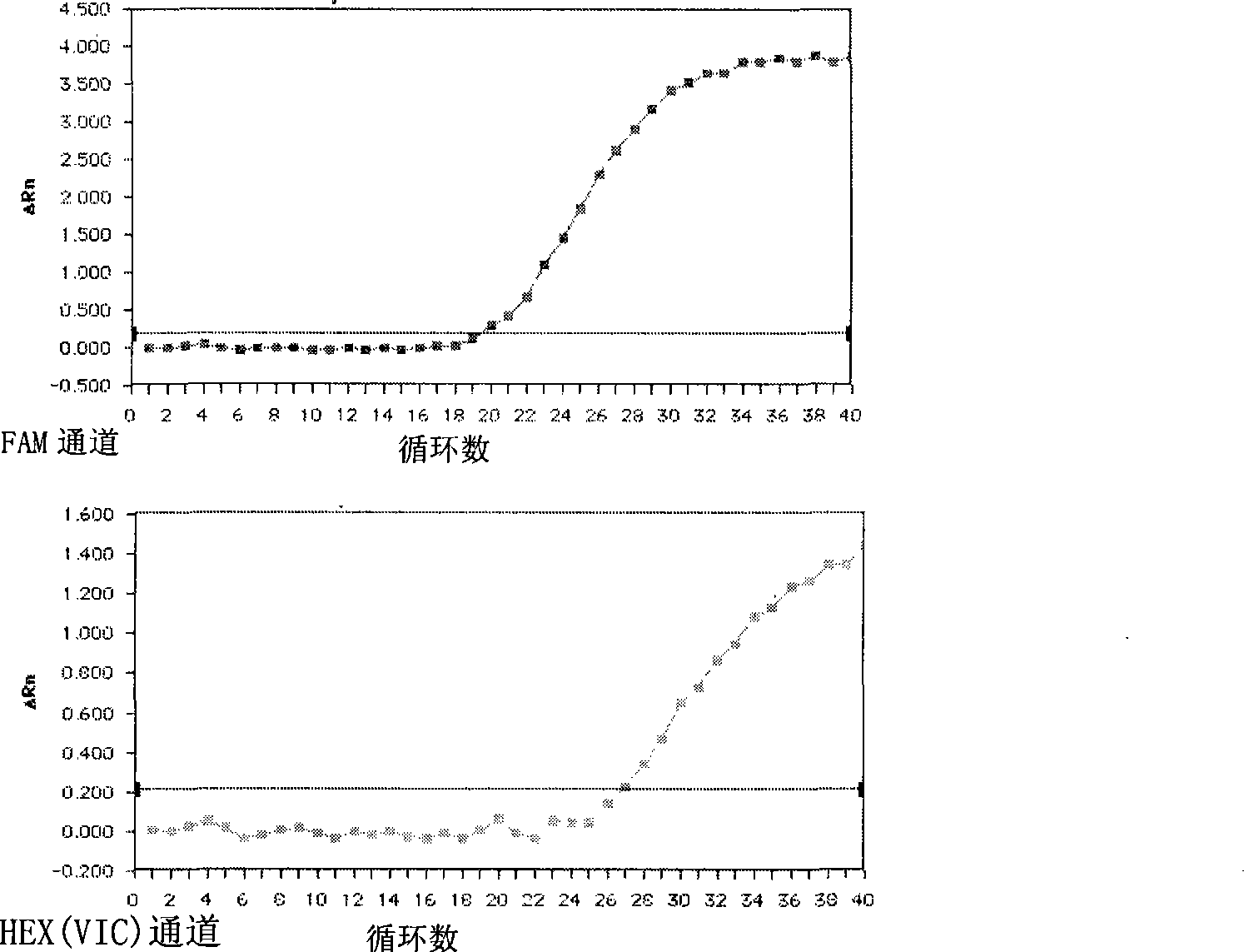
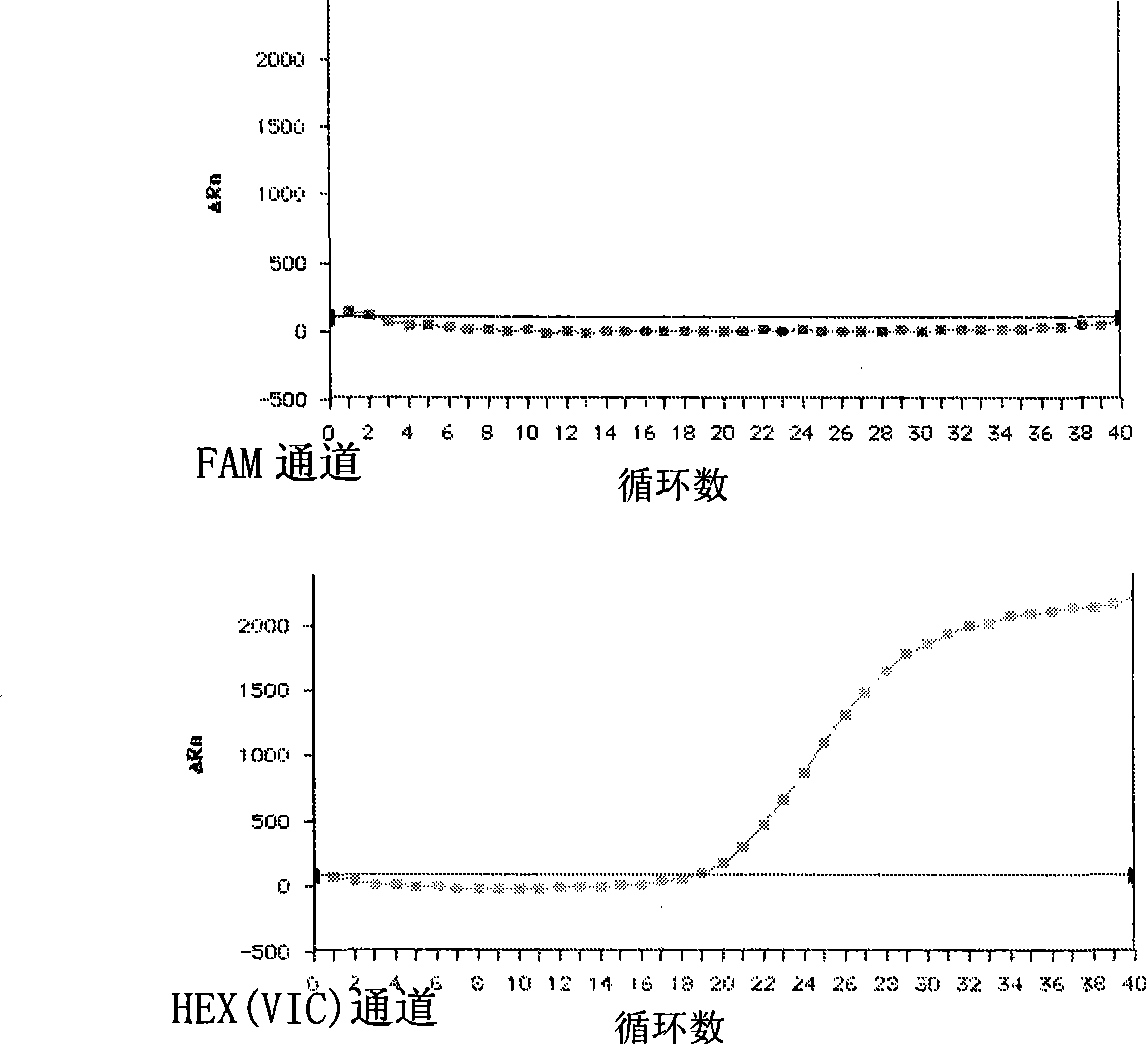
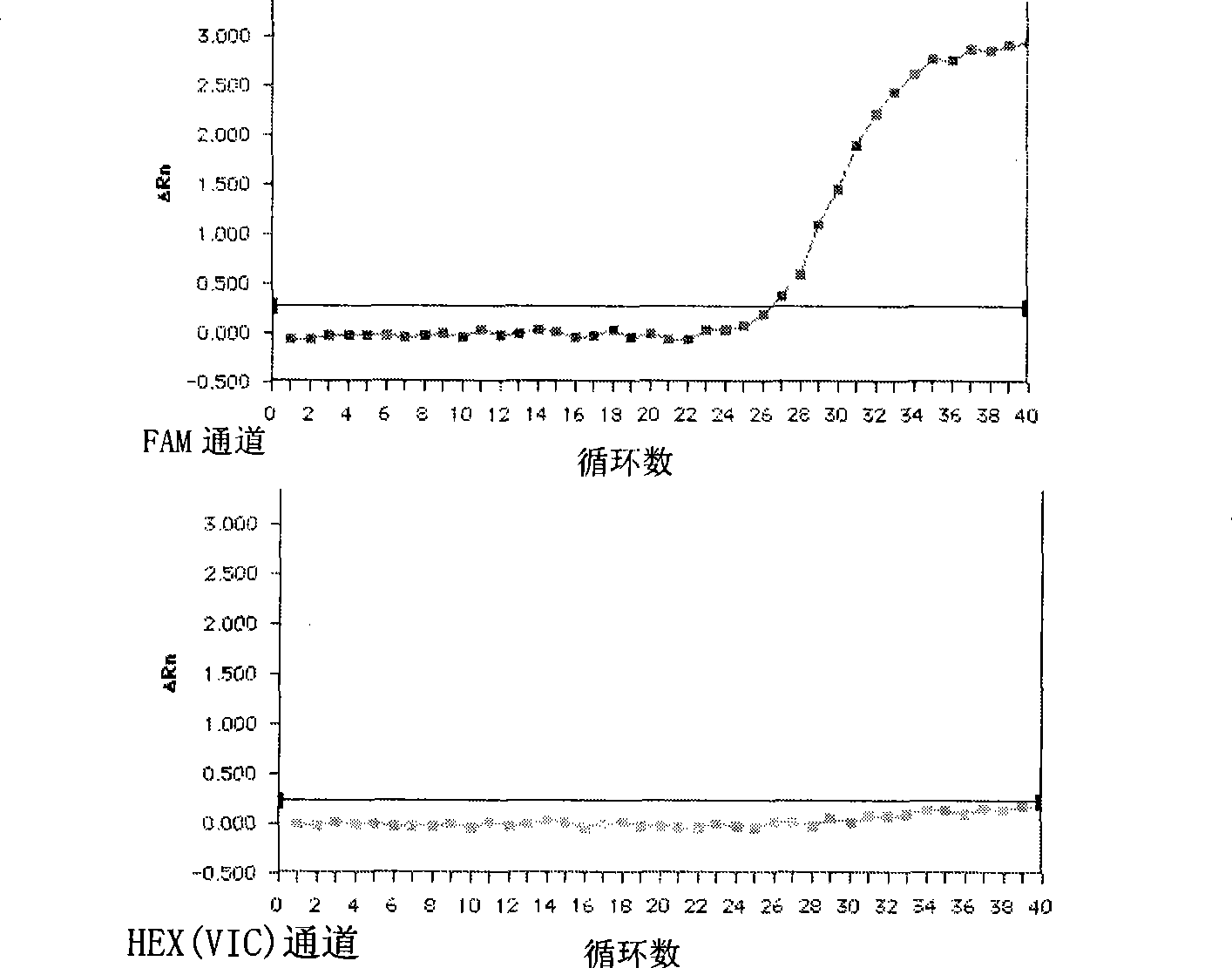
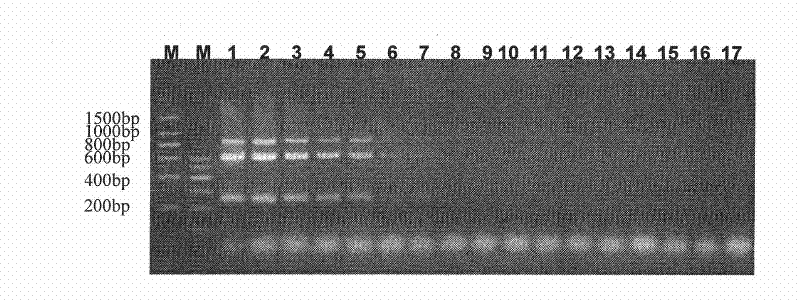
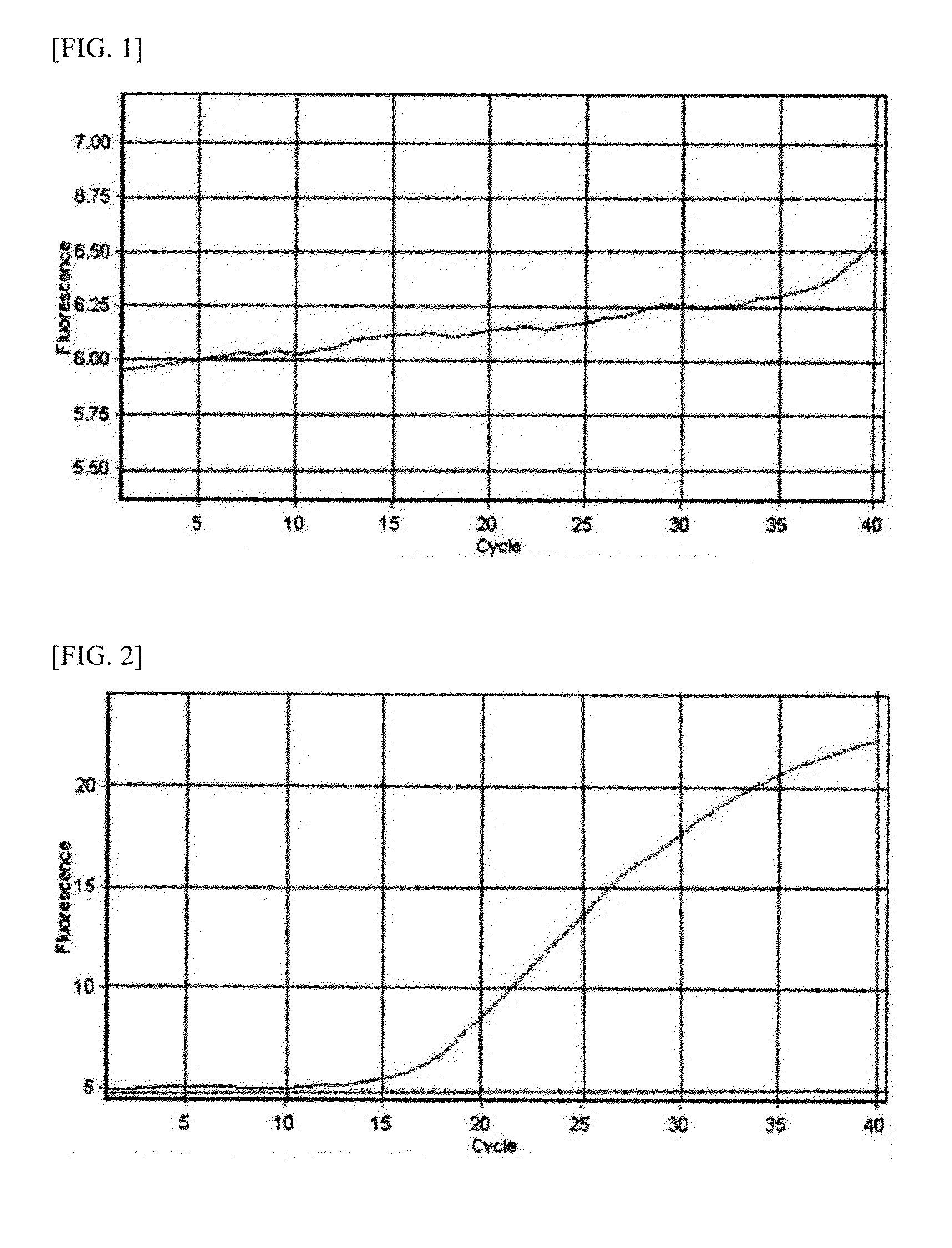
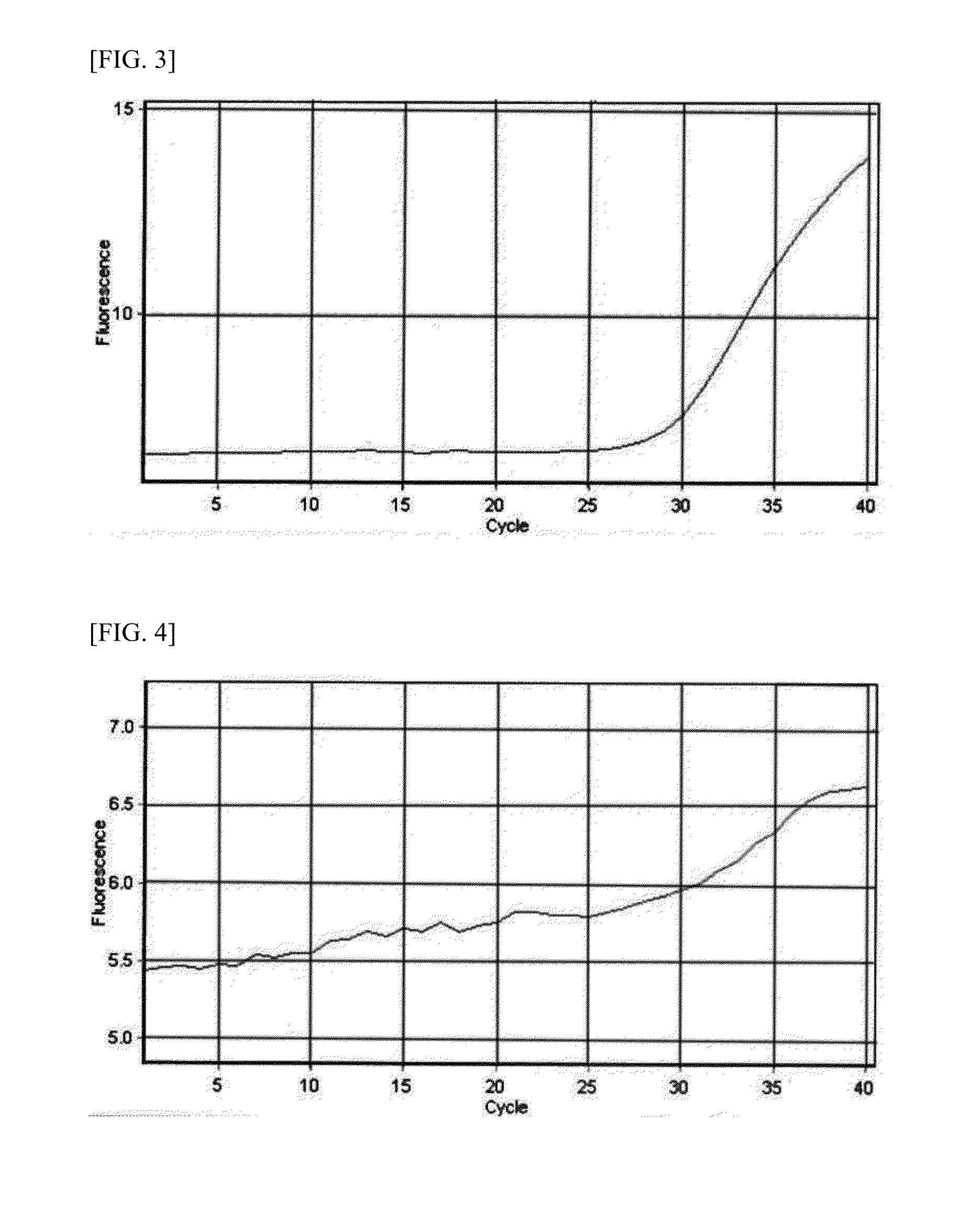
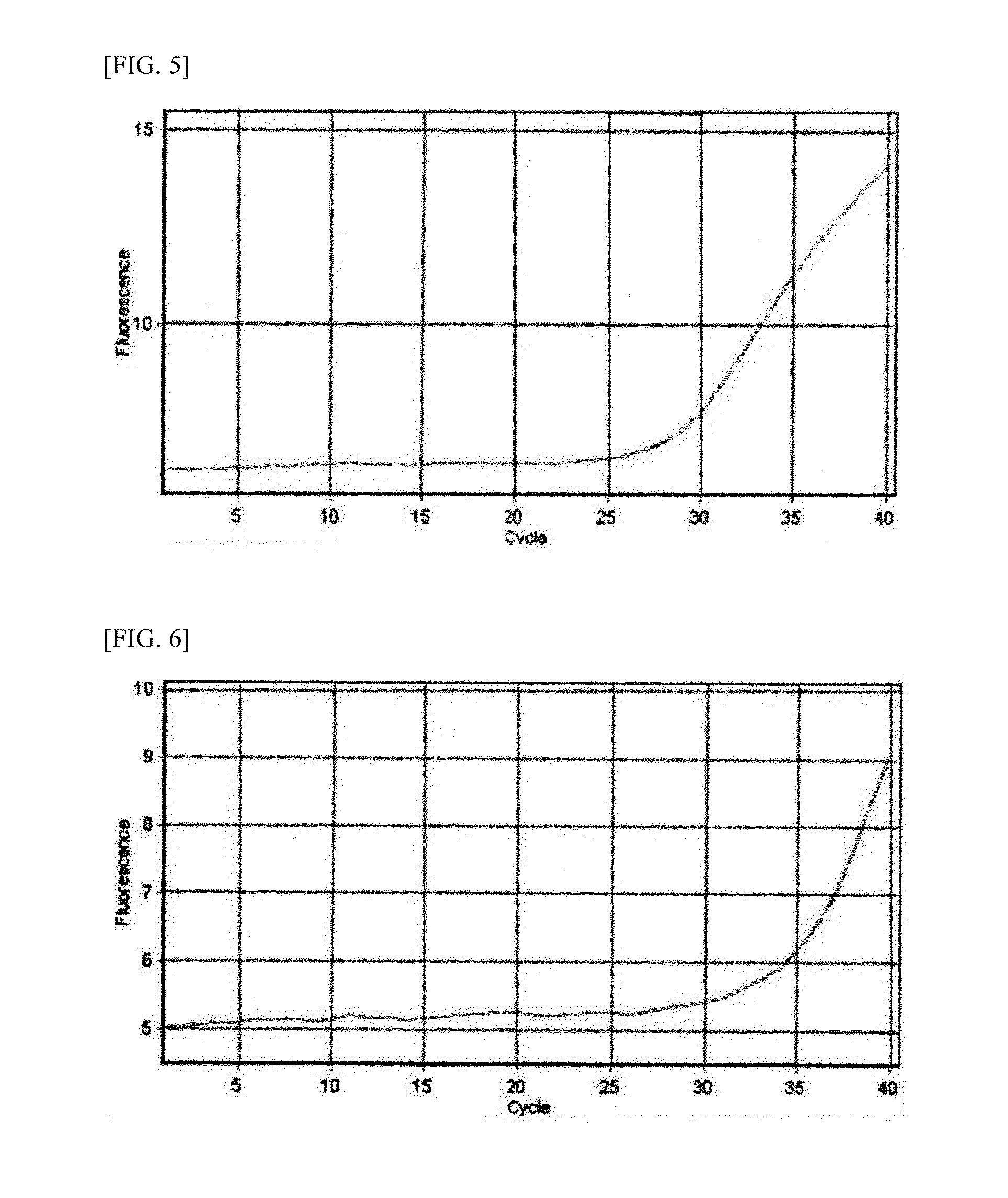
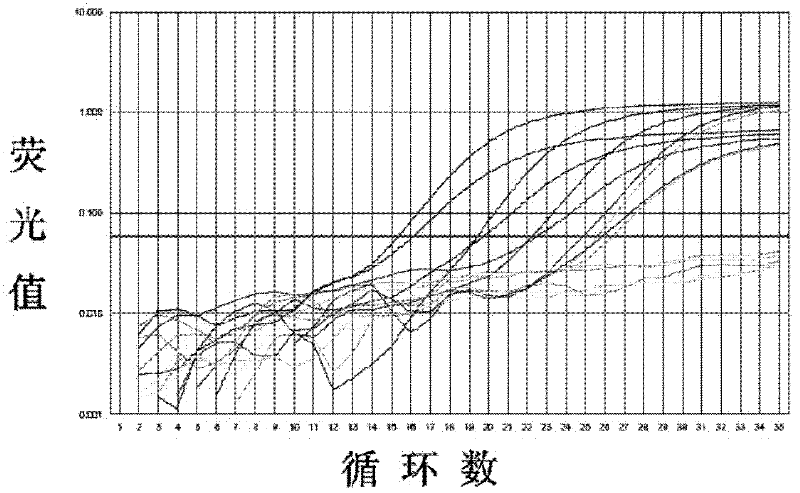
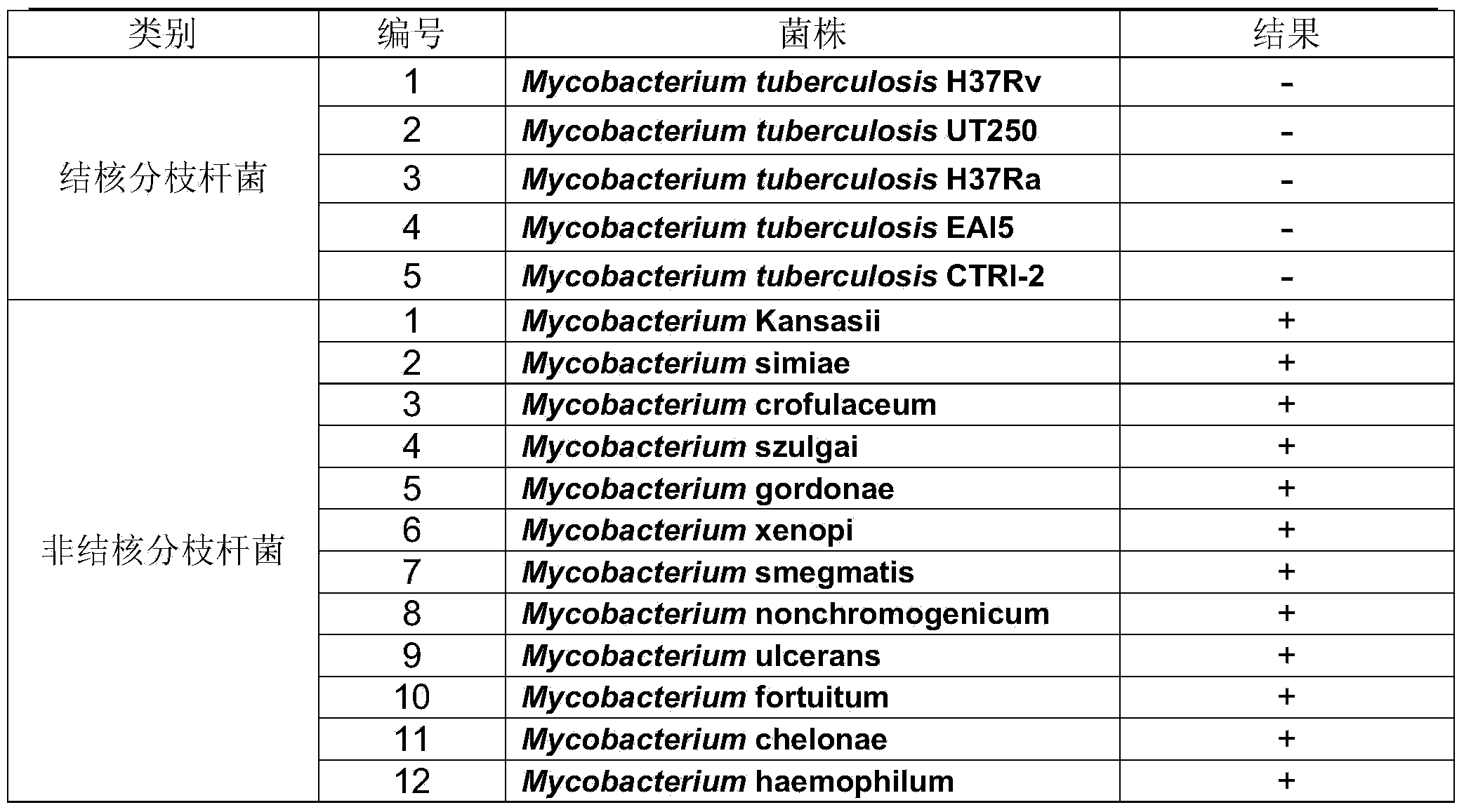
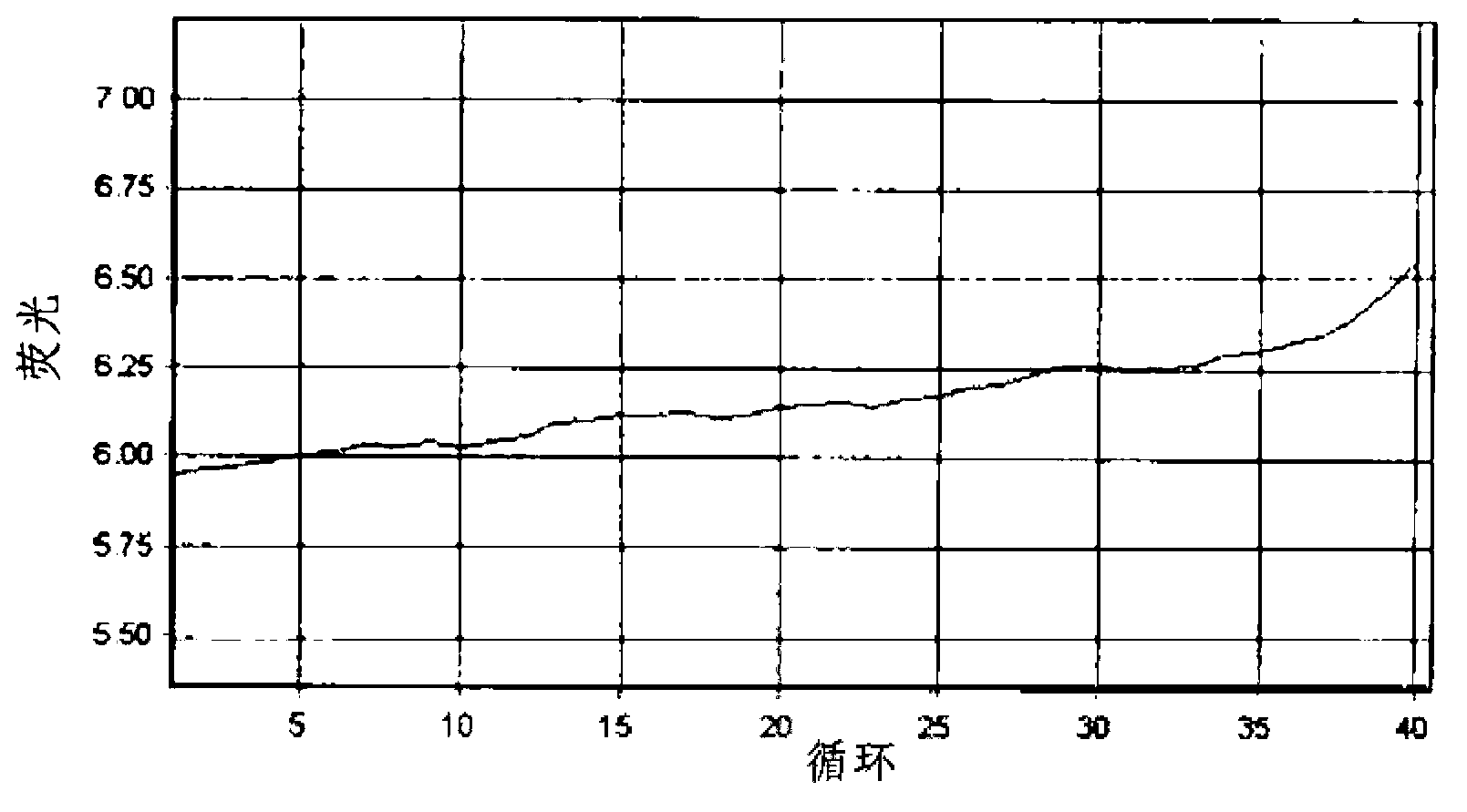
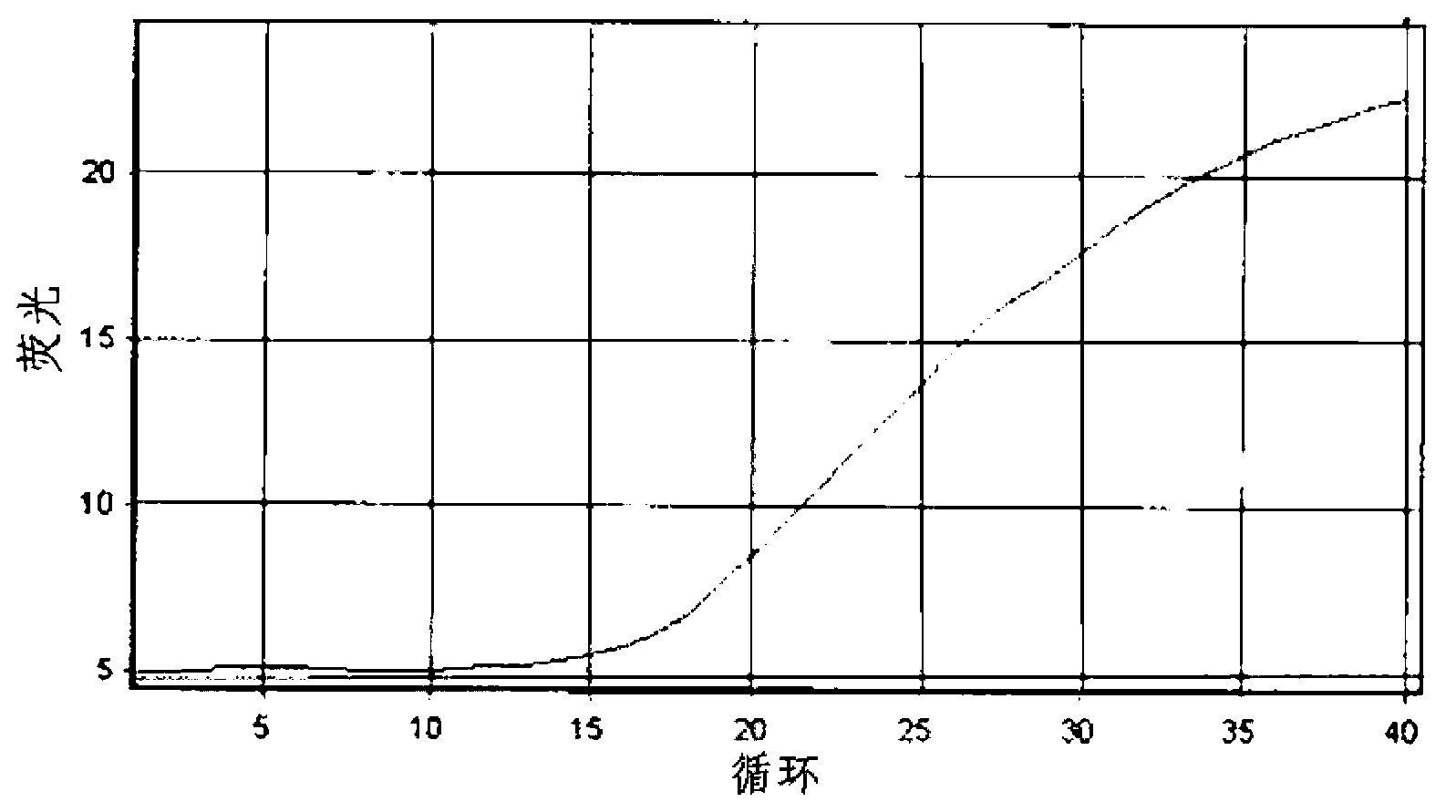
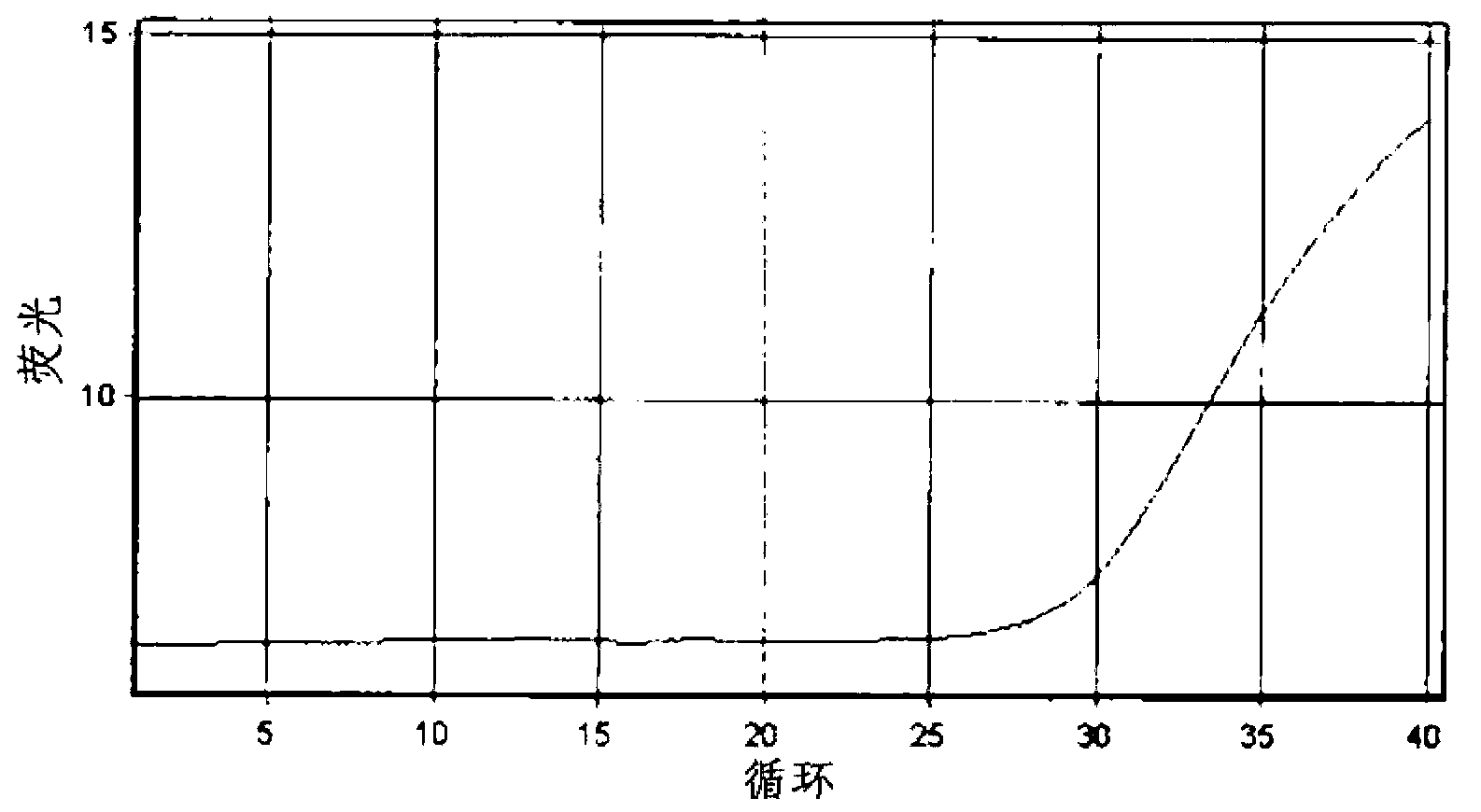
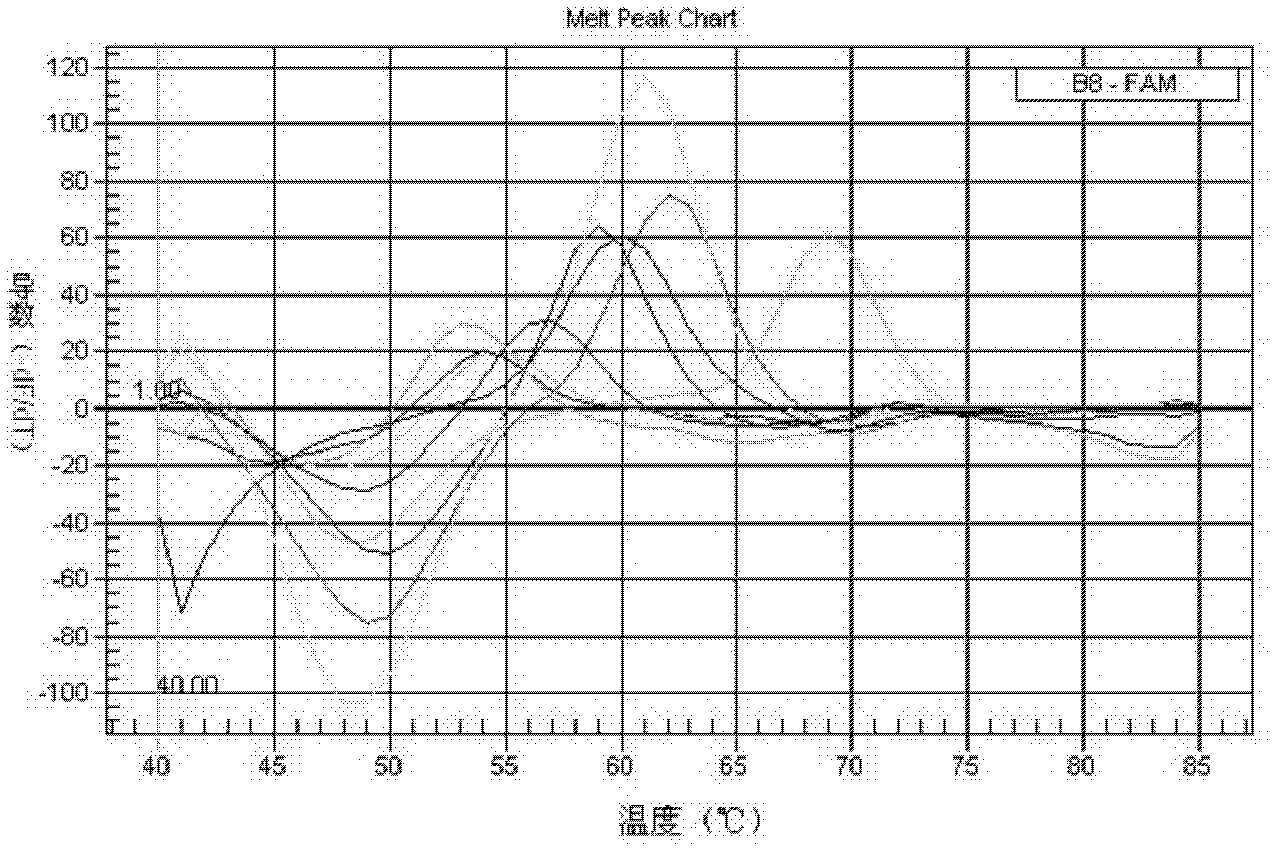
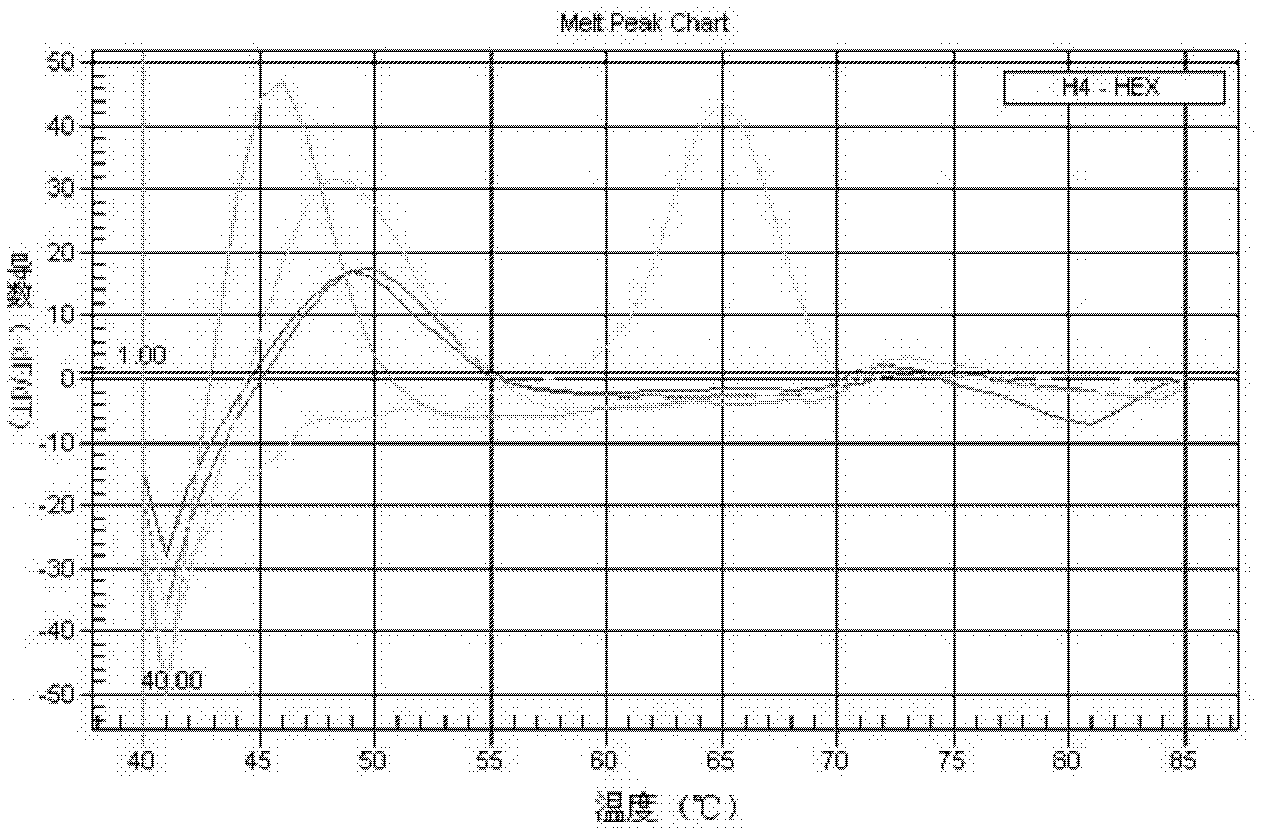
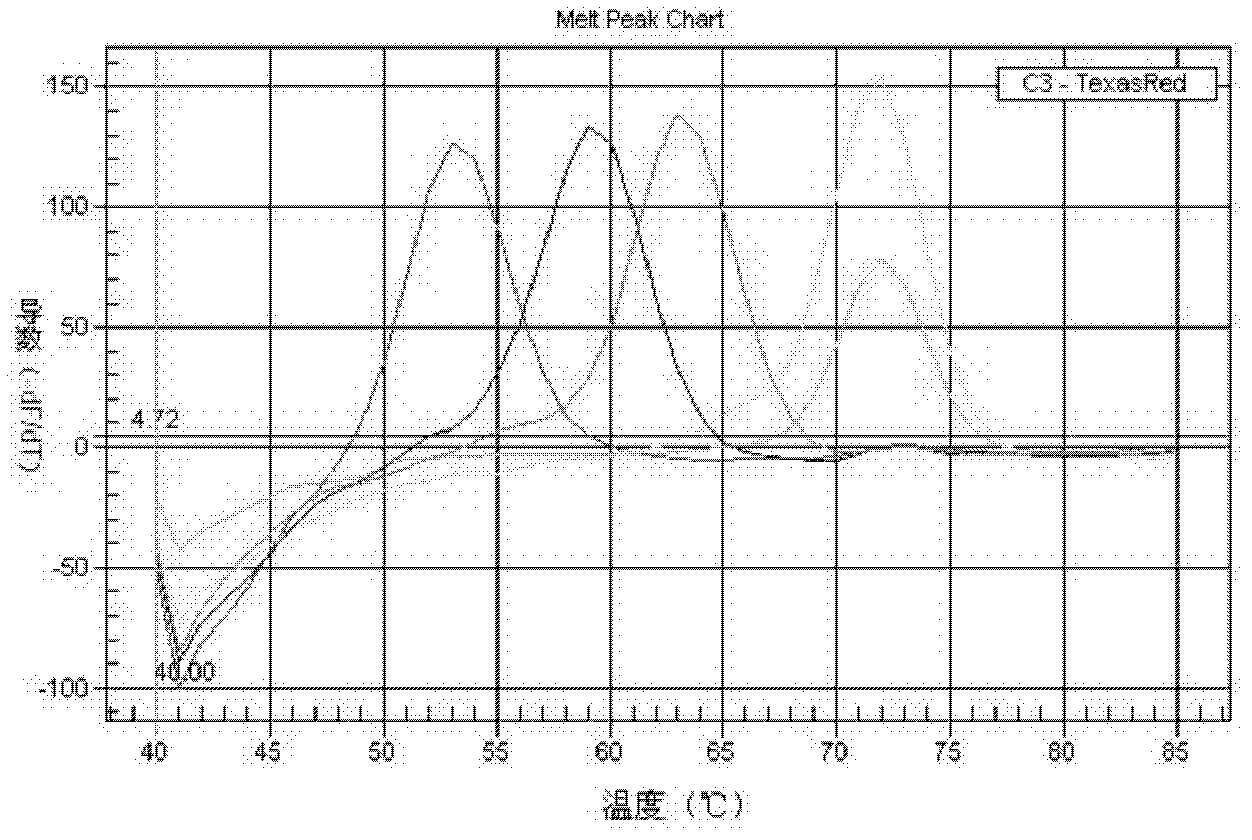
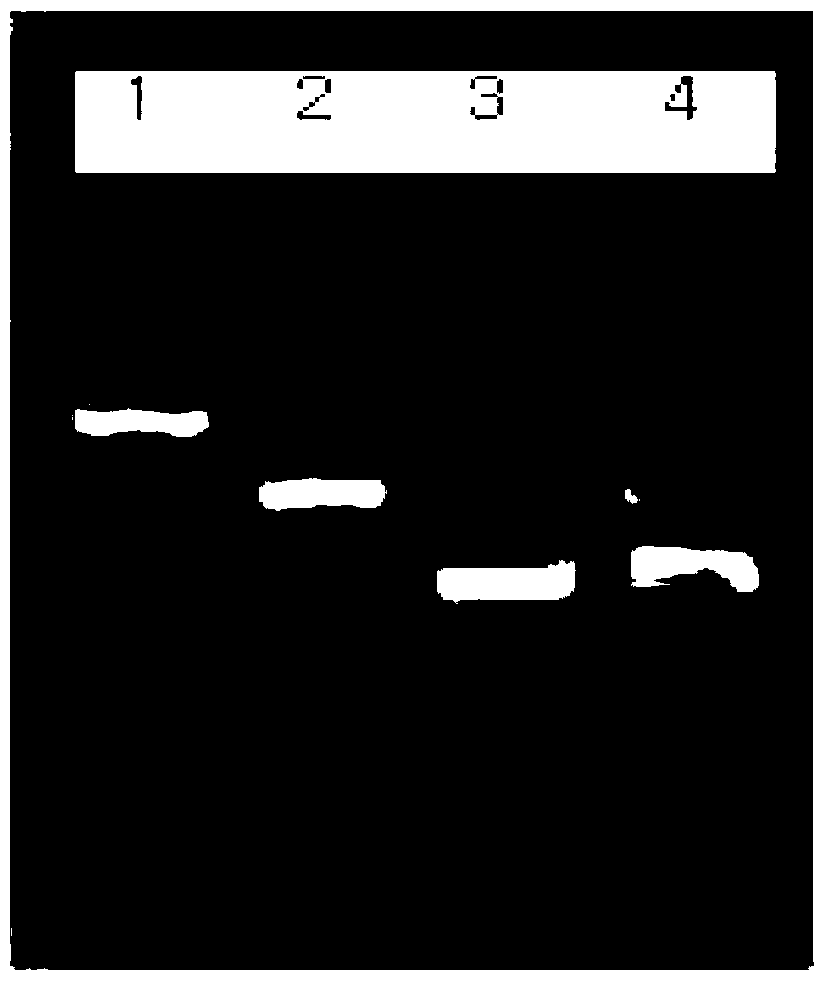
Patents
Literature
52 results about "Nontuberculous mycobacteria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nontuberculous mycobacteria (NTM), also known as environmental mycobacteria, atypical mycobacteria and mycobacteria other than tuberculosis (MOTT), are mycobacteria which do not cause tuberculosis or leprosy (also known as Hansen's disease). NTM do cause pulmonary diseases that resemble tuberculosis. Mycobacteriosis is any of these illnesses, usually meant to exclude tuberculosis. They occur in many animals, including humans.
Systems for treating pulmonary infections
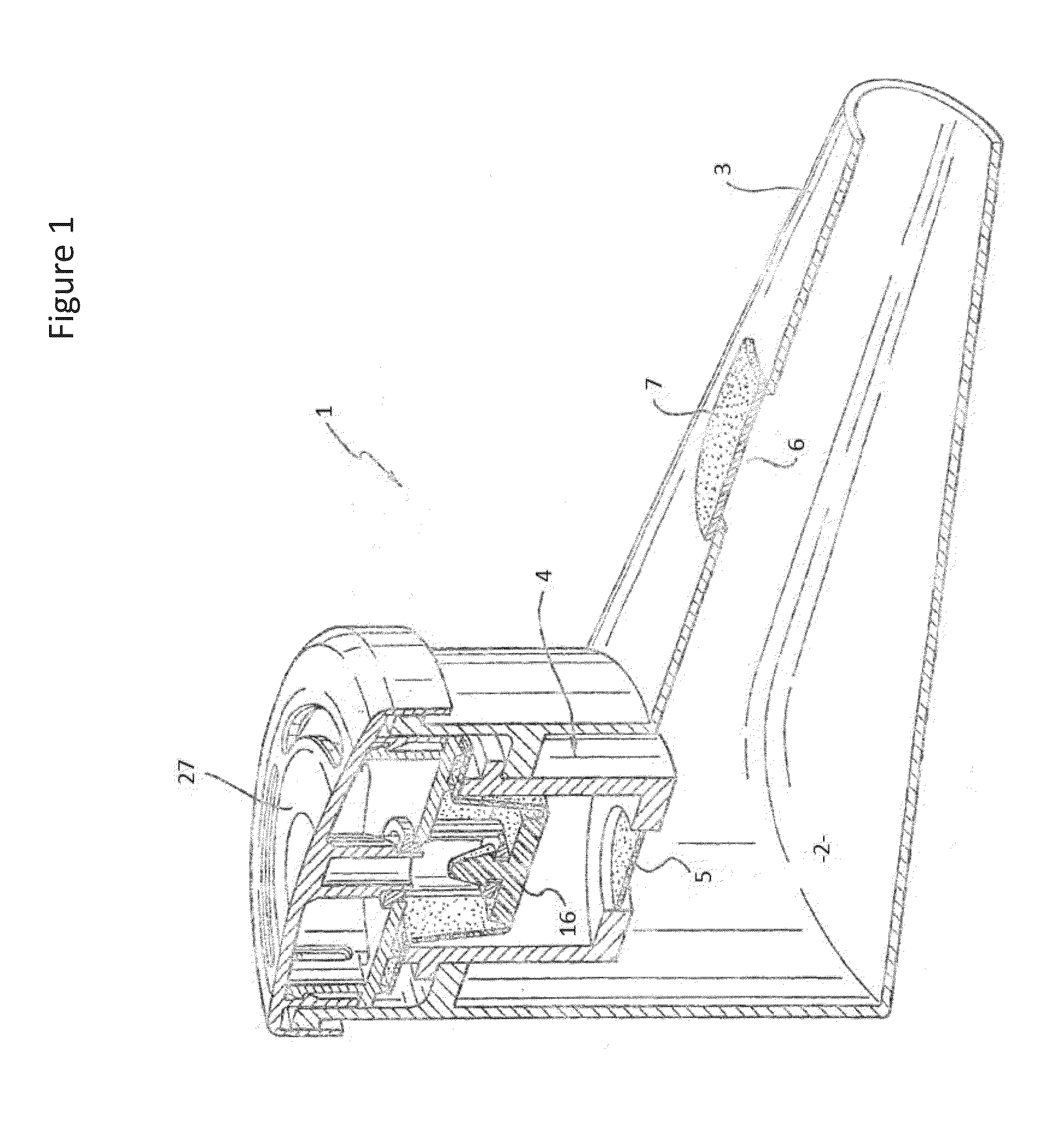
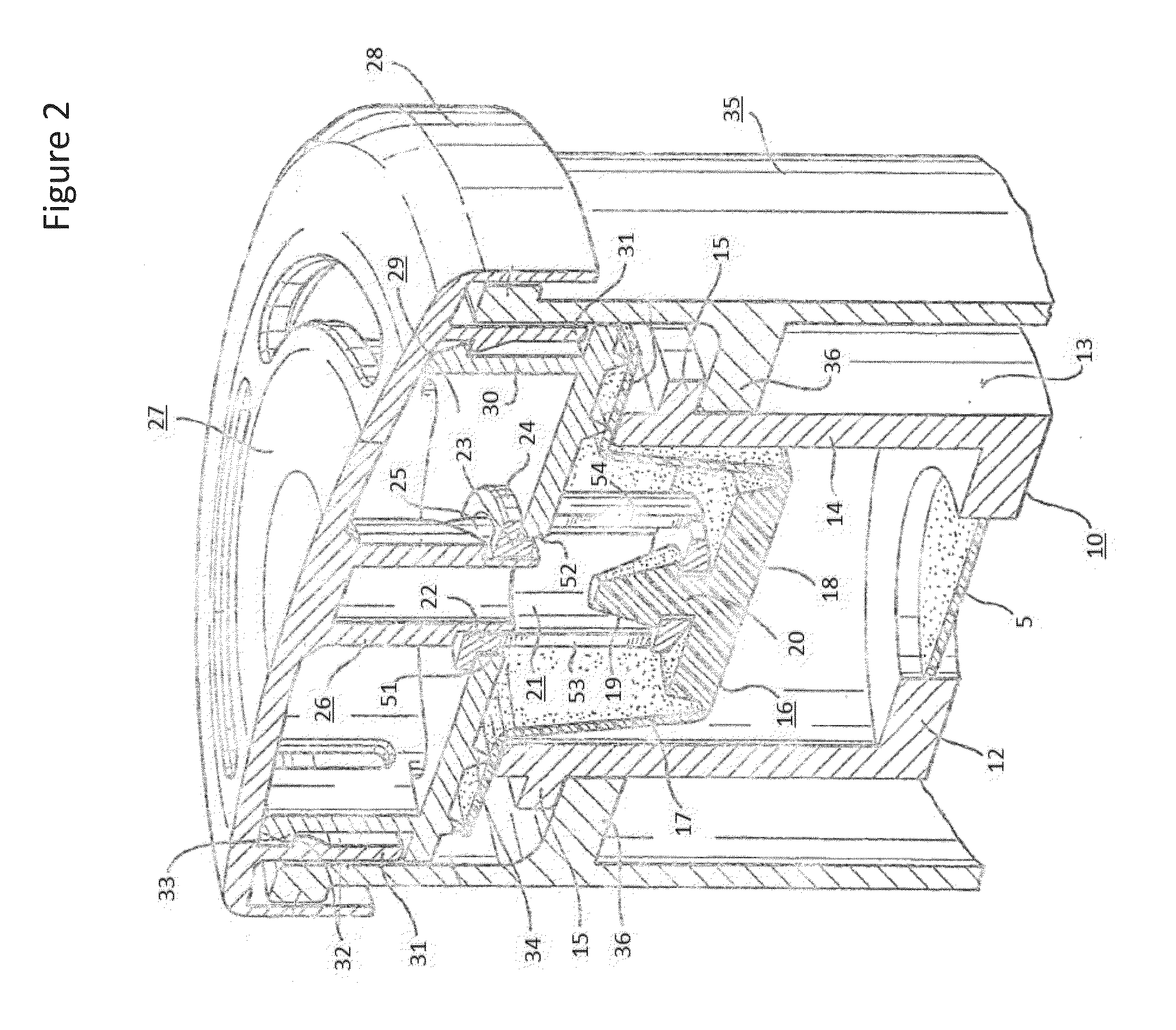
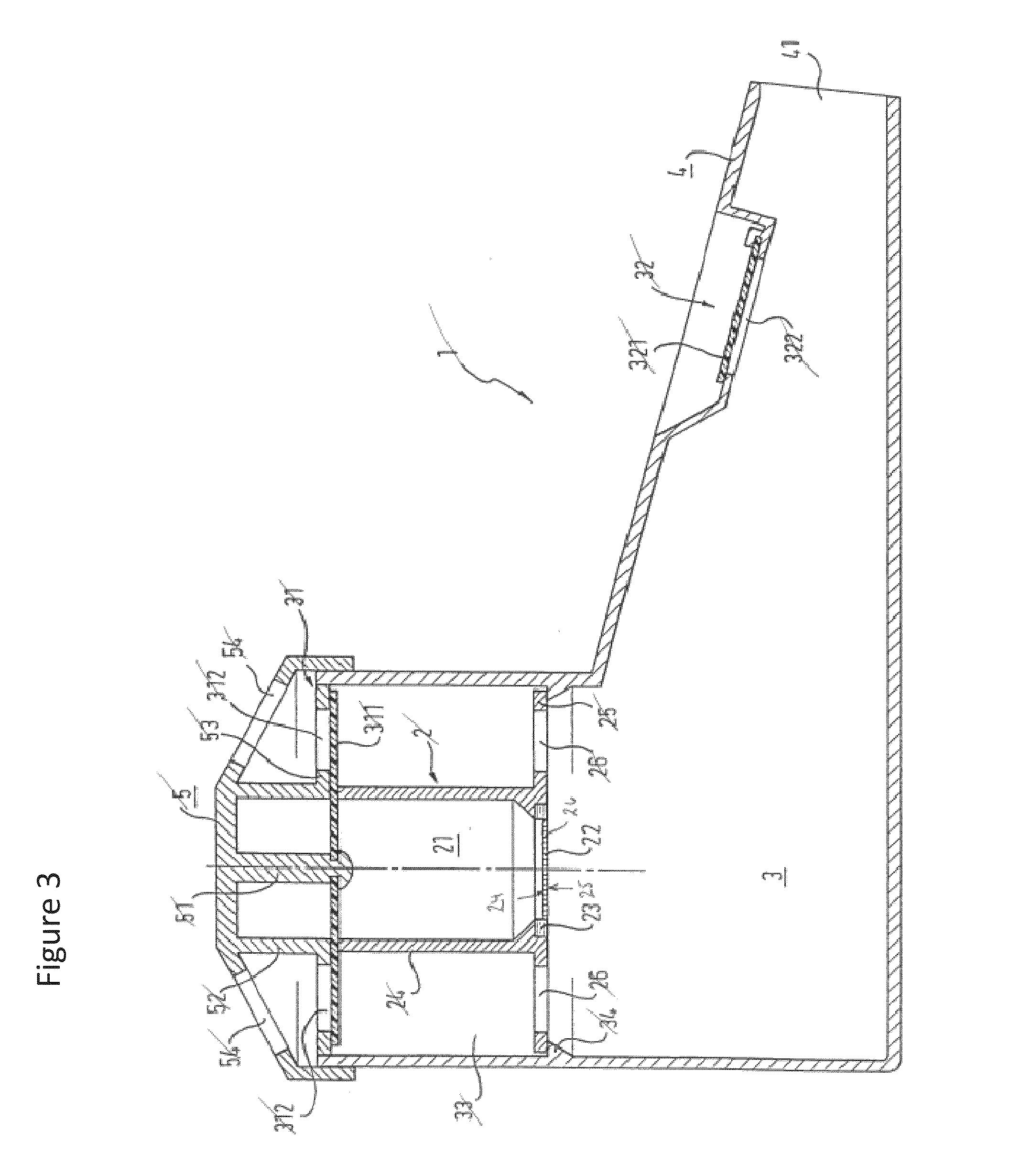
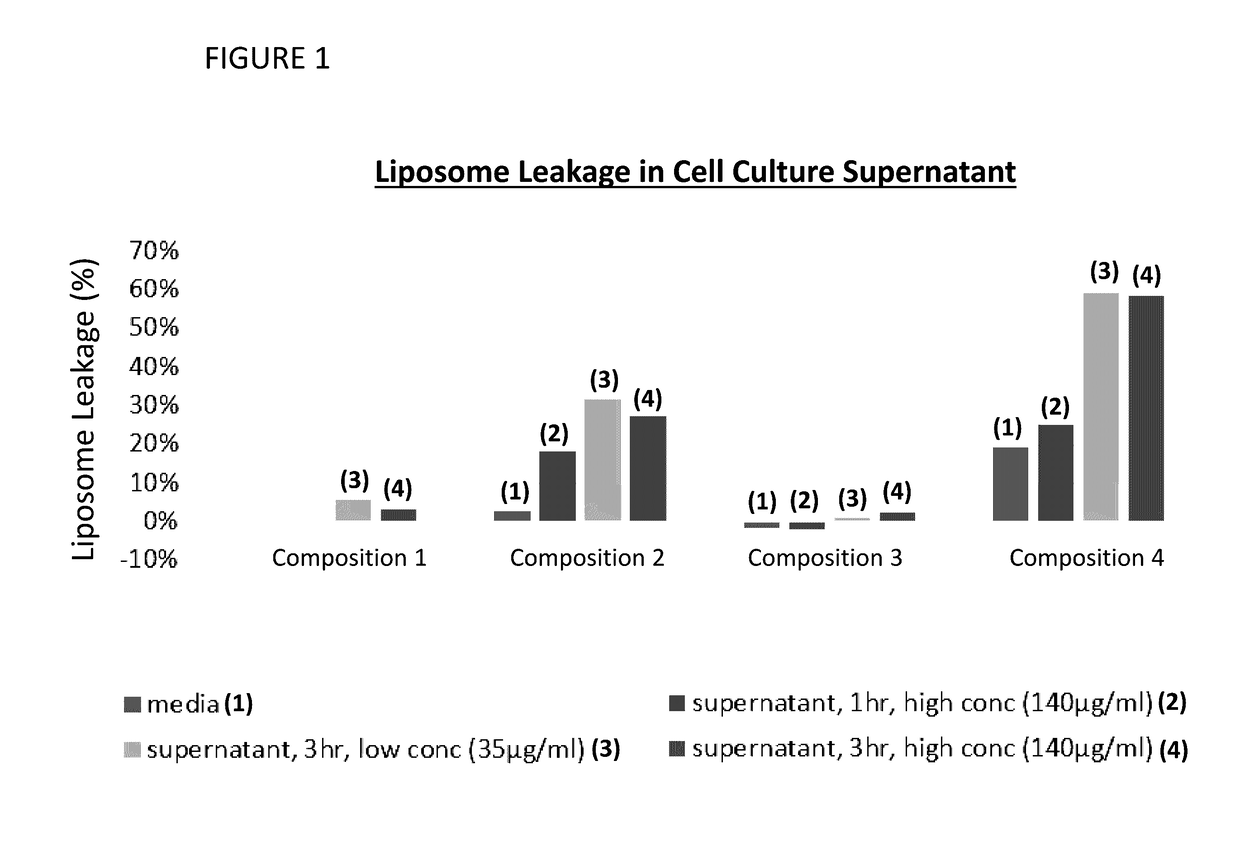
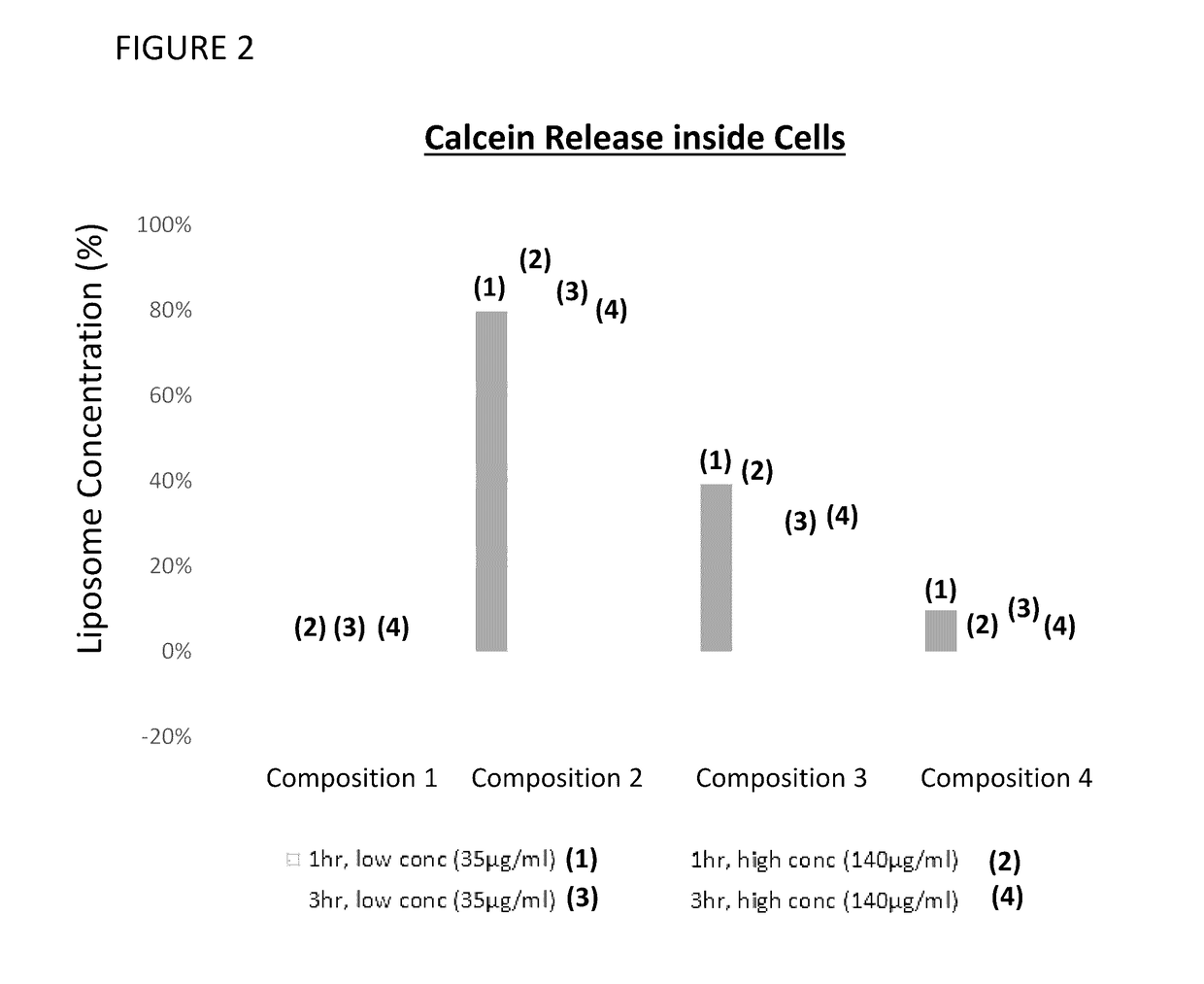
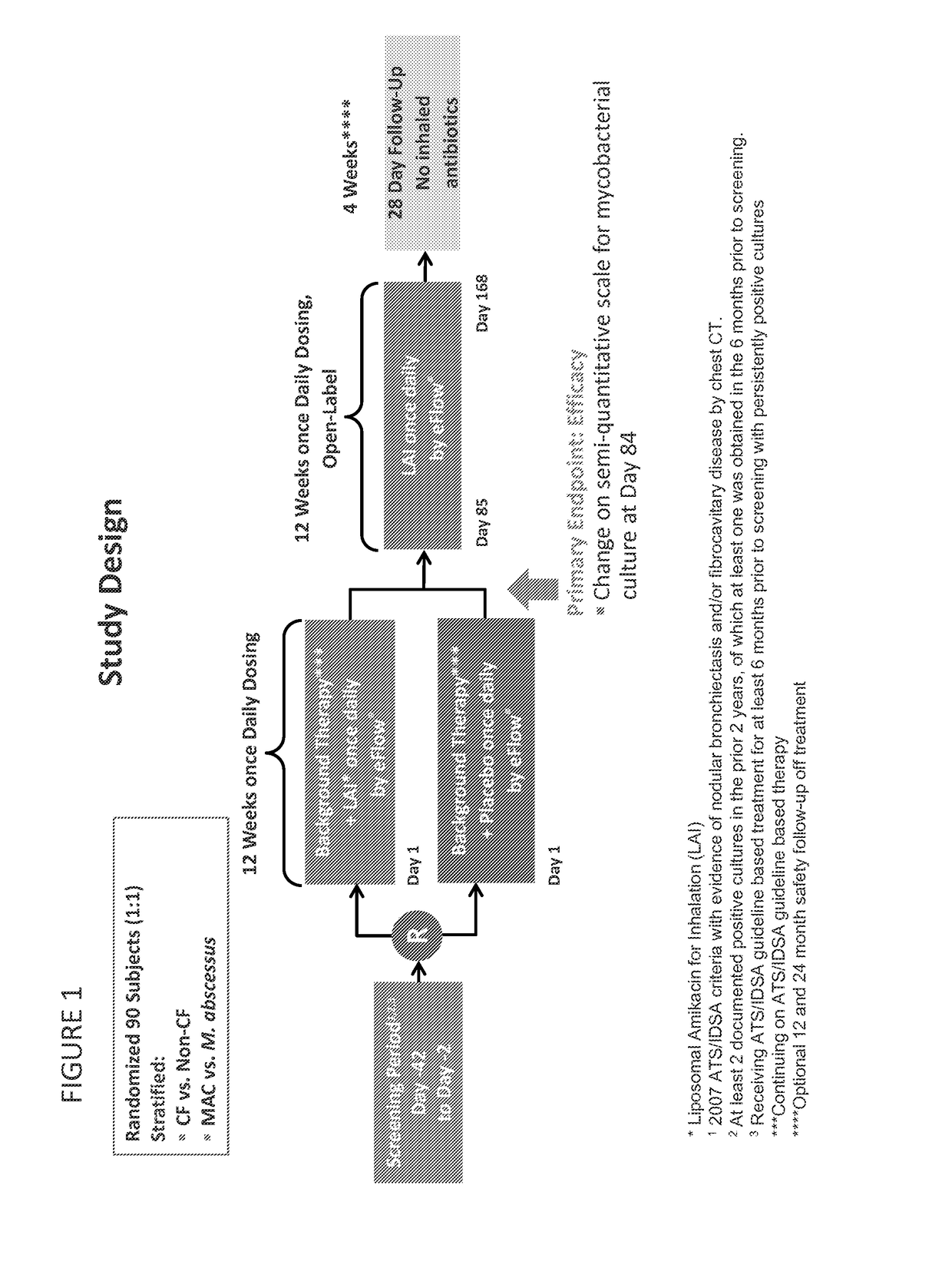
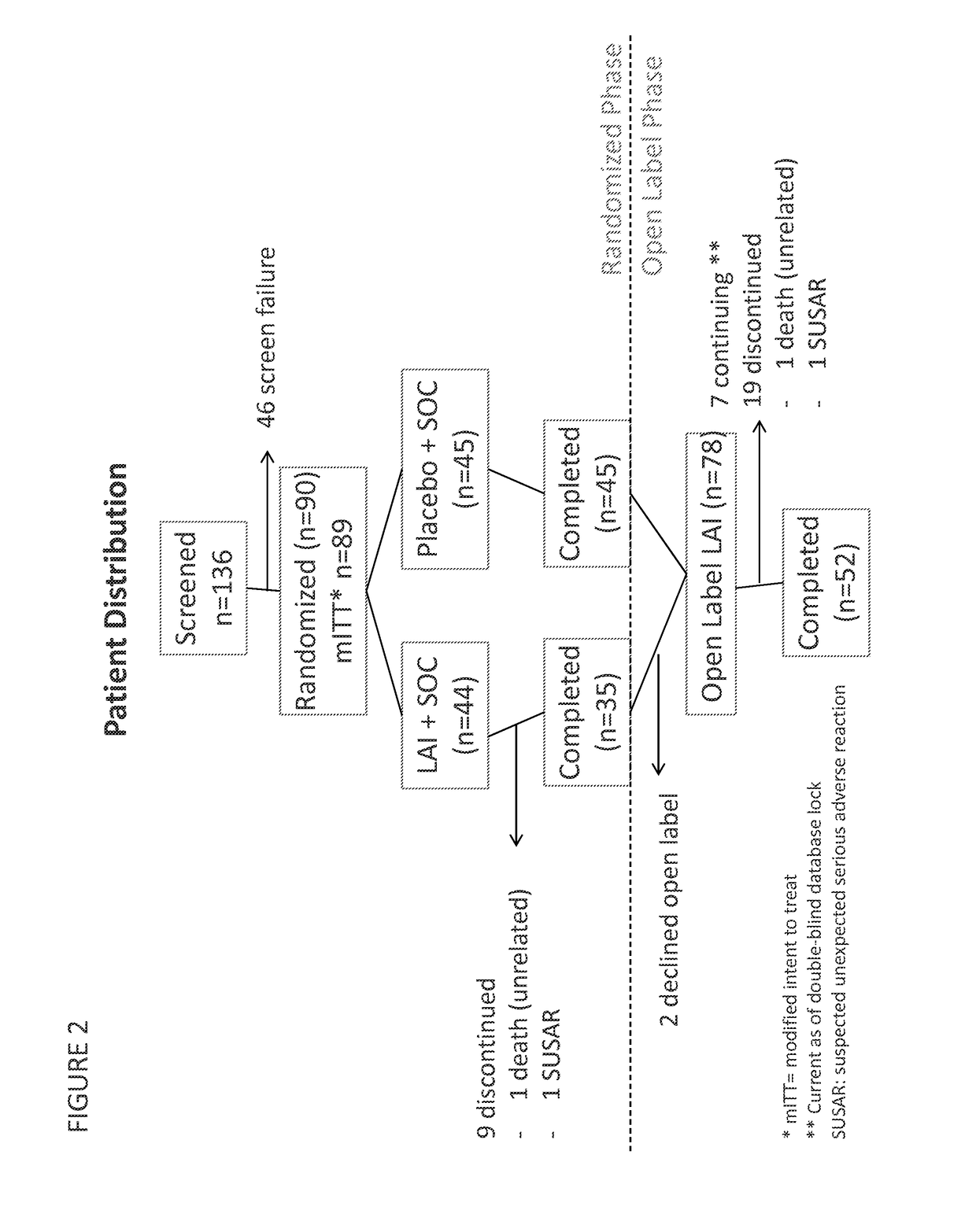
