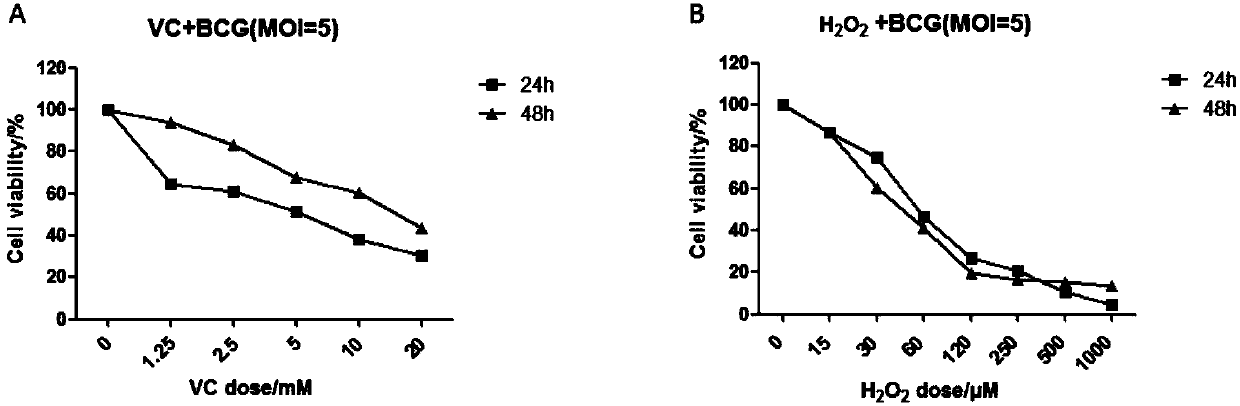
Application of vitamin C in preparation of medicines for treating and preventing tuberculosis
PendingCN108324712AAchieve therapeutic effectAntibacterial agentsOrganic active ingredientsIntervention trialPectobacterium
The invention belongs to the field of biomedicines, and relates to an application of vitamin C (ascorbic acid), as a single component, in preparation of medicines for treating and preventing tuberculosis. Through cytobiology and pharmacology experiments with combination of animal experiments, anti-tuberculosis pharmaceutical concentration of the vitamin C is determined; and on the basis of regulatory mechanism on oxidative stress status of mycobacterium tuberculosis after infection by macrophages, interference test is carried out, wherein a result proves that high-dose vitamin C can kill the mycobacterium tuberculosis, so that inhibition effect is achieved through NAC and catalase; the vitamin C treats the macrophages to generate H2O2, wherein functional effect is achieved with the signalchannel being same as H2O2; large-dose vitamin C kills mycobacteria, wherein inhibition function is achieved through a glutathione precursor NAC and CAT; high-dose vitamin C can infect the TNF-alpha signal pathway induced by RAW 264.7 cells via mediation of Bacillus Calmette-Guerin vaccine and H37Rv, thus achieving sterilization effect and influence on body immunity. The invention provides effective treatment strategy for clinical therapy and drug resistance of tuberculosis and tuberculosis in incubation period.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
A kind of recombinant BCG and its application
ActiveCN108949783BImprove defectsConsistent structureAntibacterial agentsBacterial antigen ingredientsEscherichia coliBacterial strain
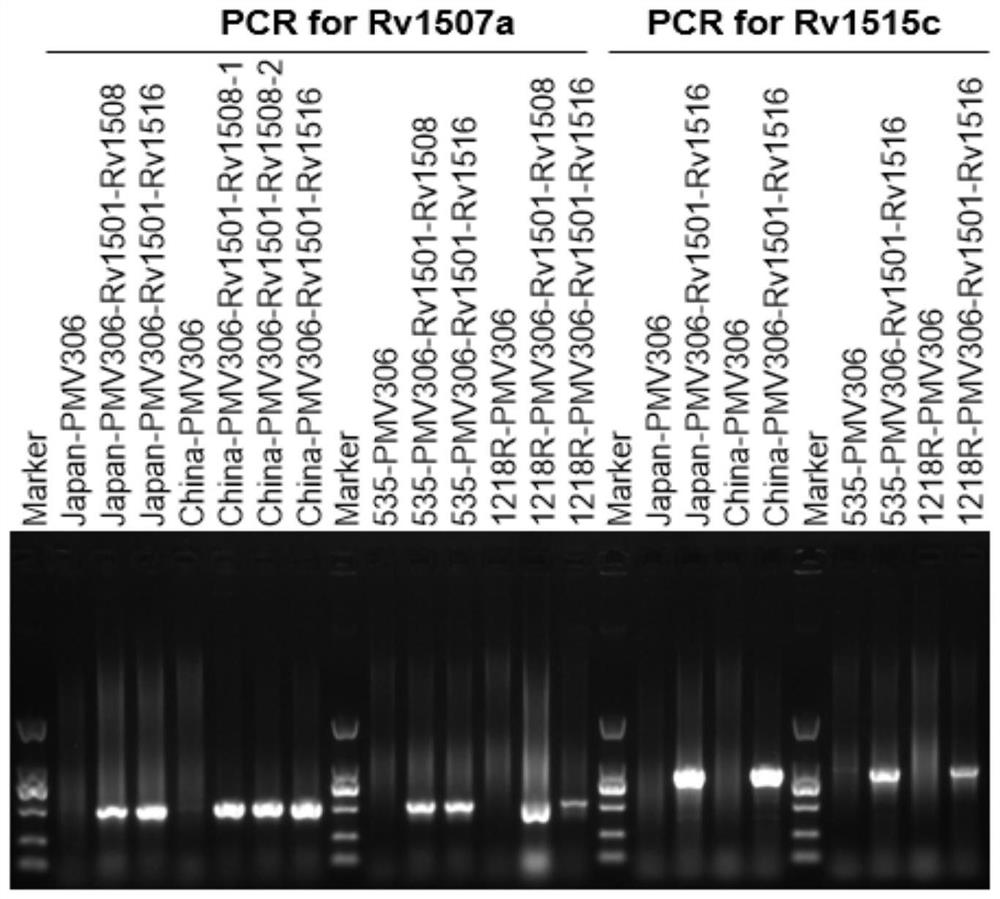
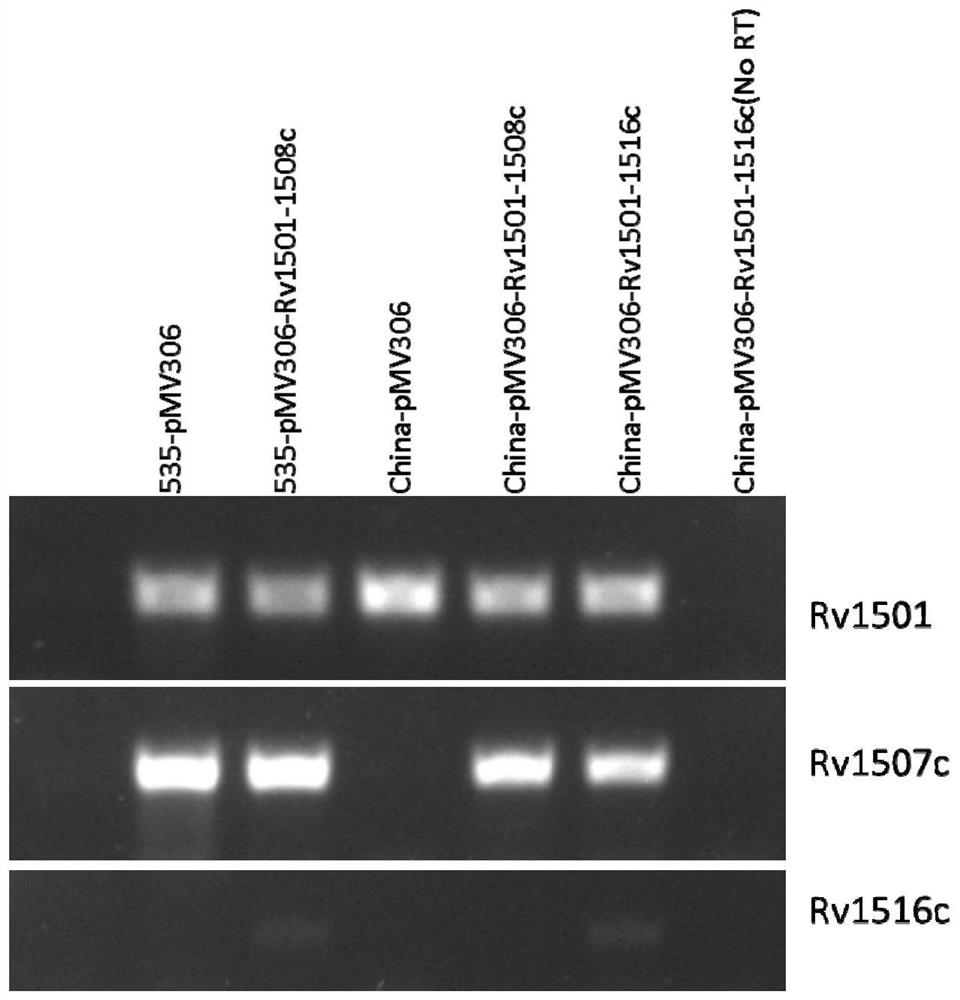
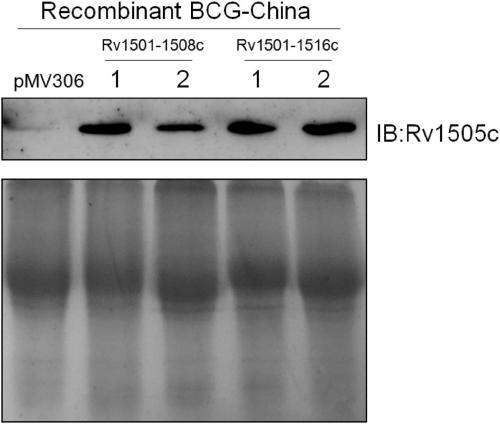
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
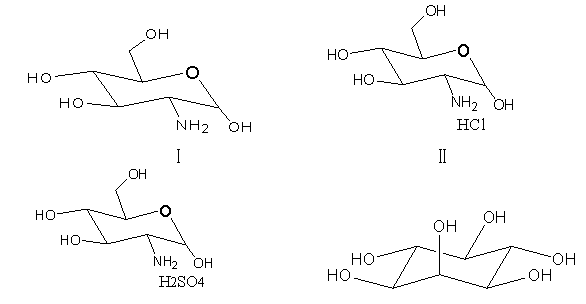
2-amino-2-deoxy-D-glucose and new application of hydrochloride, sulfate and myo-inositol thereof
InactiveCN103305586ARapid diagnosisBacteriaMicrobiological testing/measurementMycobacterium tuberculosis cultureInositol
The invention discloses 2-amino-2-deoxy-D-glucose and an application of hydrochloride, sulfate and myo-inositol thereof in preparing a mycobacterium tuberculosis culture medium or a bacillus calmette guerin vaccine mycobacterium culture medium. The 2-amino-2-deoxy-D-glucose and the hydrochloride, sulfate and myo-inositol thereof serving as a growth factor of mycobacterium tuberculosis have good application prospects in developing a novel mycobacterium tuberculosis rapid culture medium, and can be applied to the rapid diagnosis of tuberculosis, type identification and drug sensitivity detection; and by adding one of the four compounds or the mixture of several ones of the four compounds into the culture medium, the flora density in the liquid culture medium is far higher than that of a blank control group, the macroscopic bacterial colony appears one week earlier in the solid culture medium than in the blank control group, and the bacterial plaque is obviously larger than that of the blank control group.