Provided herein are systems for treating a subject with a pulmonary infection, for example, a nontuberculous mycobacterial pulmonary infection, a Burkholderia pulmonary infection, a pulmonary infection associated with bronchiectasis, or a Pseudomonas pulmonary infection. The system includes a pharmaceutical formulation comprising a liposomal aminoglycoside dispersion, and the lipid component of the liposomes consist essentially of electrically neutral lipids. The system also includes a nebulizer which generates an aerosol of the pharmaceutical formulation at a rate greater than about 0.53 gram per minute. The aerosol is delivered to the subject via inhalation for the treatment of the pulmonary infection.
Owner:INSMED INC
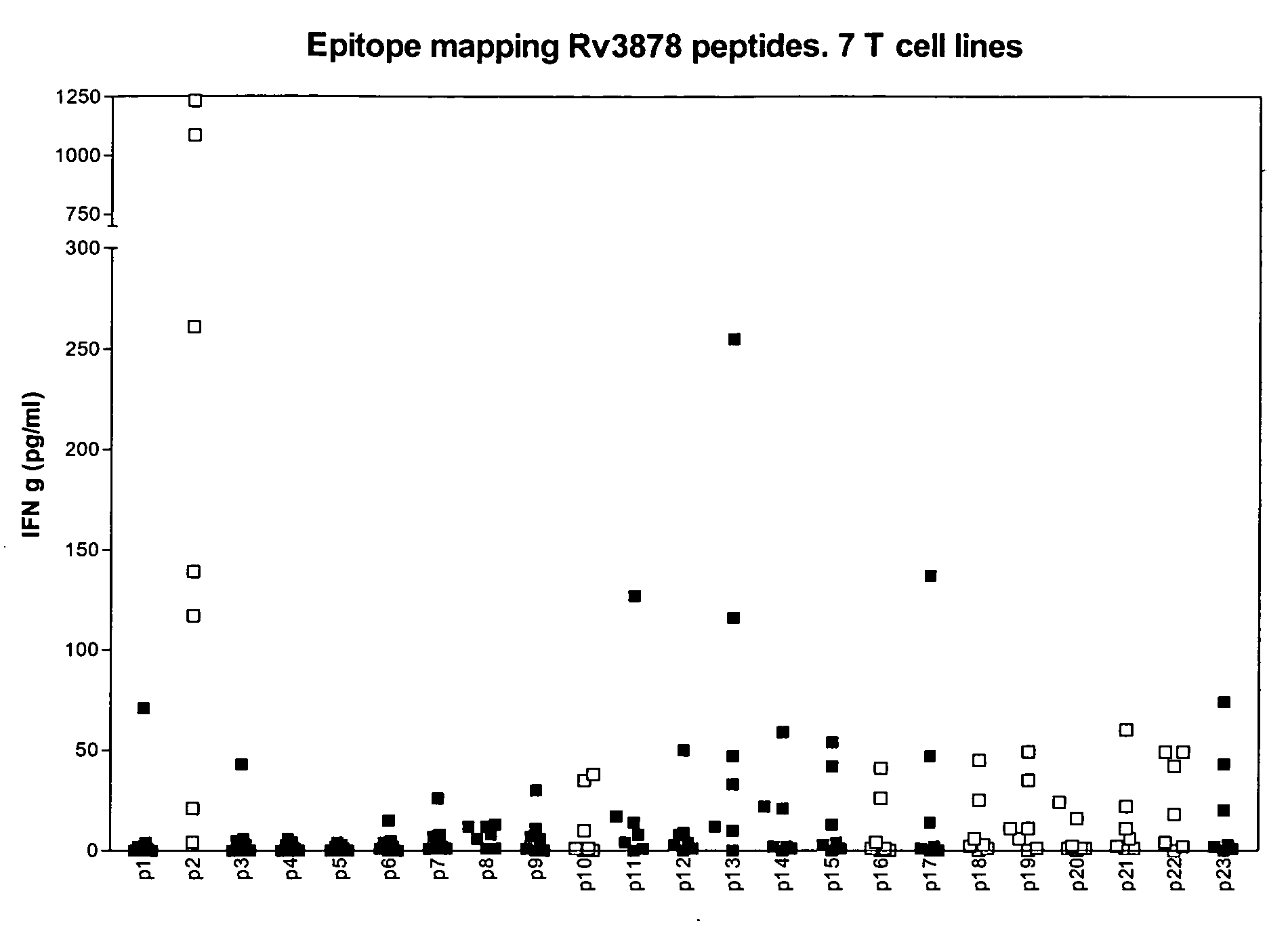
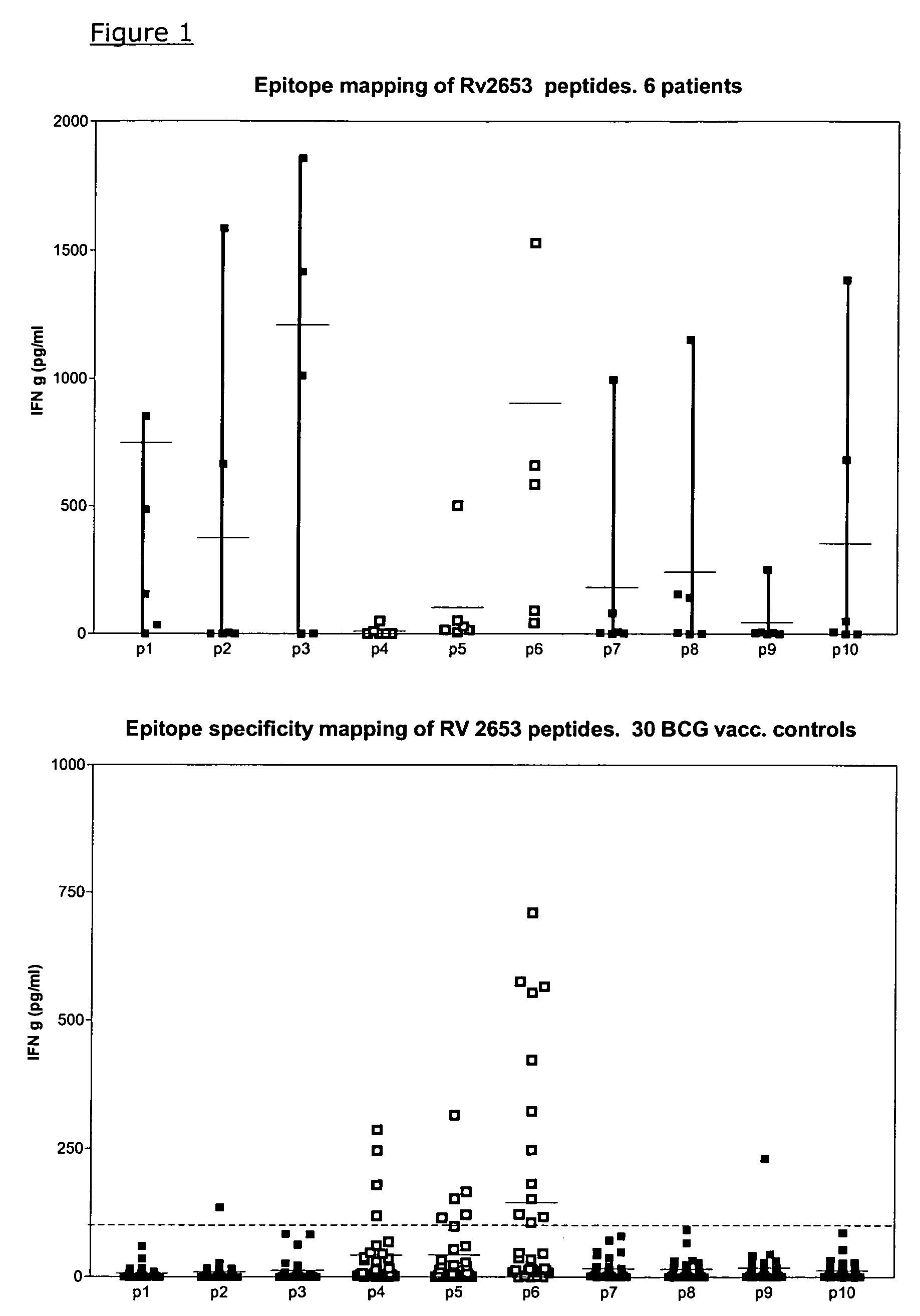
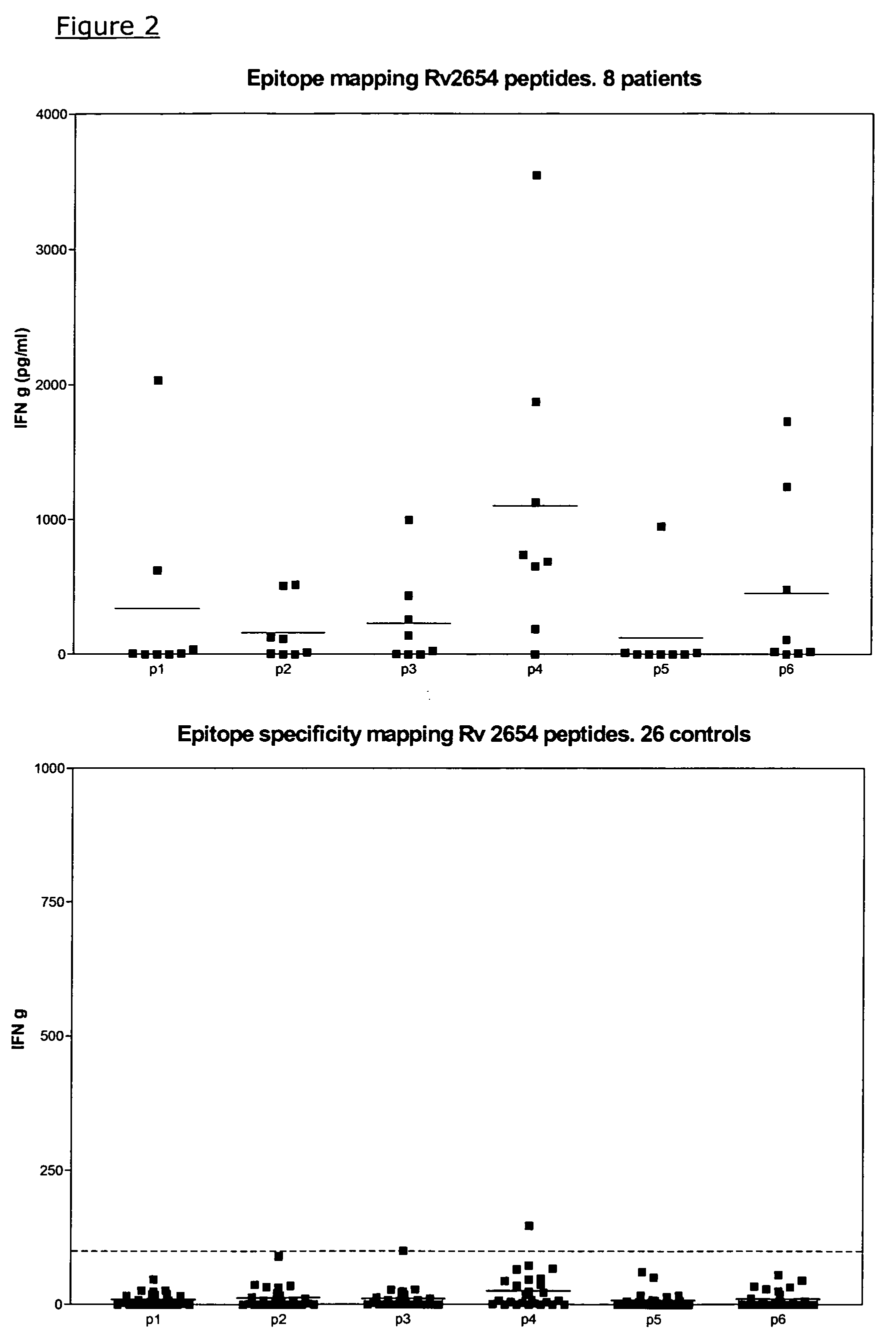
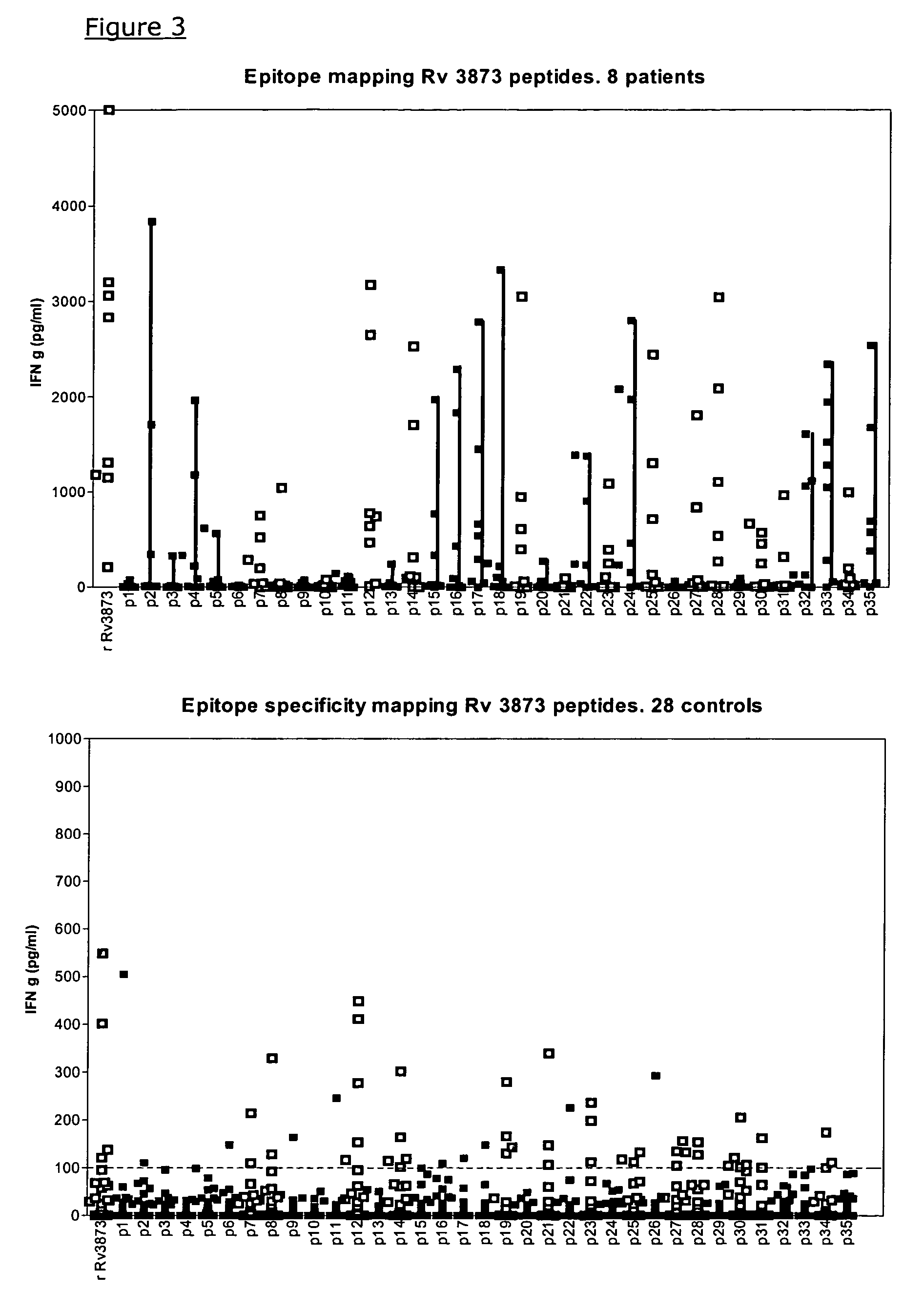
Specific epitope based immunological diagnosis of tuberculosis
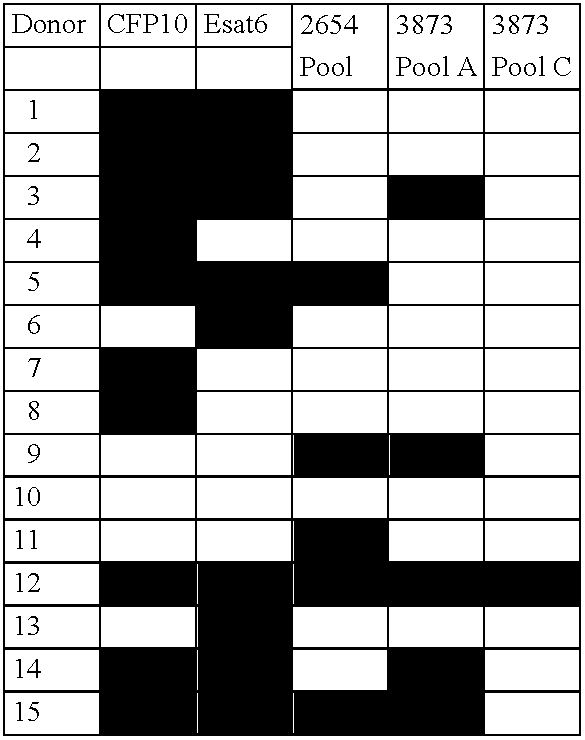
ActiveUS20060115847A1Easy to identifyTesting is superfluousBacterial antigen ingredientsMicrobiological testing/measurementSkin allergy testPeripheral blood mononuclear cell
The currently used method for immunological diagnosis of tuberculosis infection, the tuberculin skin test, is problematic for a number of reasons; it has low specificity in BCG vaccinated individuals, a high interobserver variance and requires skill to be read and interpreted. Furthermore it requires an extra visit to the clinic to have the test read. Both people vaccinated with BCG and those exposed to non-tuberculosis mycobacteria give a positive skin test result similar to that seen in a TB infected individual. This also applies for purified protein derivative (PPD) when used in a blood cell based test. The present invention discloses the development of an immunological TB diagnostic tool based on a combination of epitopes from proteins encoded by regions of the M. Tuberculosis (M. tub.) genome, that are not present in the BCG vaccine strain or in the most common non-tuberculosis mycobacteria. Four recently characterized proteins with this diagnostic potential were selected. Peptides from these proteins were tested one by one with peripheral blood mononuclear cells from microscopy or culture confirmed TB patients as well as from healthy BCG vaccinated controls. Some combinations of peptides showed a sensitivity level comparable to the level seen with the two wellknown M. tuberculosisspecific proteins ESAT 6 and CFP 10. An epitope combination with these peptides combined with ESAT 6 and CFP 10 gave a sensitivity of 93%, representing a raise in sensitivity of about 26-33% compared to using ESAT6 or CFP 10 alone. The results from a panel of TB patients, using a collection of the new specific epitopes clearly demonstrates, that addition of other specific epitopes to the already known specific antigens, increases the sensitivity of a diagnostic assay based on cell mediated immune response.
Owner:STATENS SERUM INST
Systems for treating pulmonary infections
Provided herein are systems for treating a subject with a pulmonary infection, for example, a nontuberculous mycobacterial pulmonary infection, a Burkholderia pulmonary infection, a pulmonary infection associated with bronchiectasis, or a Pseudomonas pulmonary infection. The system includes a pharmaceutical formulation comprising a liposomal aminoglycoside dispersion, and the lipid component of the liposomes consist essentially of electrically neutral lipids. The system also includes a nebulizer which generates an aerosol of the pharmaceutical formulation at a rate greater than about 0.53 gram per minute. The aerosol is delivered to the subject via inhalation for the treatment of the pulmonary infection.