Owner:GANSU AGRI UNIV
Mouse ascites inducer and application thereof
The invention provides a novel mouse ascites inducer. The mouse ascites inducer contains liquid paraffin, wool grease, bacillus calmette guerin vaccine, interleukin and B lymphocyte stimulating factors which are used as active ingredients. The inducer can directly stimulate hybridoma cells to quickly proliferate in the body of a mouse, and secrete a large amount of antibodies. The production cycle of ascites is short, a large amount of ascites is generated, and high titer is guaranteed. When a product is used for preparing a large amount of the ascites antibodies of the mouse, cost is saved; and the inducer has the considerable economic benefit.
Owner:杭州华安生物技术有限公司
Recombinant bacillus calmette guerin vaccine, and applications thereof
ActiveCN108949783ADoes not compromise or enhances securityImprove securityAntibacterial agentsBacterial antigen ingredientsBacterial strainTGE VACCINE
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
Novel water-soluble immunologic adjuvant and preparing method thereof
PendingCN109939228AAvoid harmEligible for benefitsAntibody medical ingredientsWater basedImmune cycle
The invention discloses a novel water-soluble immunologic adjuvant which comprises a PBS phosphate buffer, Poly I:C, polylysine and lentinan. The PBS phosphate buffer is composed of monopotassium phosphate, sodium dihydrogen phosphate, sodium chloride, potassium chloride and double distilled water. Each liter of PBS phosphate buffer is prepared from 0.20-0.27 g of monopotassium phosphate, 1.30-1.80 g of sodium dihydrogen phosphate, 5.0-15.0 g of sodium chloride and 0.1-0.3 g of potassium chloride and the balance double distilled water to reach the constant volume of 1 L. In this way, a Bacillus Calmette Guerin vaccine does not need to be used, an animal body is prevented from generating antibodies against the adjuvant, and the purity of the antibodies can be easily improved; in addition, an animal can be immunized after the adjuvant is evenly mixed with water-based antigens, no complicated emulsifying process is needed, the spatial conformation of the antigens is effectively protected,the antibodies for the spatial conformation epitope can be more easily obtained, the immune cycle is effectively prolonged, the antigen consumption in each injection is effectively reduced, no harm is caused to the animal, and the animal welfare is better satisfied.
Owner:苏州博特龙免疫技术有限公司
Humanized anti-mycobacterium tuberculosis complex LAM monoclonal antibody and preparation and application thereof
ActiveCN113480659AHigh sensitivityImprove featuresImmunoglobulinsBiological testingPhage antibodiesLipoarabinomannan
The invention discloses a nucleic acid sequence of a pair of humanized monoclonal antibodies specifically binding with mycobacterium tuberculosis complex (MTBC) lipoarabinomannan (LAM), and a preparation method and application of a recombinant antibody of the human monoclonal antibodies. The monoclonal antibody disclosed by the invention is obtained by screening and identifying a healthy human scFv phage antibody library inoculated with bacillus calmette guerin vaccine, an antibody sequence of the monoclonal antibody is obtained by sequencing, the antibody sequence is further cloned to an expression vector, then 293 cells are transfected, and then purification is carried out to obtain the monoclonal antibody. The recombinant antibody prepared by the invention can bind with MTBC LAM at high sensitivity, an immunochromatography reagent card for rapidly detecting LAM antigens is further developed based on monoclonal antibody pairing, MTBC and part of slowly growing mycobacteria split products can be specifically detected, and do not have cross reaction with rapidly growing mycobacteria. The immunochromatography reagent card developed by the invention can be directly used for qualitative detection (taking a colloidal gold reagent card as an example) or quantitative detection (taking a quantum dot fluorescent microsphere test paper card as an example) of a urine sample of a tuberculosis patient, and has relatively high detection sensitivity and extremely high specificity, is simple and convenient to operate, is short in time consumption, and has a wide clinical application prospect in the aspect of rapid diagnosis of tuberculosis.
Owner:迪比康上海生物科技有限公司
Special-effect drug for treating asthma
InactiveCN103655927AGood treatment effectWide range of typesBacteria material medical ingredientsRespiratory disorderPotato starchLevamisole
The invention relates to a special-effect drug for treating asthma, which is composed of a drug I, a drug II and a drug III; the drug I comprises the following components by weight: 0.016g of dead bacillus calmette guerin vaccine, 1.071g of potato starch, 0.3g of aminophylline, 0.001g of ketotifen, 0.005g of dioxopromethazine, 0.025g of levomisole, 0.03g of bromhexine and 0.8g of green tea; the drug II comprises the following components by weight: 1.4g of fructus schisandrae, 1.4g of astragalus and 0.3g of cinnamon; and the drug IIT comprises the following components by weight: 1.2g of radix codonopsis, 1.2g of astragalus, 0.3g of epimedium and 0.4g of gypsum. The special-effect drug disclosed by the invention is prepared on the basis of combination of Chinese and western medicine sciences, has remarkable treatment effect, and treats both symptoms and root causes.