Owner:INSMED INC
Rapid identification method and kit for MTBC (mycobacterium tuberculosis complex)
InactiveCN104561245AShorten diagnostic timeSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceRapid identification
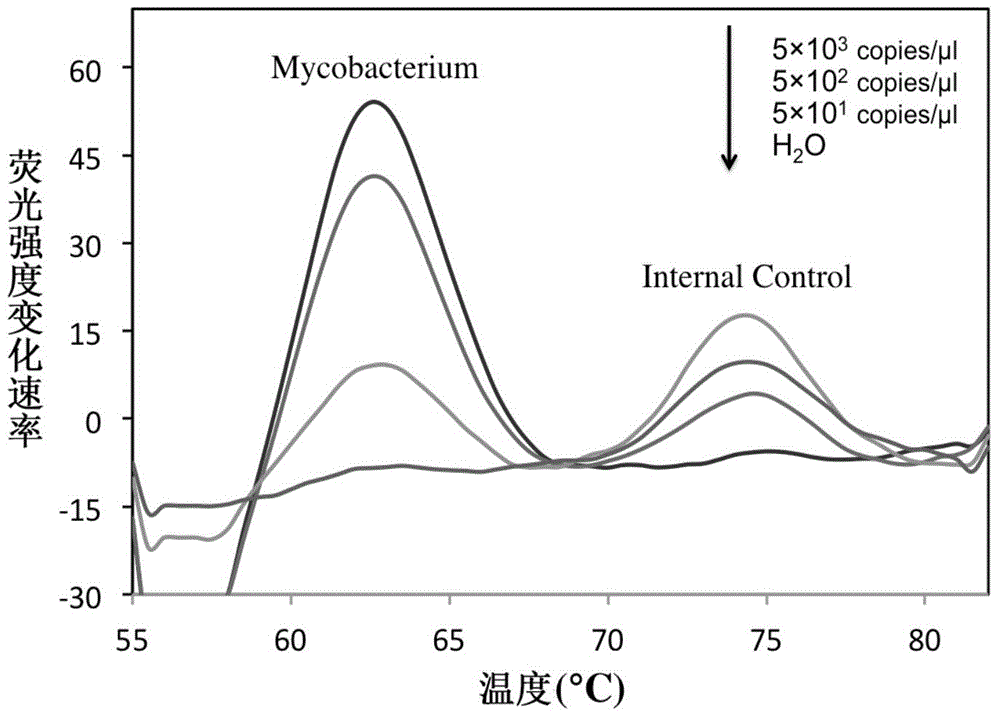
The invention belongs to the field of a detection reagent, and relates to a detection and identification kit for MTBC (mycobacterium tuberculosis complex) and an identification method of the MTBC. According to the method, fluorescence probes and amplification primers for detecting the MTBC are designed through sequence alignment, mycobacterium and the MTBC specific SNP (single nucleotide polymorphism) loci on an rrs gene are detected based on a real-time fluorescent quantitative PCR (polymerase chain reaction) platform and with an asymmetric PCR amplification technology and a probe melting curve analysis technology, so that mycobacteria are identified, and the MTBC and NTM (nontuberculosis mycobacteria) are further identified. The method has the characteristics of convenience in operation, short detection time and high specificity and sensitivity.
Owner:FUDAN UNIV
Rapid identification method and kit of novel mycobacterium strain
ActiveCN102634575AMicrobiological testing/measurementFluorescence/phosphorescenceRapid identificationNuclease
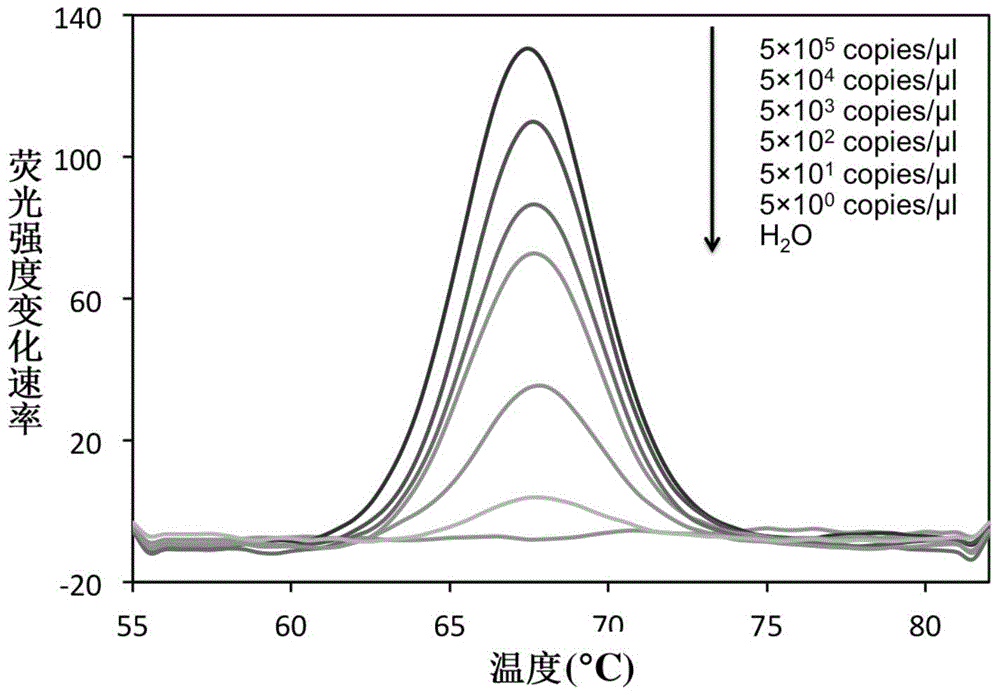
The invention relates to a double-marking probe detecting and melting curve analyzing method used for identifying mycobacterium strains and a kit using the method to identify various mycobacterium strains at the same time. The kit provided by the invention comprises a primer capable of designing mycobacterium 16S rRNA, a double-marking oligonucleotide probe capable of identifying mycobacterium strains, and a thermostable DNA polymerase without 5' nuclease activity. The detecting method and the kit, which are provided by the invention, can detect and / or identify 24 kinds of common mycobacteria. The method and the kit can judge the results according to melting peaks of different Tm values produced by the sequence hybridization of the probe and the different strains, meanwhile rapidly and accurately detect the mycobacteria, and identify the mycobacteria, non-mycobacteria, mycobacterium tuberculosis compounding groups and nontuberculosis mycobacteria, and the result of identifying the mycobacteria can be reported after 3-4 hours, thus the method and the kid can assist the clinical diagnosis, and guide efficient clinical chemotherapy at the early stage.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Compositions and methods for treating bacterial infections
Provided herein are pharmaceutical compositions comprising an antibiotic encapsulated in liposomes. The lipid membrane component of the liposomes, or portion thereof comprises an unsaturated phospholipid. The antibiotic-to-lipid component weight ratio of the liposomes ranges from about 0.5-to-1 to about 3-to-1. The pharmaceutical compositions in some embodiments also include free antibiotic, in addition to encapsulated antibiotic. Methods for treating bacterial infections, e.g., pulmonary bacterial infections such as nontuberculous mycobacterial infections with the pharmaceutical compositions are also provided.