Owner:解玉启
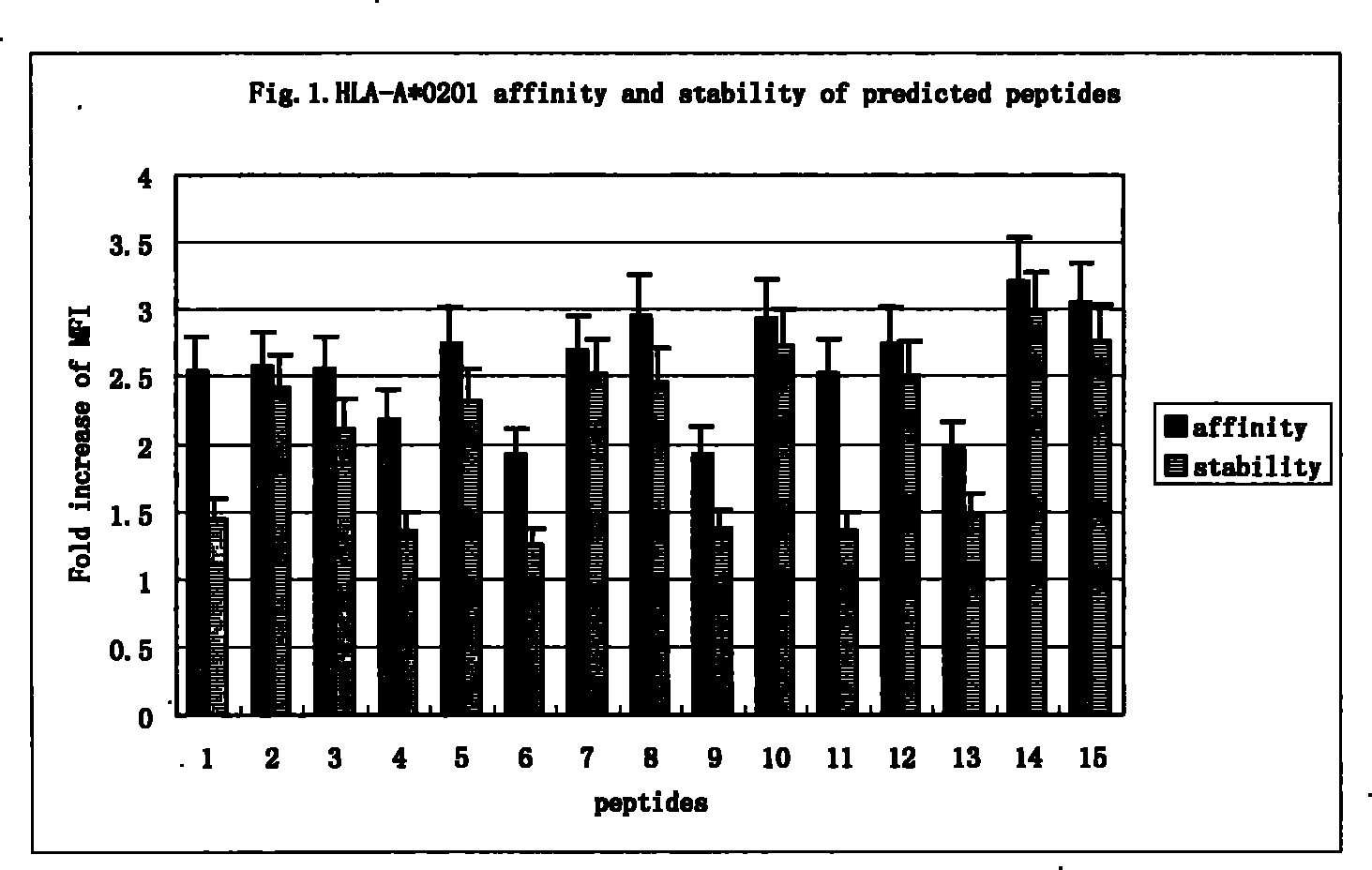
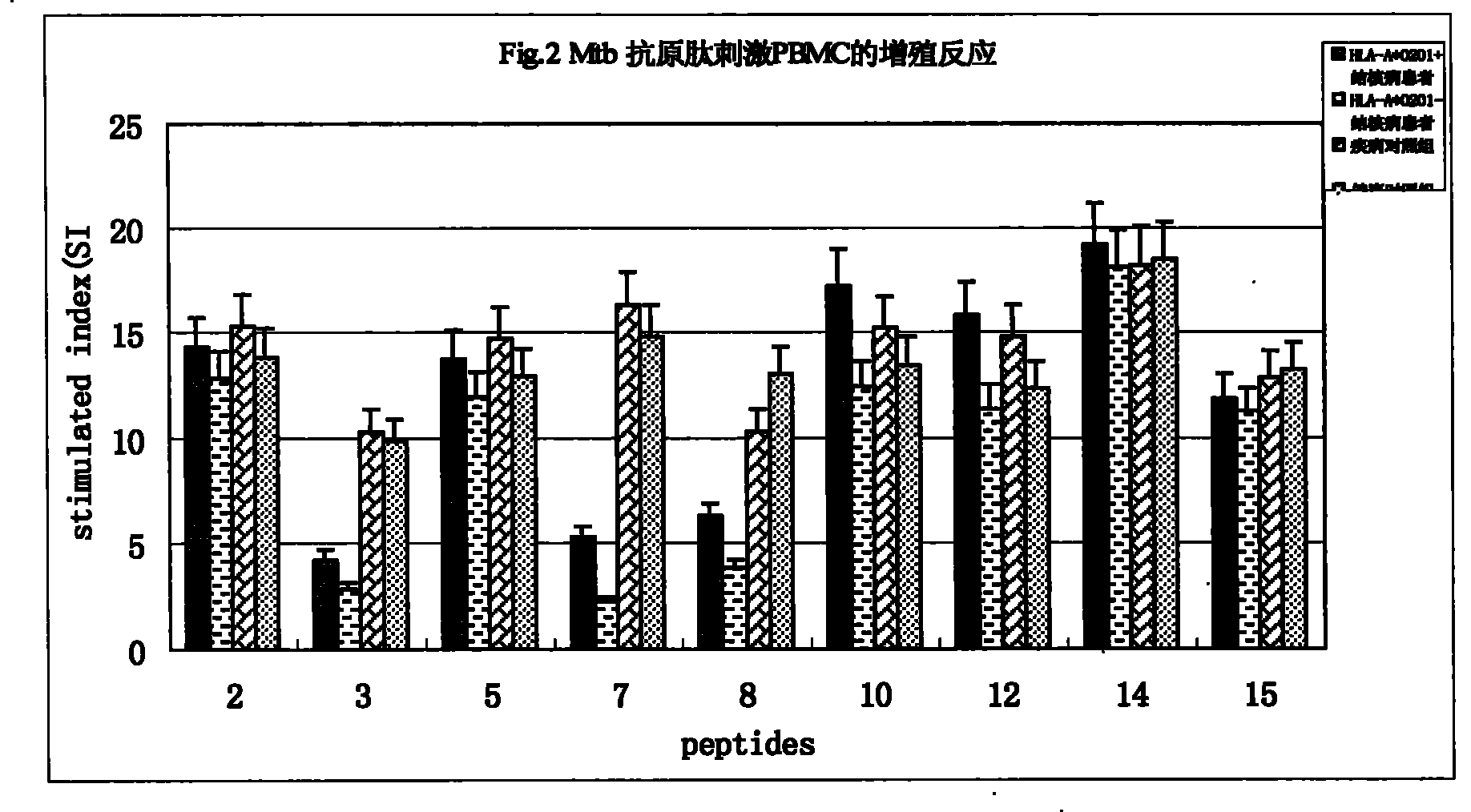
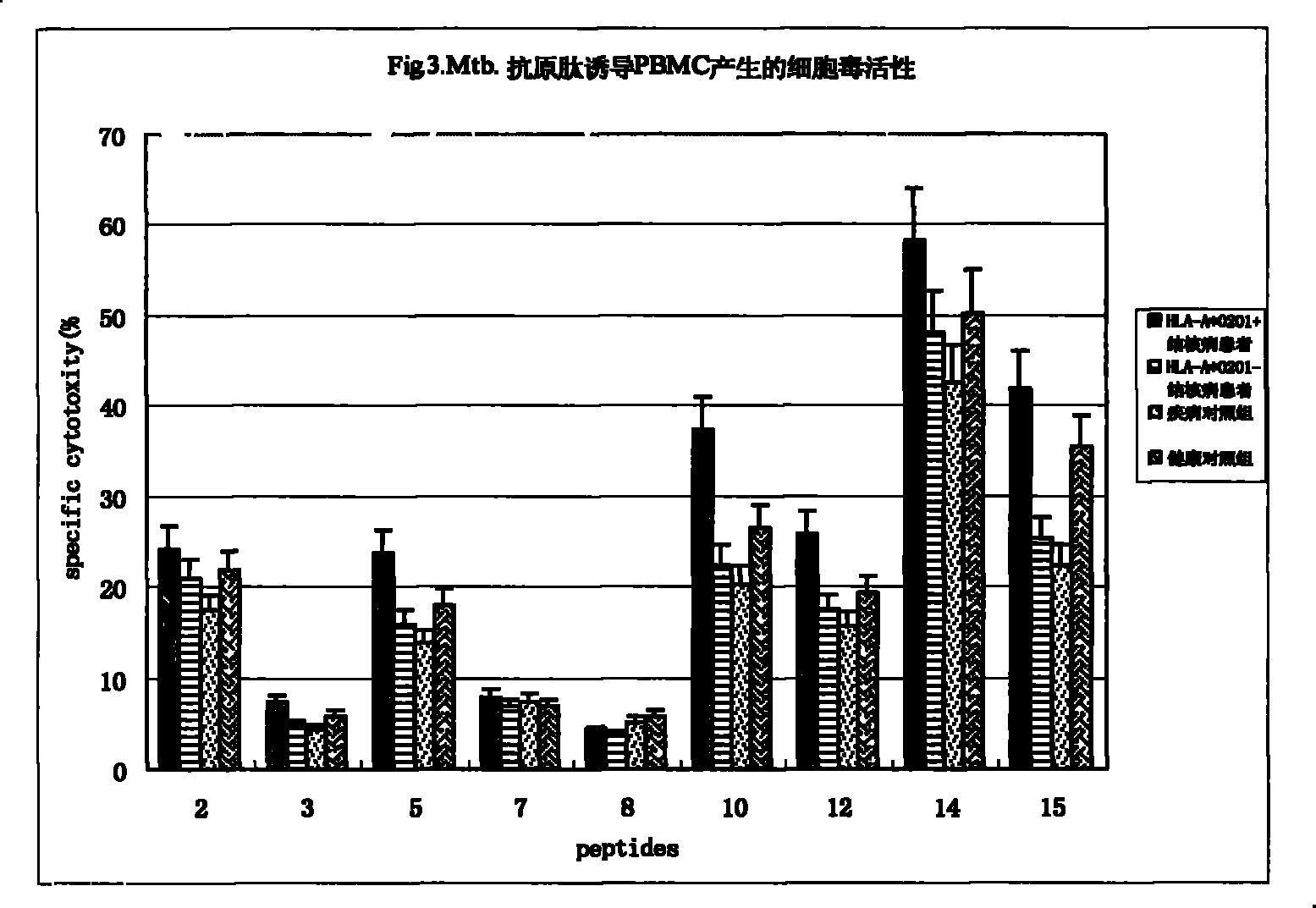
Epitope capable of exciting human body anti-mycobacterium tuberculosis protective immunity reaction and use thereof
InactiveCN101298471BMulti-drug resistance problem solvedAntibacterial agentsBacterial antigen ingredientsAntigenAntigen epitope
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Freeze-drying protective additive and application thereof, freeze-dried vaccine and production method of freeze-dried vaccine
ActiveCN111588859ASame validityImprove securityPowder deliveryBacterial antigen ingredientsBiotechnologySucrose
The invention discloses a freeze-drying protective additive and application thereof, a freeze-dried vaccine and a production method of the freeze-dried vaccine. Production concentrations of componentsof the freeze-drying protective additive are as follows: the production concentration of sucrose is 10%, the production concentration of dextran is 0.5-2.0%, the production concentration of sodium glutamate