Owner:INSMED INC
Methods for treating pulmonary non-tuberculous mycobacterial infections
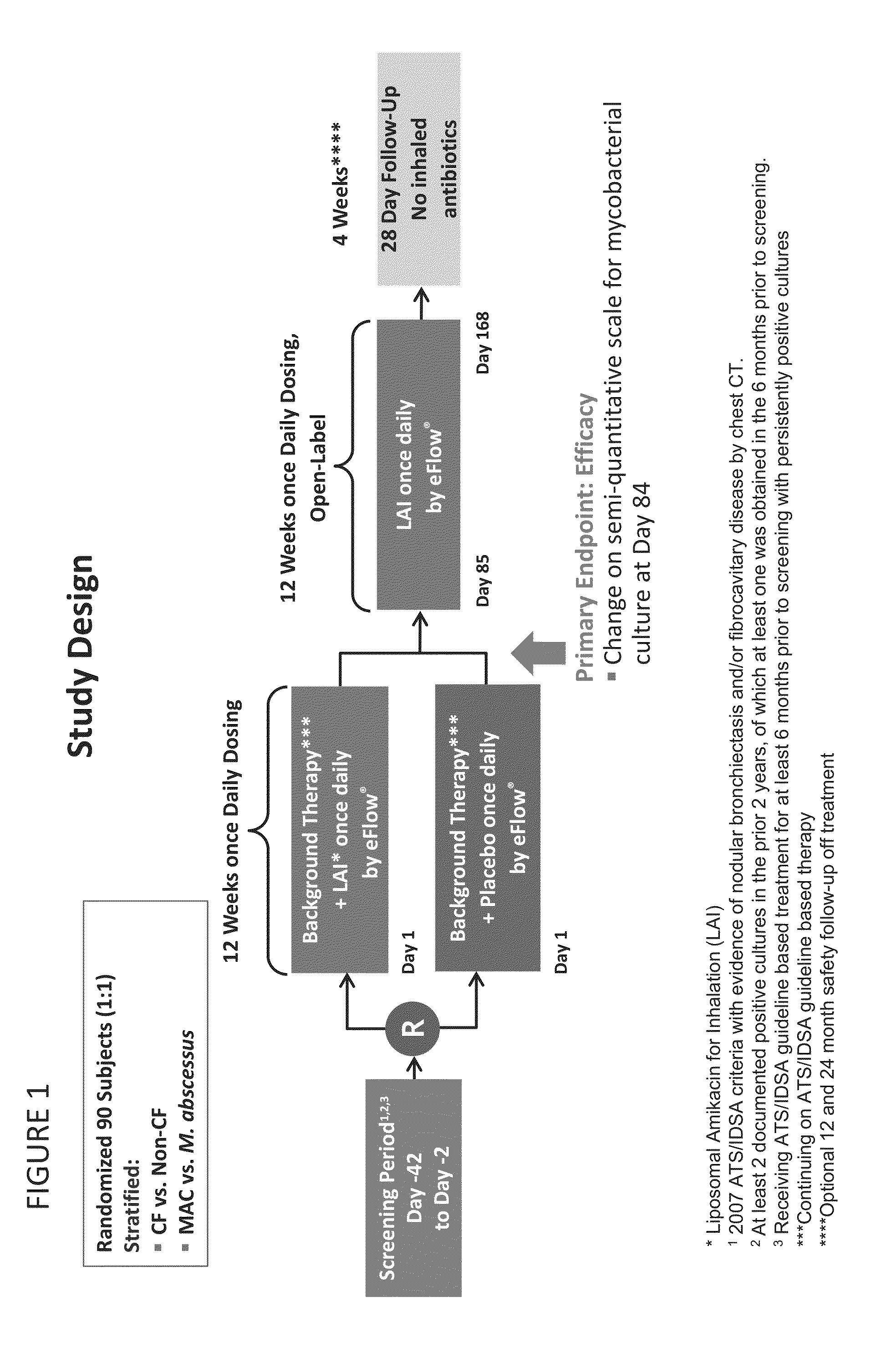
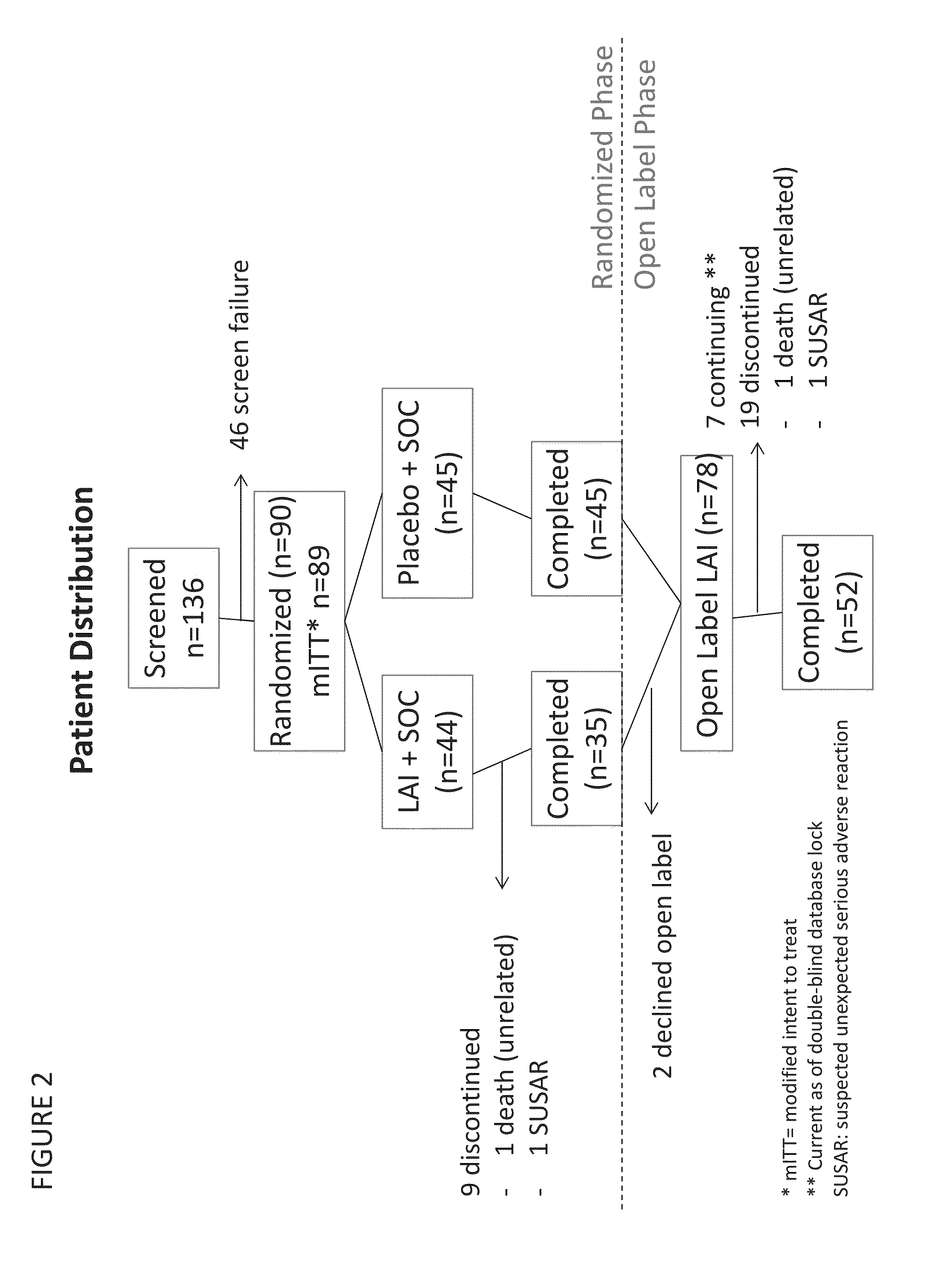
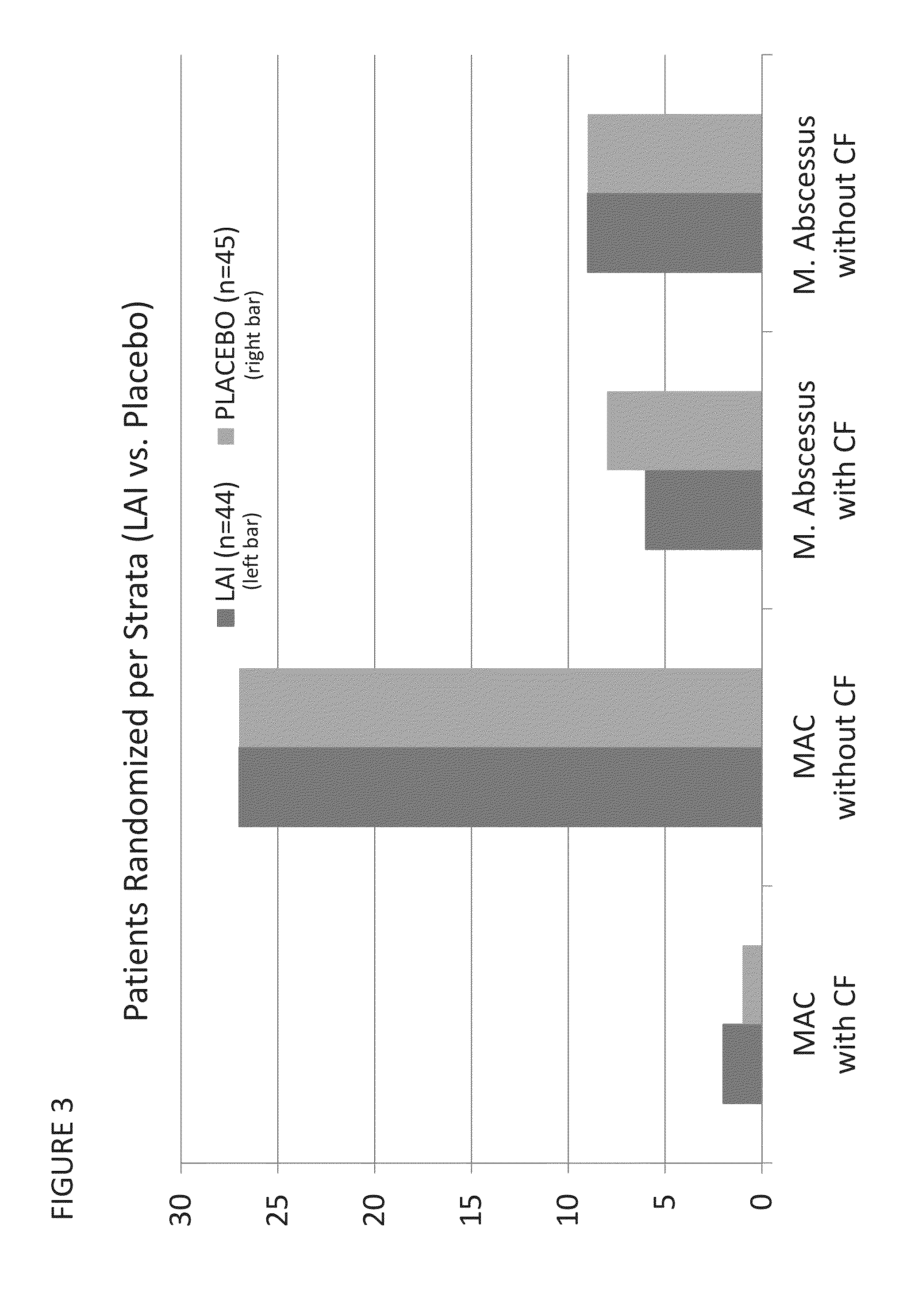
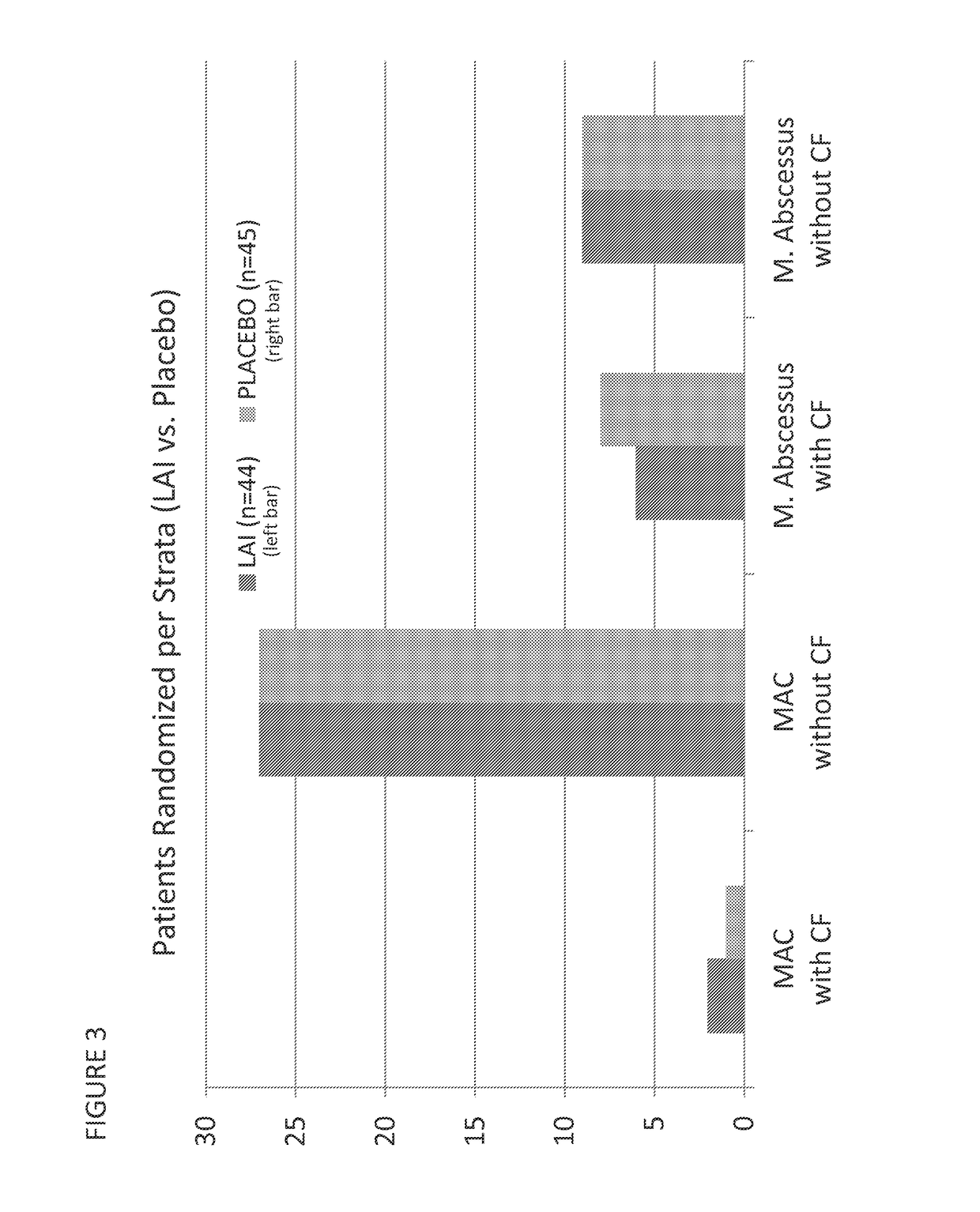
Provided herein are methods for treating a pulmonary infection in a patient in need thereof, for example, a nontuberculous mycobacterial pulmonary infection for at least one treatment cycle. The method comprises administering to the lungs of the patient a pharmaceutical composition comprising a liposomal complexed aminoglycoside comprising a lipid component comprising electrically neutral lipids and an aminoglycoside. Administration comprises aerosolizing the pharmaceutical composition to provide an aerosolized pharmaceutical composition comprising a mixture of free aminoglycoside and liposomal complexed aminoglycoside, and administering the aerosolized pharmaceutical composition via a nebulizer to the lungs of the patient. The methods provided herein result in a change from baseline on the semi-quantitative scale for mycobacterial culture for a treated patient, and / or NTM culture conversion to negative during or after the administration period.
Owner:INSMED INC
Method for identifying Mycobacterium tuberculosis and non-tuberculous mycobacteria, and special reagent kit therefor
ActiveCN101413031ARapid identificationInstrument requirements are simpleMicrobiological testing/measurementFluorescenceRibonucleotide synthesis
The invention discloses a method for identifying Mycobacterium tuberculosis and nontuberculosis mycobacteria and a special reagent kit thereof. The reagent kit for identifying the Mycobacterium tuberculosis and the nontuberculosis mycobacteria comprises a primer and a probe, wherein the primer comprises four primers, and ribonucleotide sequences of the four primers are sequence 1, sequence 2, sequence 4, and sequence 5 in a sequence table respectively; the probe comprises two probes, and ribonucleotide sequences of the two probes are sequence 3 and sequence 6 in the sequence table respectively; 5' ends of the probes are provided with fluorescent substances, and 3' ends of the probes are provided with quenching substances; and the fluorescent substances at the 5' ends of the two probes aredifferent. The reagent kit has the advantages that the reagent kit can quickly identify the Mycobacterium tuberculosis and the nontuberculosis mycobacteria, has simple requirement on instruments, lowcost and simple operation, and has broad application prospect on early diagnosis and differential diagnosis of tuberculosis.
Owner:BOAO BIOLOGICAL CO LTD +1
Multiplex polymerase chain reaction (PCR) kit for identifying mycobacterium tuberculosis
InactiveCN102533959ASimple and fast operationEasy to operateMicrobiological testing/measurementSequence designAcid-fast
Owner:HUAZHONG AGRI UNIV
Application of dicycloplatin in preparation of antiviral drug and antibacterial drug
ActiveCN104127402AImprove antibacterial propertiesGood antiviral effectAntibacterial agentsAntiviralsBacteroidesAdjuvant
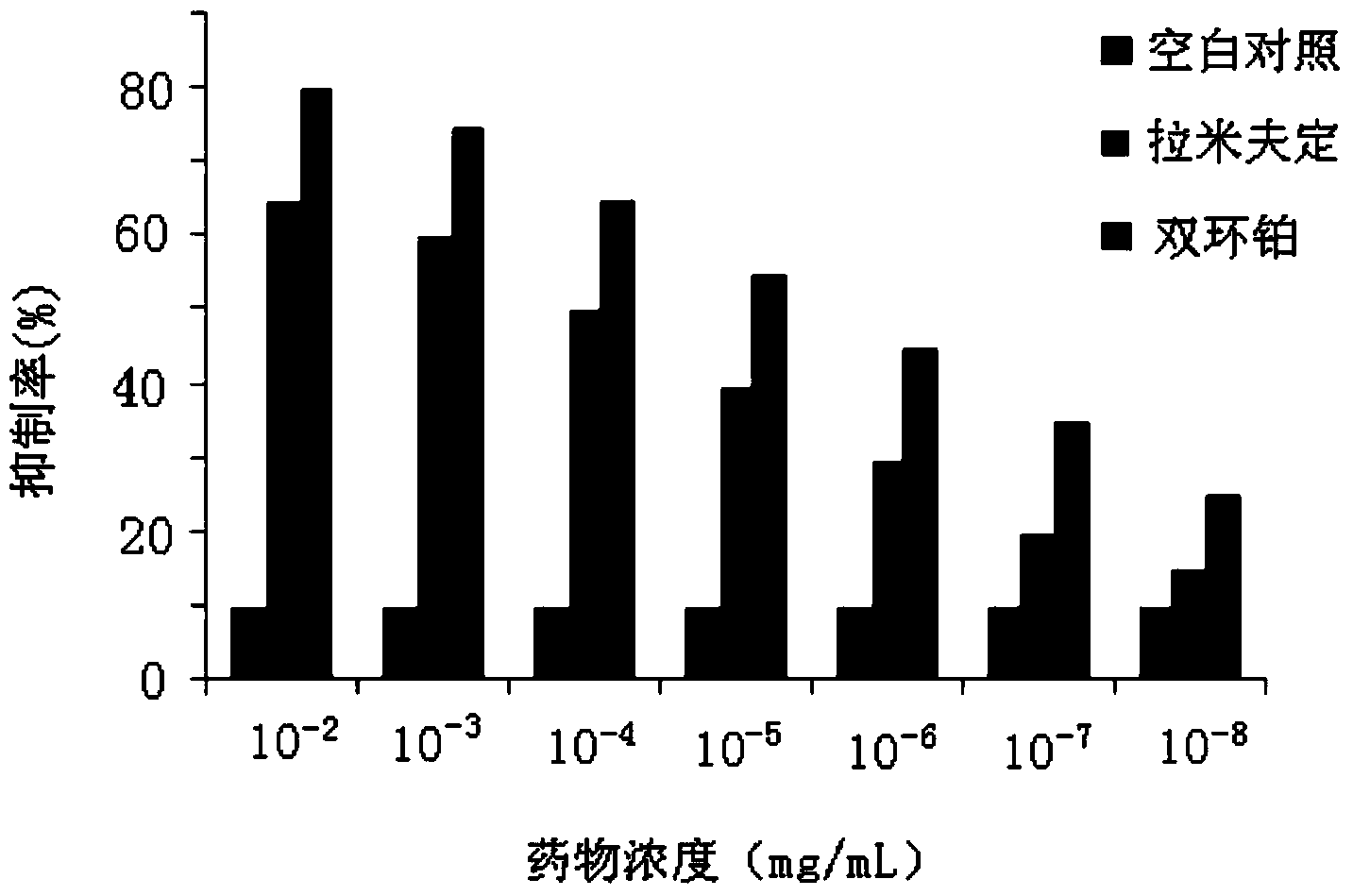
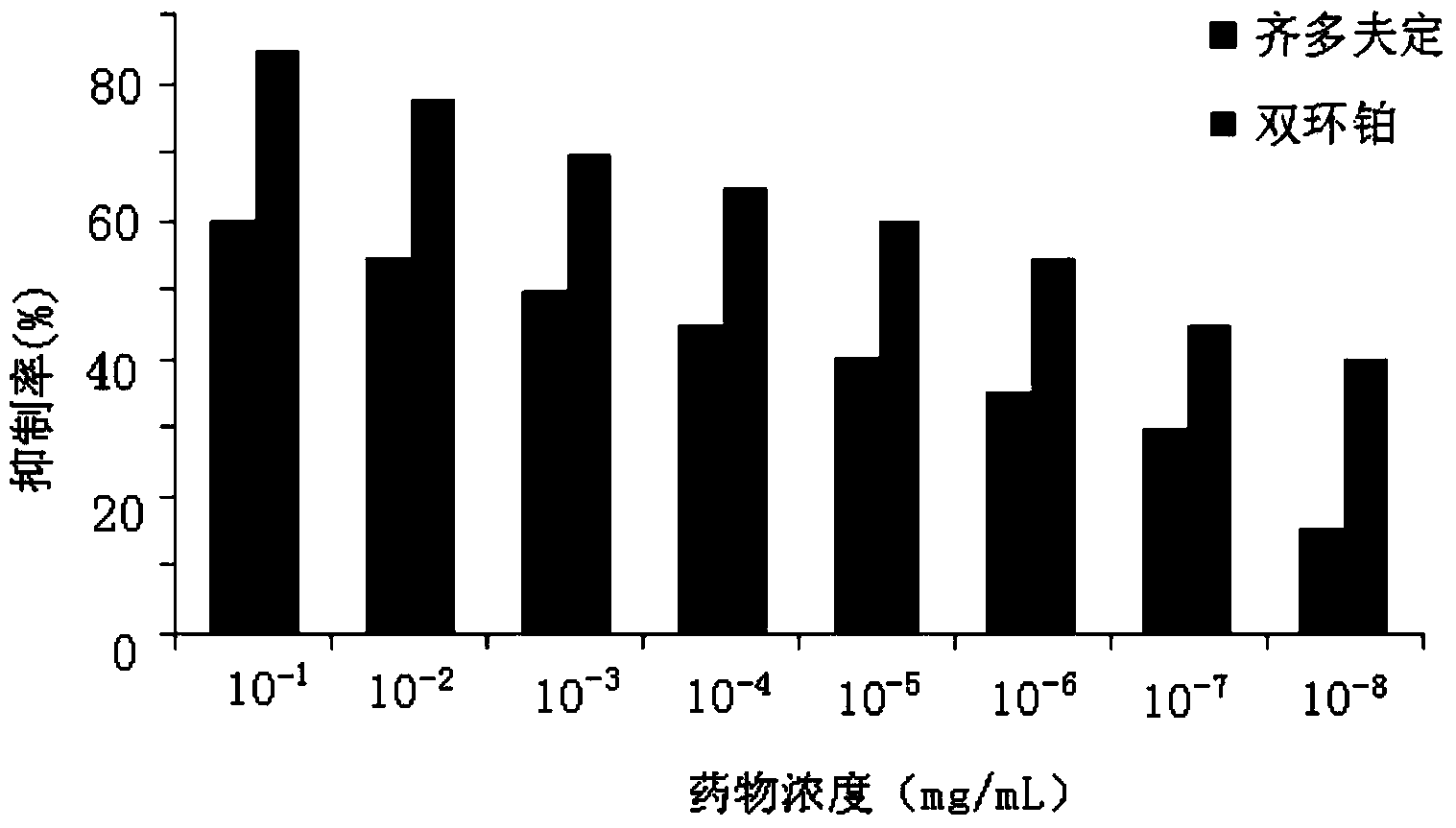
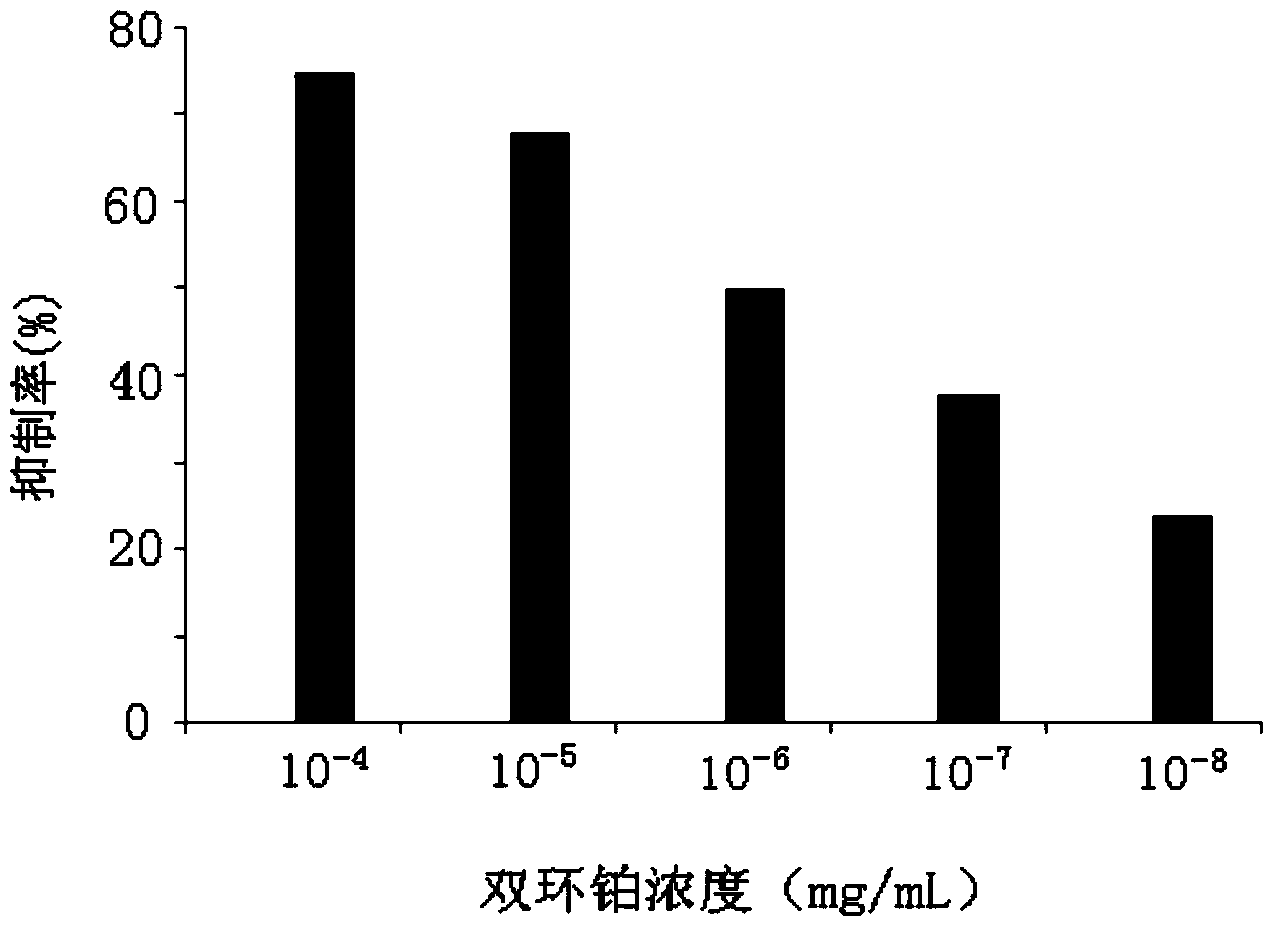
The invention provides application of dicycloplatin in preparation of an antiviral drug and / or antibacterial drug and application of the dicycloplatin in preparation of an antiviral adjuvant drug and / or antibacterial adjuvant drug. Viruses related in the invention are RNA viruses such as hepatitis B virus, hepatitis C virus, human immunodeficiency virus and influenza virus, and the antibacterial drug is resistant to mycobacterium tuberculosis and / or nontuberculosis mycobacteria, Research results prove that dicycloplatin has the effect of resisting multiple bacteria and viruses, especially has obvious inhibitory effect on drug-resistance bacteria, no obvious cytotoxicity and high medication safety.