is 1%, the production concentration of KCL is 1% or the production concentration of sucrose is 10%, the production concentration of dextran is 1%, the production concentration of trehalose is1-5%, the production concentration of sodium glutamate is 1%, and the production concentration of KCL is 1%; and a solvent for producing the freeze-drying protective additive is injection water, and apH value of the freeze-drying protective additive is adjusted by means of a 10% sodium hydroxide solution to be 7.3-7.5. The freeze-drying protective additive does not contain gelatin, is used for producing a bacillus calmette guerin vaccine used for freeze-drying treatment, can reduce an endotoxin content, is not liable to cause anaphylases, and can still maintain high stability and a long validity period.
Owner:成都可恩生物科技有限公司
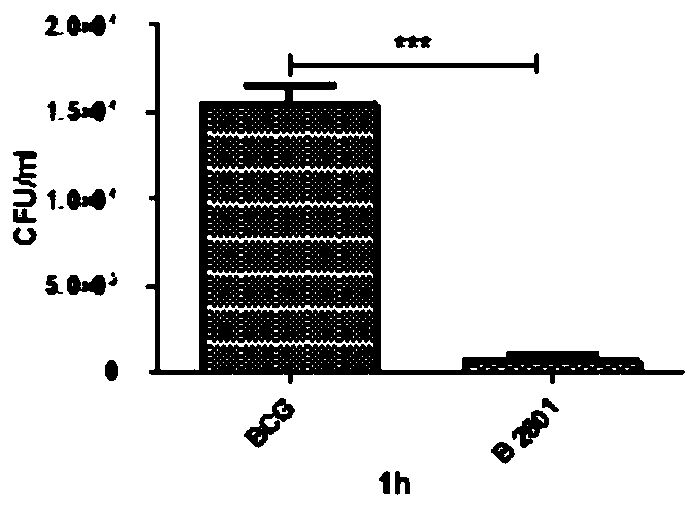
Mycobacterium bovis bacillus calmette guerin vaccine low-invasiveness mutant strain B2801
ActiveCN109825515AReduce the ability to invadeSignificantly low intracellular viabilityBacteriaMicroorganism based processesNucleotideMycobacterium
The invention belongs to the technical field of infectious disease prevention and control of animals, and relates to a mycobacterium bovis bacillus calmette guerin vaccine low-invasiveness mutant strain B2801. The mutant strain contains a mutant gene of a mycobacterium bovis BCG_2658, the nucleotide sequence of the mutant gene is shown as SEQ ID NO.1, a mutation site is located behind a site 2922763 of a genome and behind a site 748 of the BCG_2658 gene sequence, and the mutant strain is a novel structural gene and a functional factor. The mutant strain has low invasiveness, low intracellularsurvival capacity, high growth speed, no cord-like structure and microcolony morphology. The mutant strain is preserved at the China Center for Type Culture Collection, with the preservation CCTCC NO:M 2018864. The seperated mutant gene and the mutant strain thereof can be expected to be applied to research on the pathogenic mechanism and immune mechanism of mycobacterium bovis and preparation ofdrugs for preventing and treating bovine tuberculosis.