Owner:北京默加农生物技术发展有限公司
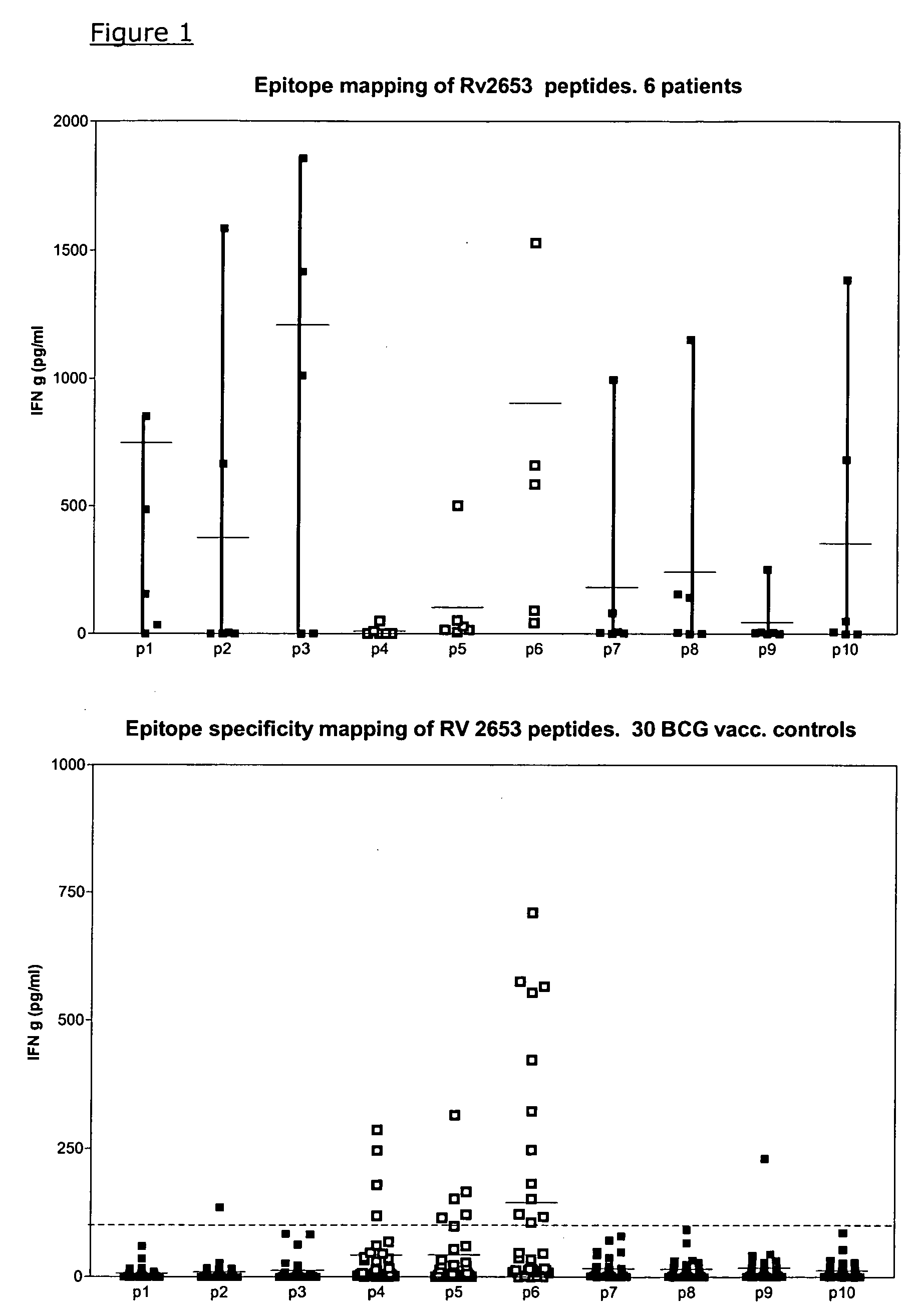
Specific epitope based immunological diagnosis of tuberculosis
ActiveUS7838013B2Easy to identifyTesting is superfluousBacterial antigen ingredientsMicrobiological testing/measurementBCG vaccineDiagnostic tools
The currently used method for immunological diagnosis of tuberculosis infection, the tuberculin skin test, is problematic for a number of reasons; it has low specificity in BCG vaccinated individuals, a high interobserver variance and requires skill to be read and interpreted. Furthermore it requires an extra visit to the clinic to have the test read. Both people vaccinated with BCG and those exposed to non-tuberculosis mycobacteria give a positive skin test result similar to that seen in a TB infected individual. This also applies for purified protein derivative (PPD) when used in a blood cell based test. The present invention discloses the development of an immunological TB diagnostic tool based on a combination of epitopes from proteins encoded by regions of the M. tuberculosis (M. tub.) genome, that are not present in the BCG vaccine strain or in the most common non-tuberculosis mycobacteria.
Owner:STATENS SERUM INST
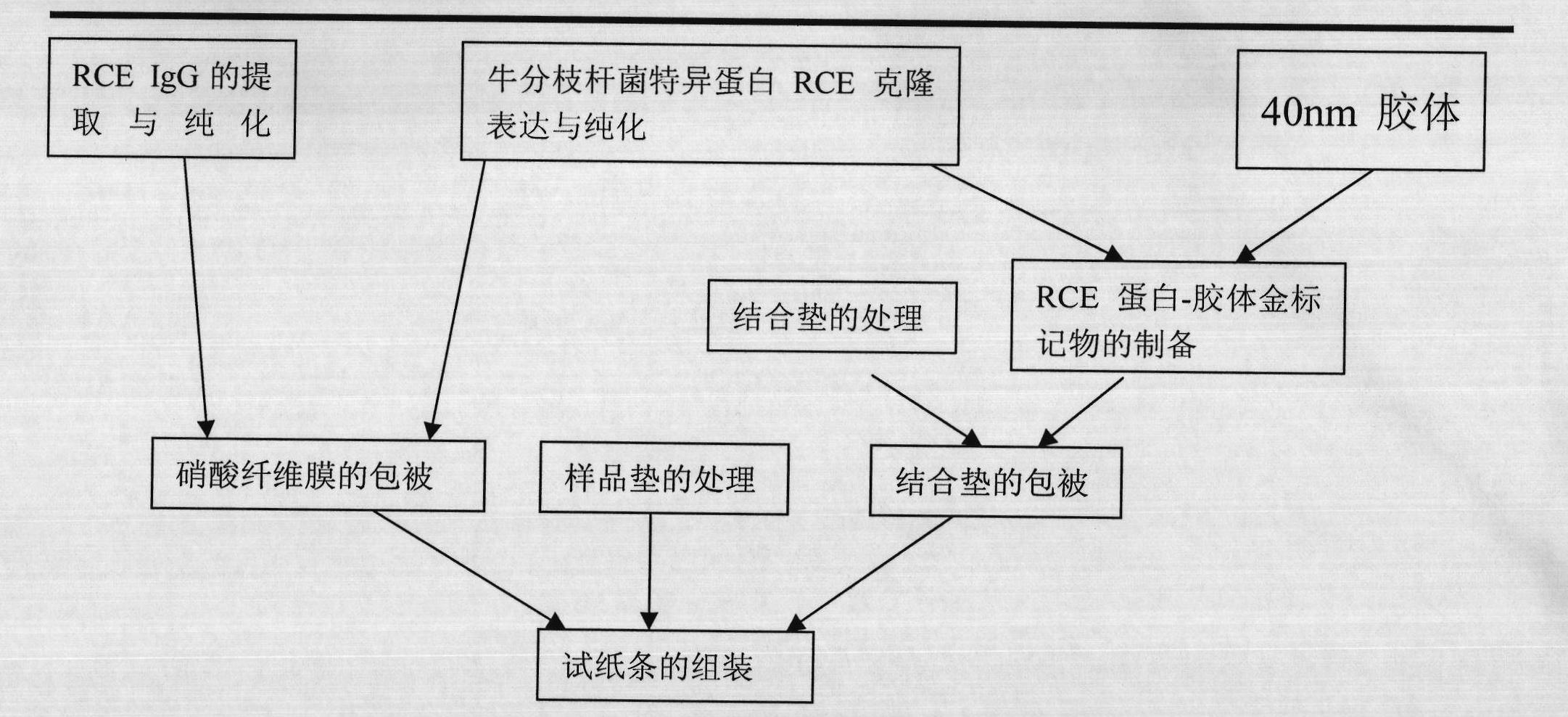
Bovine tuberculosis antibody identifying and detecting test strip prepared by applying Rv3872 novel fusion protein
InactiveCN101900727AWith identificationFunctionalBacteriaMicroorganism based processesAntigenNitrocellulose
The invention belongs to the technical field of animal infectious disease gene engineering and discloses a bovine tuberculosis antibody detecting immune colloidal gold test strip prepared by utilizing RV3872, ESAT6 and CFP10 fusion protein, and a preparation method and application. A colloidal gold immunochromatographic test strip is established by using the fusion protein as a colloidal gold labeled antigen and a capture antigen in a detection region of a nitrocellulose membrane. The detection of the bovine tuberculosis antibody by using test strip has prominent advantages of strong specificity and high sensitivity, and simultaneously bacillus calmette-guerin immunity and nontuberculosis mycobacteria infection can be identified and detected. The test strip comprises recombinant Escherichia coli BL21 / pET28a-MPBrce, and the strain expresses mycobacterium bovis RCE proteins and is preserved in the China Center for Type Culture Collection with the collection number of CCTCC No:M208244.
Owner:HUAZHONG AGRI UNIV
Test paper and method for checking mycobacteria tuberculosis and non-mycobacteria tuberculosis nucleic acid amplifying products
The invention provides a test paper of nucleic acid amplification product for detecting tubercle mycobacterium (TB) and Nontuberculous mycobacteria (NTM), comprising (a) (avidin-gold release region) and (b) test belt. The invention further provides a method of detecting TB and NTM, comprising (a) amplifying the sample DNA of primer having mark; (b) mixing the amplified DNA product by electrophoretic buffer; (c) dipping the test paper in the mixture; and (d) flowing the mixture in the reaction area of the paper.
Owner:ASIAGEN CORP
Method for detecting mycobacterium tuberculosis and nontuberculous mycobacteria by using dual real-time polymerase chain reaction
InactiveUS20130210005A1Improve efficiencySugar derivativesMicrobiological testing/measurementNucleotideNucleotide sequencing
Disclosed are a primer set and / or a probe capable of detecting specific nucleotide sequences of MTC and NTM, a kit for the detection of MTC and NTM, comprising the same, and a method for detecting MTC and NTM by duplex real-time PCR using the same. Useful in detecting genes characteristic of MTC and NTM, the primer sets and / or probes, detection kits, and detection methods can be applied as the clinical diagnosis of diseases caused by MTC and NTM, and therefore find applications in the medical fields including hospitals, research institutes, etc.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Kit for identifying Mycobacterium tuberculosis and nontuberculous mycobacteria and application method thereof
InactiveCN102229999AIssues that take up to 4-6 weeks to resolveShort detection timeMicrobiological testing/measurementFluorescence/phosphorescenceBacteroidesMycobacterium tuberculosis culture
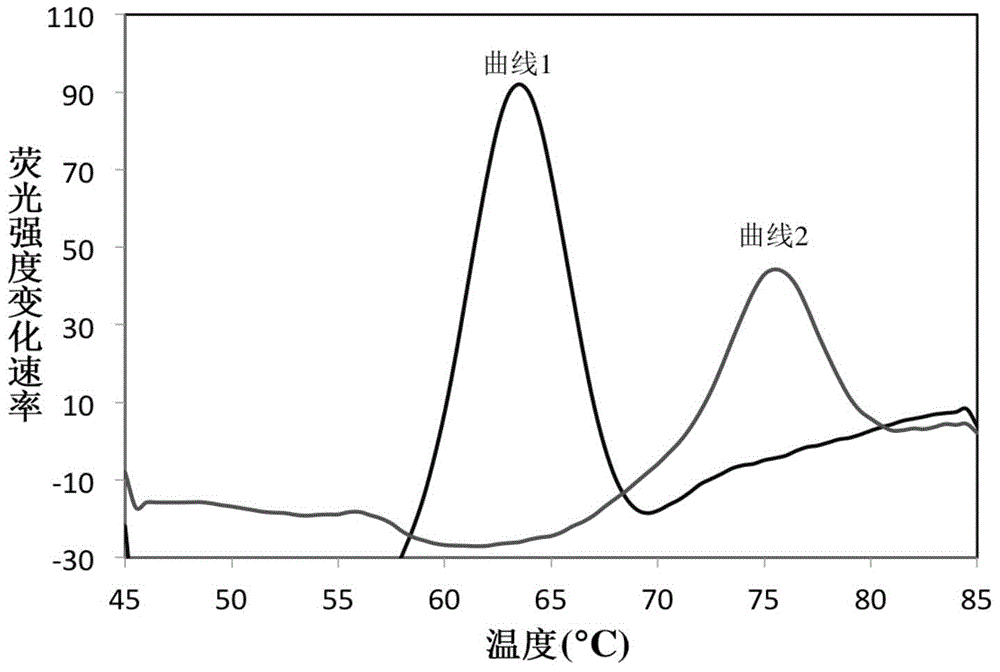
The invention discloses a fluorescence quantitative nucleic acid detection technique for differentiating and identifying Mycobacterium tuberculosis and nontuberculous mycobacteria in one step based on the features of dual-channel fluorescence quantitative detection, which has a short detection time, provides reliable detection results and achieves the quantitative detection. The invention adopts the technical scheme as follows: a kit for identifying Mycobacterium tuberculosis and nontuberculous mycobacteria is provided, which comprises primers for PCR (polymerase chain reaction) amplification of strains to be detected and probes for fluorescence quantitative detection. According to the invention, according to the difference in gene sequence of different types of mycobacteria, the fluorescence probes are designed to differentiate Mycobacterium tuberculosis from nontuberculous mycobacteria; and the differentiation and identification is performed at the levels of gene sequence and molecular structure of bacterial strains, so as to ensue more accurate and more reliable classification. The invention solves the problem that in the conventional identification method, the differentiation based on the growth forms of bacterial strains needs a long period of time up to 4 to 6 weeks.
Owner:亚能生物技术(深圳)有限公司
Fluorescent PCR reaction liquid and kit for mycobacterium parting identification
ActiveCN104131100ASimple and fast operationAccurate detectionMicrobiological testing/measurementFluorescenceMicrobiology
The invention discloses a fluorescent PCR reaction liquid for mycobacterium parting identification. The fluorescent PCR reaction liquid contains amplification primers and a molecular beacon probe. Specifically, the molecular beacon probe includes at least one of 12 mycobacterial molecular beacon probes with both ends provided with fluorescent groups and quenching groups respectively, and the sequences are shown as SEQ ID NO.1-SEQ ID NO.12. The invention also discloses a kit having the PCR reaction liquid. With the fluorescent PCR reaction liquid and kit provided by the invention, 12 mycobacteria including mycobacterium tuberculosis and nontuberculosis mycobacteria can be quickly and accurately detected and identified.
Owner:新疆亿立方生物技术有限公司
Primers, probes, method and kit for detecting mycobacterium tuberculosis specific gene
InactiveCN107841568AImprove accuracyNo cross reactionMicrobiological testing/measurementMicroorganism based processesFluorescencePcr method
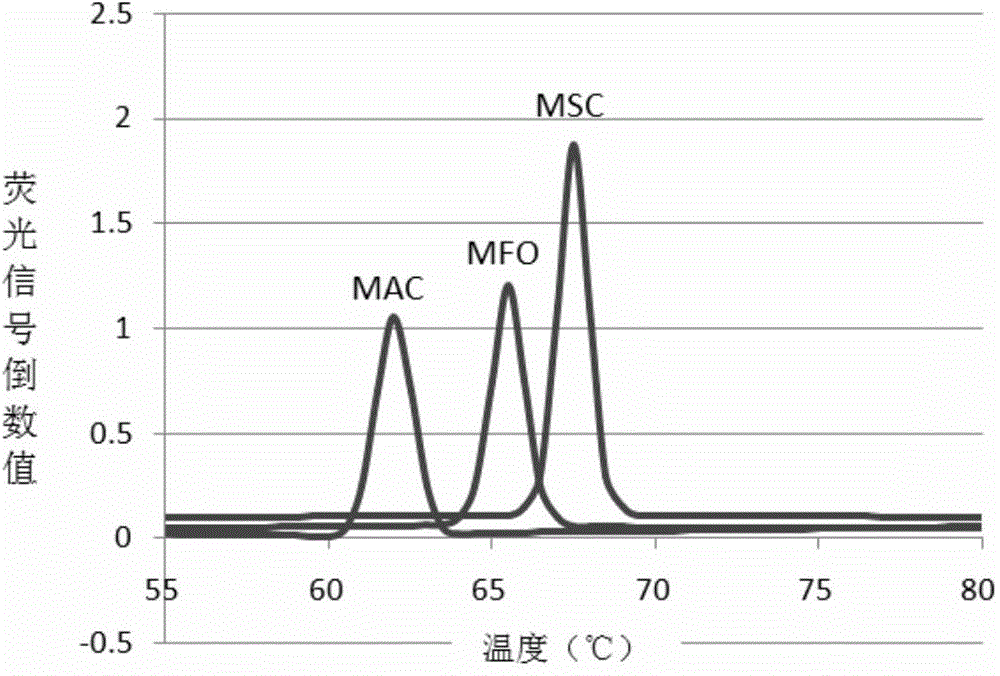
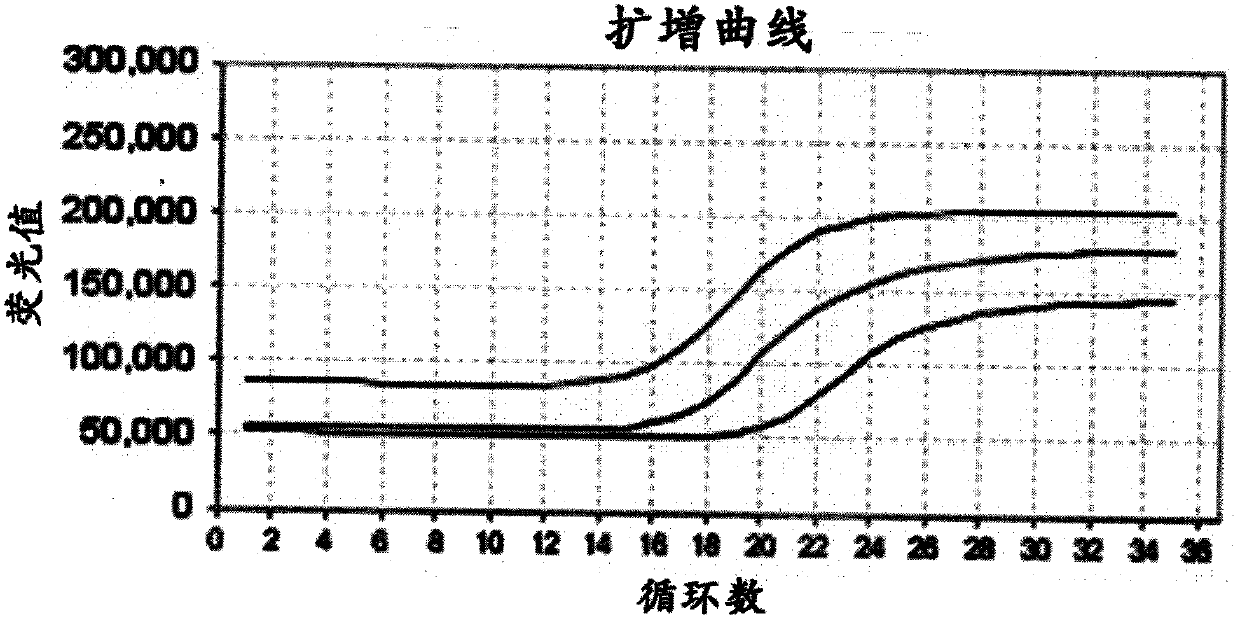
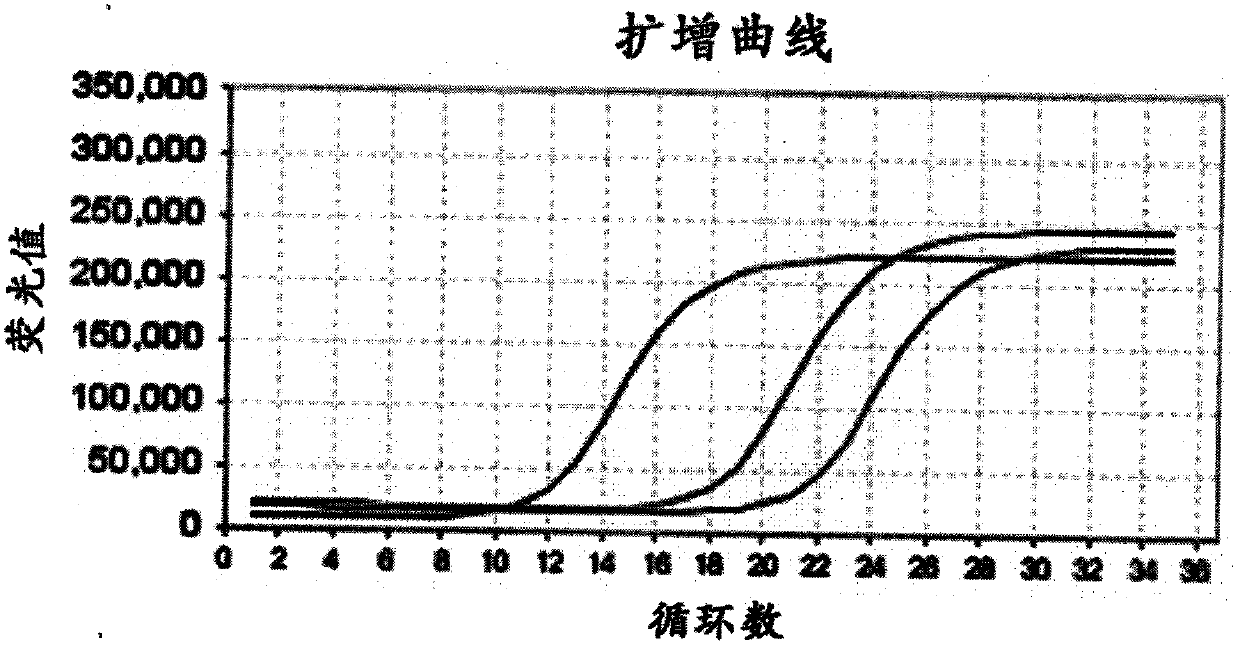
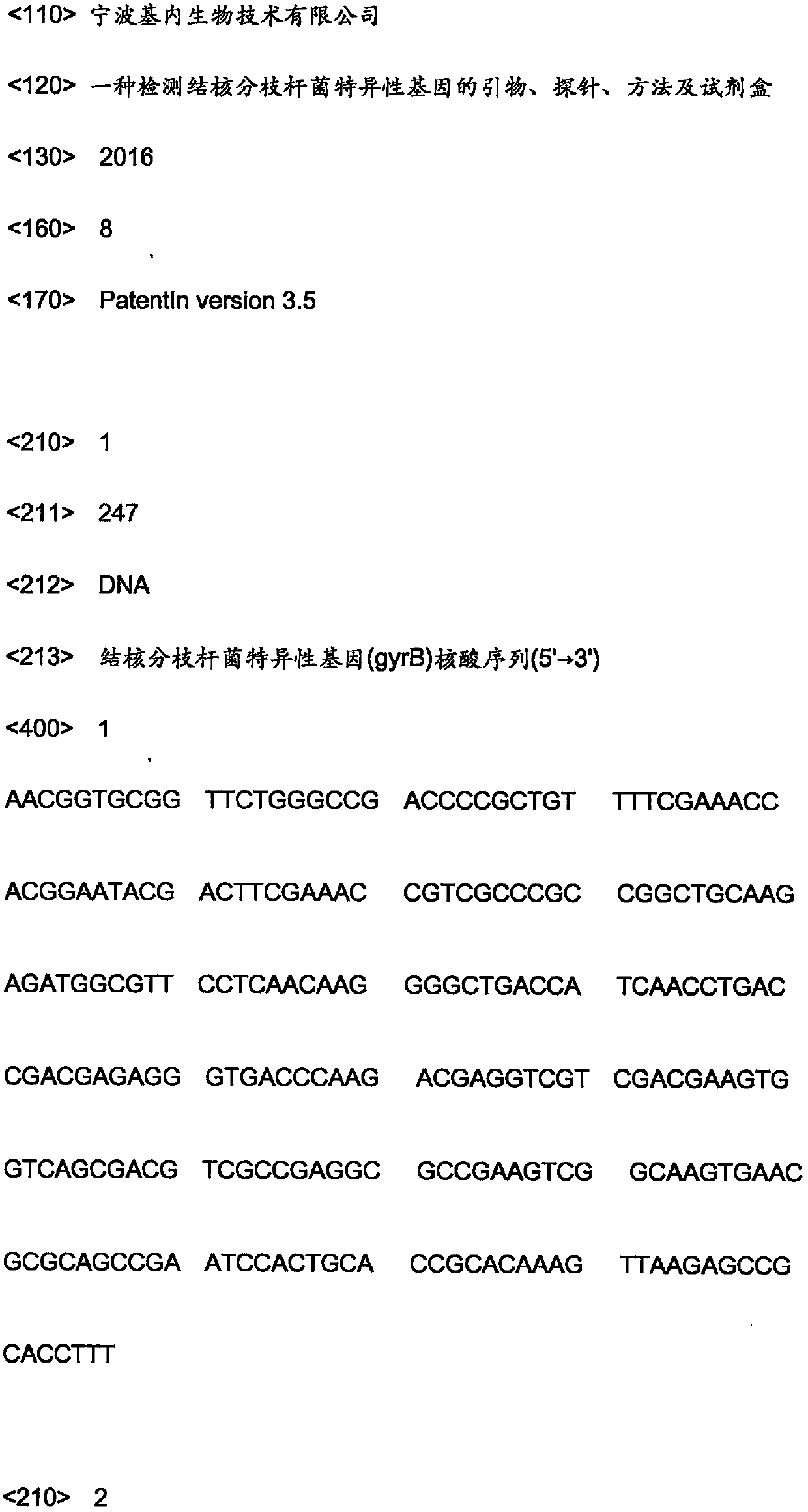
The invention relates to the technical field of molecular biology and discloses primers, probes, method and kit for detecting a mycobacterium tuberculosis specific gene. Through a Taqman probe real-time fluorescence PCR method, primers and fluorescent labeled probes are designed according to (gyrB) and 16S rDNA nucleic acid conserved regions. An enzyme and a sample nucleic acid are added into a PCR detection mixed solution of the primers and probes, a FAM channel of a fluorescence PCR device is used for amplification and a desired gene is detected through change of a fluorescence signal. The primers, probes, method and kit have the characteristics of high accuracy, strong specificity and high sensitivity and can realize fast and accurate detection of mycobacterium tuberculosis (MTB) and non-tuberculous mycobacteria (NTM).