Owner:HUAZHONG AGRI UNIV
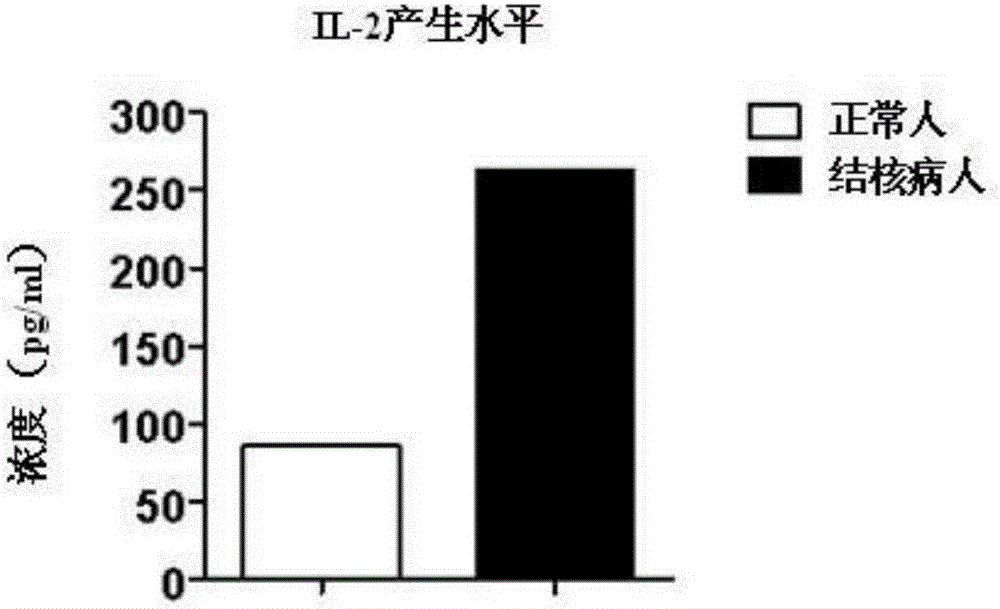
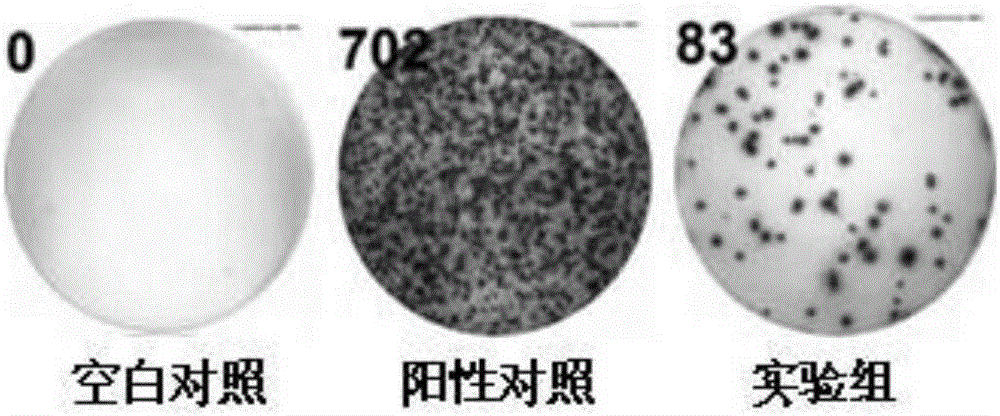
Fusion protein for inducing peripheral blood mononuclear cells to produce tuberculosis specific cytokines
ActiveCN105218681AIncreased sensitivityStrong specificityBiological testingHybrid peptidesPeripheral blood mononuclear cellCytokine
The invention discloses fusion protein for inducing peripheral blood mononuclear cells to produce tuberculosis specific cytokines. The fusion protein includes CFP-10 protein, ESAT-6 protein and EspC protein which are connected through connecting peptides. Compared with an existing stimulus, the fusion protein is more efficient in effect, higher in sensitivity, higher in specificity and good in stimulating effect. Meanwhile, the fusion protein stimulates PBMC to produce lots of tuberculosis related factors such as IFN-gamma, IL-2 and TNF-alpha with mycobacterium tuberculosis antigenic specificity, and the stimulus-induced reaction is not interfered with by bacillus calmette guerin vaccine. By means of the fusion protein, the relevance ratio of tuberculosis is more effectively improved, and the fusion protein has positive significance in control over tuberculosis. The fusion protein as a stimulus can be applied to research on a tuberculosis pathopoiesia and immunity prevention mechanism and further control over tuberculosis.
Owner:SUN YAT SEN UNIV
A recombinant bacillus calmette-guerin vaccine used for preventing toxoplasmosis in pigs and a preparing method thereof
InactiveCN106047914AImprove securityGood immune protectionProtozoa antigen ingredientsBacteriaAdjuvantSpecific immunity
A recombinant bacillus calmette-guerin vaccine used for preventing toxoplasmosis in pigs is disclosed. A preparing method of the vaccine is also provided. The characteristic that a bacillus calmette-guerin living-vector vaccine has strong cell immunity and body fluid immunity adjuvant functions is utilized, an exogenous protein can be expressed efficiently so that the expressed protein can exert good immunity protection functions, and the above two advantages are combined to achieve the objective of preventing toxoplasmosis. The recombinant BCG vaccine combines an adjuvant and the vector, and integrates a plurality of exogenous genes and a live vaccine. Strong and durable specific immunity can be obtained through one time of inoculation of the recombinant BCG vaccine. The recombinant BCG vaccine is stable, high in safety and easy in transportation and storage.
Owner:JILIN UNIV
Vaccine traditional Chinese medicine composition for treating infectious diseases through transdermal administration and preparation method thereof
PendingCN113350494ASignificant effectShort treatment cycleSenses disorderBacterial antigen ingredientsInfective disorderTGE VACCINE
The invention discloses a vaccine traditional Chinese medicine composition for treating infectious diseases through transdermal administration. The vaccine is bacillus calmette guerin vaccine, and the traditional Chinese medicine composition comprises the following traditional Chinese medicine raw materials in parts by weight of 0-20 parts of coptis chinensis, 5-20 parts of rheum officinale, 0-9 parts of aloe, 0-16 parts of dandelion, 8-20 parts of isatis root, 6-20 parts of flos lonicerae, 5-20 parts of fructus forsythiae, 0-8 parts of andrographis paniculata, 1-10 parts of radix bupleuri, 2-11 parts of artemisia apiacea, 0-9 parts of cortex phellodendri and 0-9 parts of scutellaria baicalensis. According to the vaccine, transdermal administration is realized in a manner of the acillus calmette guerin vaccine and traditional Chinese medicines, a painful acillus calmette guerin vaccine intradermal injection method is replaced, the traditional Chinese medicine composition has obvious curative effects on various infectious diseases such as amygdalitis, pneumonia, nasosinusitis, appendicitis, otitis media, cholecystitis, influenza and the like, the treatment period is short and is 2-11 days, the traditional Chinese medicine composition is simple to use, injection and medicine taking are not needed, side effects are avoided, the effect is quick, and the traditional Chinese medicine replaces antibiotics.