Owner:宁波基内生物技术有限公司
Molecular beacon probe for quickly detecting non-Mycobacterium tuberculosis and detection method using same
ActiveCN104032023AQuick checkSensitive detectionMicrobiological testing/measurementMicroorganism based processesHigh signal intensityLength wave
The invention discloses a molecular beacon probe for quickly detecting non-Mycobacterium tuberculosis. The invention is characterized in that the base sequence of the molecular beacon probe is BeaconNTM: 5'-CY3-(b)CATTG( / b)TACGCCCATAATTCGGAC(b)CAATG( / b)-BHQ1-3', wherein the 5' terminal of the probe is marked with Cy3, the 3' terminal is marked with BHQ1, the fluorophore excitation wavelength is 552nm, and the detection wavelength is 570nm. The molecular beacon probe has the advantages of high signal intensity and high specificity, and can effectively and quickly detect non-Mycobacterium tuberculosis. The invention also discloses a kit and detection method for quickly detecting non-Mycobacterium tuberculosis.
Owner:SUZHOU ZHONGSHENG DAMAIDI MOLECULE DIAGNOSTICS TECH
Method for detecting mycobacterium tubericulosis and nontuberculous mycobacteria by using dual real-time polymerase chain reaction
InactiveCN103038348AEfficient detectionMicrobiological testing/measurementMicroorganism based processesMicrobiologyClinical diagnosis
The present invention provides: a primer set and / or a probe for detecting Mycobacterium tubericulosis and nontuberculous mycobacteria specific for a Mycobacterium tubericulosis-specific IS6110 gene or 16S rRNA gene and a nontuberculous mycobacteria-specific 16S rRNA gene; a kit for detecting Mycobacterium tubericulosis and nontuberculous mycobacteria, containing the same; and a method for detecting Mycobacterium tubericulosis and nontuberculous mycobacteria through dual real-time polymerase chain reaction by using the same. The present invention can provide a clinical diagnosis means capable of more effectively detecting and analyzing Mycobacterium tubericulosis and / or nontuberculous mycobacteria at the same time.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Mycobacterium culture medium, preparation method and method for culture, identification and drug sensitivity test
InactiveCN101560490AEasy to operateEasy to useBacteriaMaterial analysis by observing effect on chemical indicatorGlycerolAntibiotic Y
The invention relates to a mycobacterium culture medium, a preparation method and a method for culture, identification and drug sensitivity test. The culture medium contains buffer solution, glycerol, trace element, albumin, glucose, antibiotic and a colour-changing agent and the like. The first fourteenth components are added in distilled water, pH is adjusted, high-pressure sterilization is carried out, methyl thiazolyldiphenyl tetrazolium, albumin-oleic acid-glucose liquid and four types of antibiotics are added and the culture medium is obtained. Specimen after pre-treatment is taken and added into the culture medium for culture, and after the color of ae culture hole is changed, the mycobacterium tuberculosis or nontuberculous mycobacteria and drug sensitivity are judged according to the condition of color change of identification holes and drug-sensitivity testing holes. The invention can obtain the results of mycobacterium culture, identification and drug-sensitivity measurement by only one-time inoculated culture, has simple operation, practicability, easy standardization, high positive rate, fastness, strong practicability, low cost, easy promotion and wide application, and is applicable to mycobacterium culture, identification and drug-sensitivity test in the environments of human bodies, animals and sewage and the like.
Owner:熊礼宽
Identification method, kit and universal primer pair for mycobacterium, and application of rpsA gene
ActiveCN104164475AMicrobiological testing/measurementDNA/RNA fragmentationForward primerMicroorganism
The invention relates to an identification method, a kit and a universal primer pair for mycobacterium and application of an rpsA gene, belonging to the field of molecular strain identification of microorganisms. The rpsA gene is applied in strain identification of mycobacterium and can be obtained through amplification of the universal primer pair including a forward primer and a reverse primer. The universal primer pair comprises the forward primer and the reverse primer and is used for amplification of the rpsA gene of mycobacterium. The species identification method for mycobacterium employs the universal primer pair for PCR amplification so as to obtain an rpsA gene fragment of a to-be-tested strain; and after sequencing, comparison is carried out so as to identify the species of the to-be-tested strain. The classification and identification kit for mycobacterium utilizes the rpsA gene for mycobacterium species identification and comprises PCR technology used for direct detection of the rpsA gene or other detection techniques based on the PCR technology. According to the invention, classification and identification results of the species of mycobacterium are accurate and reliable, the method is simple, and an identification speed is fast; and the universal primer pair can identify most common clinical isolates of non-tuberculosis mycobacteria.
Owner:BEIJING TUBERCULOSIS & THORACIC TUMOR RES INST
Rapid identification method and kit of novel mycobacterium strain
ActiveCN102634575BMicrobiological testing/measurementFluorescence/phosphorescenceRapid identificationNuclease
The invention relates to a double-marking probe detecting and melting curve analyzing method used for identifying mycobacterium strains and a kit using the method to identify various mycobacterium strains at the same time. The kit provided by the invention comprises a primer capable of designing mycobacterium 16S rRNA, a double-marking oligonucleotide probe capable of identifying mycobacterium strains, and a thermostable DNA polymerase without 5' nuclease activity. The detecting method and the kit, which are provided by the invention, can detect and / or identify 24 kinds of common mycobacteria. The method and the kit can judge the results according to melting peaks of different Tm values produced by the sequence hybridization of the probe and the different strains, meanwhile rapidly and accurately detect the mycobacteria, and identify the mycobacteria, non-mycobacteria, mycobacterium tuberculosis compounding groups and nontuberculosis mycobacteria, and the result of identifying the mycobacteria can be reported after 3-4 hours, thus the method and the kid can assist the clinical diagnosis, and guide efficient clinical chemotherapy at the early stage.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Method for detecting mycobacterium tuberculosis and nontuberculous mycobacteria using duplex polymerase chain reaction
InactiveCN103038346AEfficient clinical diagnostic toolsMicrobiological testing/measurementMicroorganism based processesMicrobiologyClinical diagnosis
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
DNA aptamer of mycobacterium tuberculosis standard strain H37Rv and preparation method thereof
InactiveCN104946655AHigh affinityStrong specificityDNA preparationMaterial analysisNontuberculous mycobacteriaA-DNA
The invention provides a DNA aptamer of a mycobacterium tuberculosis standard strain H37Rv and a preparation method thereof. The ssDNA aptamer of the mycobacterium tuberculosis standard strain H37Rv provided by the invention is high in affinity and specificity, capable of detecting mycobacterium tuberculosis, nontuberculosis mycobacteria and nonmycobacteria with a high specificity in case of being used in microbiological detection, and capable of providing a beneficial basis for laboratory diagnosis on tuberculosis.
Owner:SHANGHAI PULMONARY HOSPITAL
Specific skin regent for diagnozing tuberculomyces infection and active tuberculosis
InactiveCN1683009ABacterial antigen ingredientsDrug compositionsMycobacterium InfectionsCalmette-Guerin Bacillus
The present invention relates to specific skin reagent for diagnosing tuberculous infection and active tuberculosis, and belongs to the field of medical immunological diagnosis technology. The specific skin reagent consists of diluent liquid and dissolved tuberculous allergen, and the tuberculous allergen is tuberculous mycobacterium ESAT6 protein. The present invention also proposes applying tuberculous mycobacterium ESAT6 protein for human skin test as tuberculous allergen to detect tuberculous infecting person and tuberculosis patient and identify the specific reaction of inoculated Calmette-Guerin bacillus vaccine. The present invention can induce the immune response of tuberculous infecting person. The reagent can induce delayed allergic reaction of tuberculous infecting person and can identify BCG inoculation from the allergic reaction of non-tuberculous mycobacterium and tuberculous mycobacterium infection.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Gold-labeled diagnosis reagent based on combined protein for tubercle bacillus
InactiveCN1844929AIncreased sensitivityImprove featuresSugar derivativesBiological testingAntigenSpecific test
The invention discloses a medical immunity diagnose technique, especially providing a bacillus tubercle mycobacterium gold mark diagnose agent based on combined protein. Wherein, it uses B cell antigen determinants of bacillus tubercle mycobacterium main excrete proteins as ESAT6, MPT64, PstS-1, Ag85B to combine a new group of protein as antigen, as the specific test antibody in the clinic serology diagnosis of phthisis. The invention combines the B cell antigen determinants of bacillus tubercle mycobacterium main excrete proteins to be displayed by gene project, and purified to attain one new protein, and via gold mark filter method to test the serum of tuberculosis patient. The invention has high sensitivity, simple operation, and high speed, which can identify the sensitized condition caused by contacting the non- bacillus tubercle mycobacterium or inoculating beg vaccine, and the real bacillus tubercle mycobacterium. It can be used in the clinic serology diagnosis of clinic tuberculosis.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
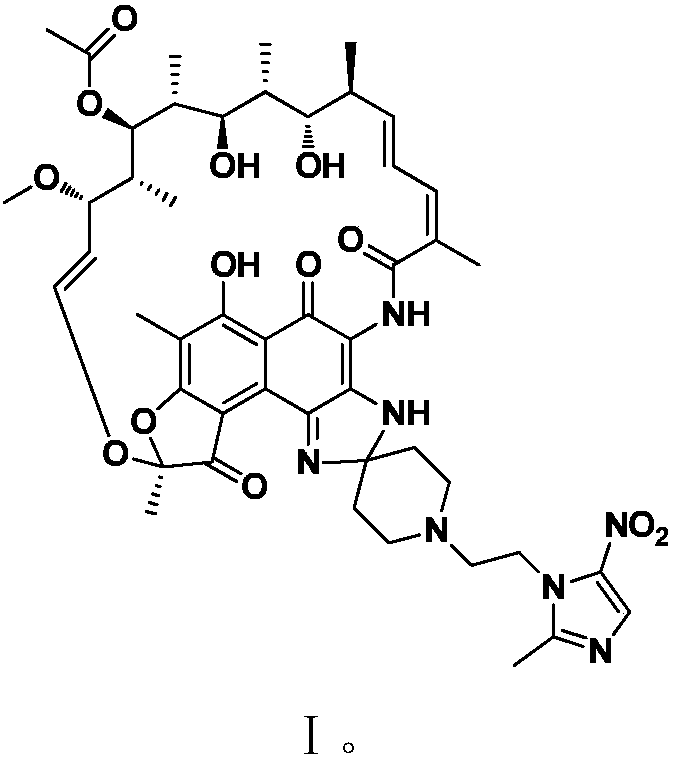
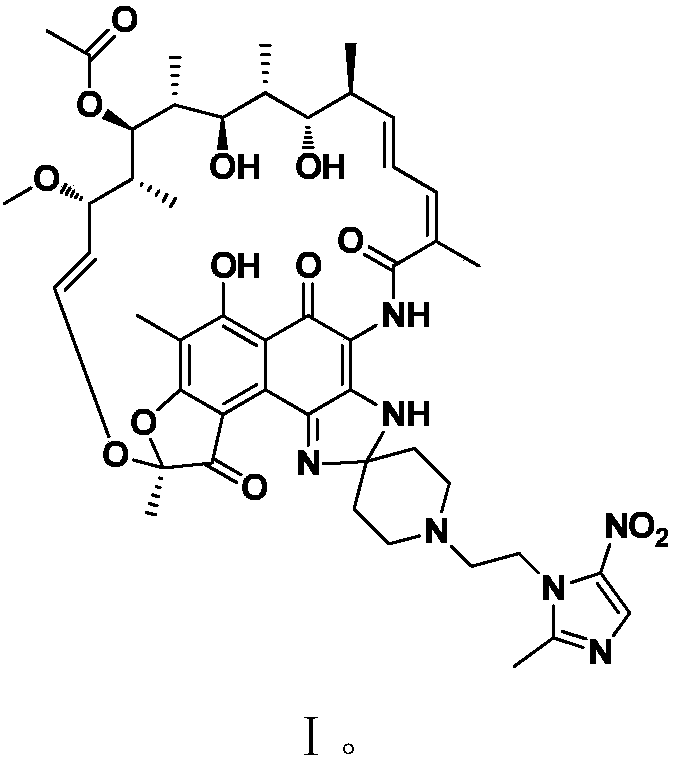
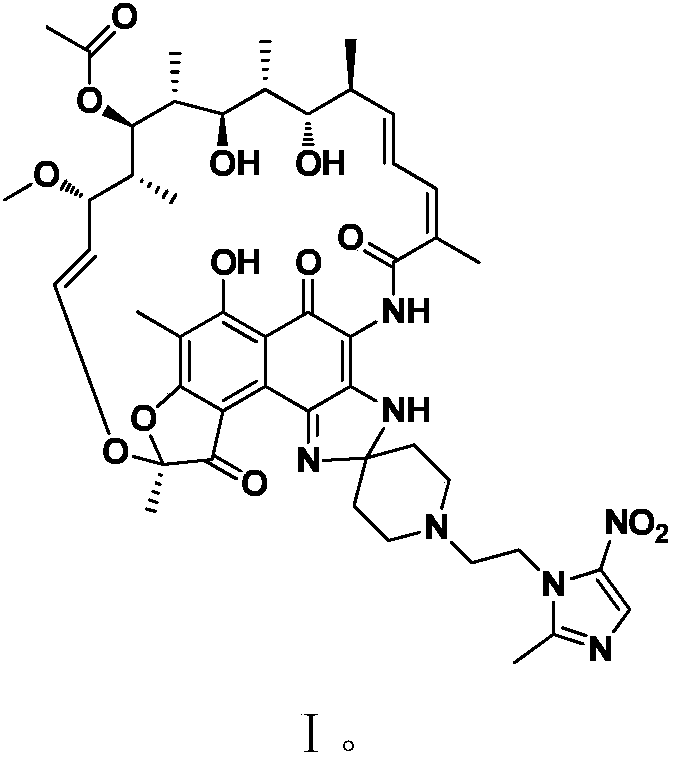
Application of rifamycin-nitroimidazole coupling molecule
ActiveCN108047250AEffective againstAntibacterial agentsOrganic active ingredientsNitroimidazoleMetabolite
The invention provides application of a rifamycin-nitroimidazole coupling molecule, or a stereoisomer, an aquo-complex, a deuterated material, an ester, a solvate, a crystal form, a metabolite, pharmaceutically acceptable salt or prodrug thereof in resisting non-mycobacterium tuberculosis, the rifamycin-nitroimidazole coupling molecule has a structure shown in Formula I. The rifamycin nitroimidazole coupling molecule, or the stereoisomer, the aquo-complex, the deuterated material, the ester, the solvate, the crystal form, the metabolite, the pharmaceutically acceptable salt or the prodrug thereof can effectively resist non-mycobacterium tuberculosis and can be used for treating infections caused by non-mycobacterium tuberculosis.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Method for detecting mycobacterium tuberculosis and nontuberculous mycobacteria using duplex real-time polymerase chain reaction and melting curve analysis
InactiveCN103038347AMicrobiological testing/measurementMicroorganism based processesMicrobiologyMelting curve analysis
The present invention provides: primers for detecting mycobacterium tuberculosis and nontuberculous mycobacteria, the primers being specific respectively to a mycobacterium tuberculosis-specific IS6110 gene and a nontuberculous mycobacteria-specific 16S rRNA; a mycobacterium tuberculosis and nontuberculous mycobacteria detection kit including the primers; and a method for detecting mycobacterium tuberculosis and nontuberculous mycobacteria by means of a duplex real-time polymerase chain reaction using the primers and by means of a melting curve analysis. The present invention can provide a clinical diagnostic means that can quickly detect mycobacterium tuberculosis and / or nontuberculous mycobacteria simultaneously and more effectively, at a lower cost, since the invention does not use a sequence-specific probe.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Kit and method for detecting mycobacterium tuberculosis and non-mycobacterium tuberculosis
InactiveCN107058522AShort timeReliable classificationMicrobiological testing/measurementMicroorganism based processesNontuberculous mycobacteriaMycobacterium tuberculosis
The invention relates to a kit and a method for detecting mycobacterium tuberculosis and non-mycobacterium tuberculosis. The kit comprises a primer pair 1 and a probe 1 for detecting the mycobacterium tuberculosis and a primer pair 2 and a probe 2 for detecting the non-mycobacterium tuberculosis; the primer pair 1 comprises sequences as shown in SEQ ID No.1 and SEQ ID No.2, and the primer pair 4 comprises sequences as shown in SEQ ID No.3 and SEQ ID No.4; the probe 1 comprises a sequence as shown in SEQ ID No.5 and the probe 2 comprises a sequence as shown in SEQ ID No.6. Fluorescent probes are designed by means of difference of gene sequences of different mycobacteria and mycobacterium tuberculosis and non-mycobacterium tuberculosis can be differentiated at one time. Therefore, the detection method is short in time and more accurate and reliable to classify, and solves the problem that in the prior art, the detection method is tedious and long in consumed time.
Owner:HEBEI CHEST HOSPITAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com