Owner:王琳扬 +1
Immune preparation method of antituberculous polypeptide
ActiveCN103059134AIncrease productionImprove specific immunityImmunoglobulins against bacteriaUltrafiltrationFreeze-drying
The invention relates to an immune preparation method of antituberculous polypeptide. The method is characterized by comprising the following steps of carrying out subcutaneous injection of bacillus calmette guerin vaccine lipidosome on healthy pigs so as to carry out immune response, wherein the bacillus calmette guerin vaccine lipidosome is prepared by mixing bacillus calmette guerin vaccine with an immune intensifier; taking spleens and lymph glands of the immune healthy pigs, and removing the fat and mucosa to obtain objects to be treated; adding normal saline into the objects to be treated, smashing and preparing homogenate; repeatedly freezing and melting the homogenate at minus 20 to minus 30 DEG C and carrying out ultrafiltration by using an ultrafiltration film so as to obtain the filtrate as a primary antituberculous polypeptide product; and further carrying out white blood cell adhesion inhibition experiments to detect that the white blood cell adhesion inhibition rate meets the requirements of range values of 33.9-38%, subsequently carrying out crosslinked glucan gel chromatography separation and purification, and freeze-drying so as to obtain the antituberculous polypeptide. The preparation process is simple, convenient and controllable; and the white blood cell adhesion inhibition rate of the prepared antituberculous polypeptide can be 33.9-38%.
Owner:广东龙帆生物科技有限公司
a medicine for asthma
InactiveCN103655927BGood treatment effectWide range of typesBacteria material medical ingredientsRespiratory disorderPotato starchLevamisole
The invention relates to a special-effect drug for treating asthma, which is composed of a drug I, a drug II and a drug III; the drug I comprises the following components by weight: 0.016g of dead bacillus calmette guerin vaccine, 1.071g of potato starch, 0.3g of aminophylline, 0.001g of ketotifen, 0.005g of dioxopromethazine, 0.025g of levomisole, 0.03g of bromhexine and 0.8g of green tea; the drug II comprises the following components by weight: 1.4g of fructus schisandrae, 1.4g of astragalus and 0.3g of cinnamon; and the drug IIT comprises the following components by weight: 1.2g of radix codonopsis, 1.2g of astragalus, 0.3g of epimedium and 0.4g of gypsum. The special-effect drug disclosed by the invention is prepared on the basis of combination of Chinese and western medicine sciences, has remarkable treatment effect, and treats both symptoms and root causes.
Owner:解玉启
Preparation and identification method of taenia solium TSOL18 gene recombinant bacillus calmette guerin vaccine
InactiveCN104498522AImprove immunityLow priceAntibacterial agentsBacterial antigen ingredientsBacteroidesWater baths
Owner:ZUNYI MEDICAL UNIVERSITY
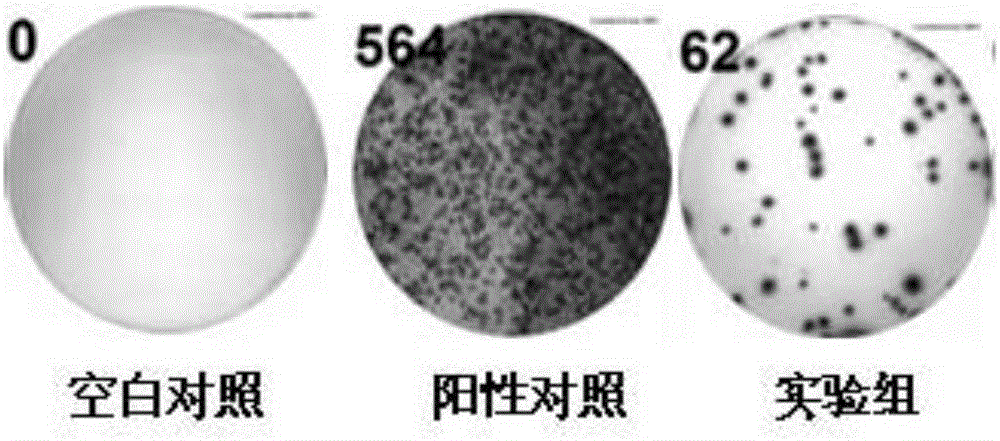
A Mycobacterium tuberculosis fusion protein for inducing cytokine production in peripheral blood mononuclear cells
ActiveCN105218680BIncreased sensitivityStrong specificityAntibacterial agentsBacterial antigen ingredientsPeripheral blood mononuclear cellFactor ii
The invention discloses mycobacterium tuberculosis fusion protein for inducing peripheral blood mononuclear cells to produce cytokines. The fusion protein includes CFP-10 protein, EsxG-6 protein and EsxH protein which are connected through connecting peptides. Compared with an existing stimulus, the fusion protein is more efficient in effect, higher in sensitivity, higher in specificity and good in stimulating effect. Meanwhile, the fusion protein stimulates PBMC to produce lots of tuberculosis related factors such as IFN-gamma, IL-2 and TNF-alpha with mycobacterium tuberculosis antigenic specificity, and the stimulus-induced reaction is not interfered with by bacillus calmette guerin vaccine. By means of the fusion protein, the relevance ratio of tuberculosis is more effectively improved, and the fusion protein has positive significance in control over tuberculosis. The fusion protein as a stimulus can be applied to research on a tuberculosis pathopoiesia and immunity prevention mechanism and further control over tuberculosis.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com