Systems for treating pulmonary infections
a technology for pulmonary infections and systems, applied in the direction of antibacterial agents, drug compositions, aerosol delivery, etc., can solve problems such as entrapment, size, and physical characteristics chang
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison Of Nebulizer Reservoir Volumes
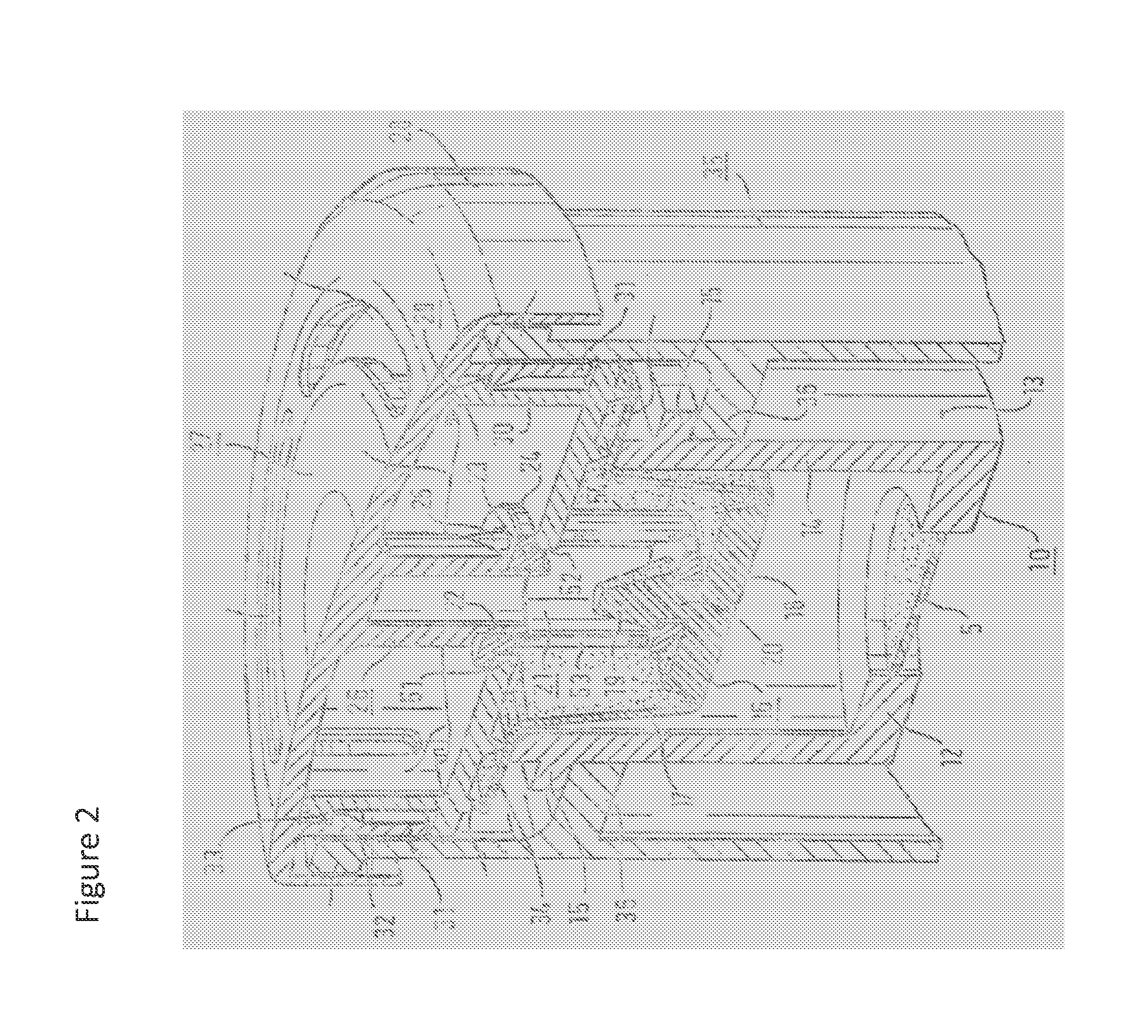
[0174]In this example, the aerosol generator was an investigational eFlow® nebulizer, modified for use with liposomal aminoglycoside formulations provided herein, of Pari Pharma GmbH, Germany. A first aerosol generator had an initial volume of the liquid reservoir VRI of 13 mL (A), a second one of 17 mL (B), a third one of 22 mL (C) and a fourth one of 20 mL (D). That is the increased volume VRN of the first one had 15.5 mL, the second one 19.5 mL, the third 24.5 mL and the fourth 22.5 mL.
[0175]8 mL of a liposomal amikacin formulation was poured into the liquid reservoir 10. As shown in FIG. 8, an air cushion of 8 mL resulted in an aerosol generation time period upon complete emission of 8 mL of the formulation in the liquid reservoir of between 14 and 16 minutes. An air cushion of 12 mL, however, decreased the aerosol generation time to a range between 12 and approximately 13 minutes. The air cushion of 17 mL further decreases the aerosol ge...
example 2
Aerosol Properties of Amikacin Formulation
[0182]Eleven different lots of the liposomal amikacin formulation were examined with the modified eFlow® nebulizer (i.e., modified for use with the liposomal aminoglycoside formulations described herein) having a modified 40 mesh membrane fabricated as described herein, and a reservoir with an 8 mL liquid capacity and aforementioned air cushion. Cascade impaction was performed using either the ACI (Anderson Cascade Impactor) or the NGI (Next Generation Impactor) to establish aerosol properties: mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), and Fine-Particle-Fraction (FPF). Mass Median Aerodynamic Diameter (MMAD) measurement with ACI
[0183]An Anderson Cascade Impactor (ACI) was used for MMAD measurements and the nebulization work was conducted inside a ClimateZone chamber (Westech Instruments Inc., Ga.) to maintain temperature and relative humanity % during nebulization. The ClimateZone was pre-set to a temperatu...
example 3
Nebulization Rate Study
[0194]Nebulization rate studies (grams of formulation nebulized per minute) were conducted in a biosafety cabinet (Model 1168, Type B2, FORMA Scientific). The assembled nebulizer (handset with mouth piece and aerosol head) was first weighed empty (W1), then a certain volume of formulation was added and the nebulizer device was weighed again (W2). The nebulizer and timer were started and the formulations nebulized were collected in a chilled impinger at a flow rate of ˜8 L / min (see FIG. 14 for details of experimental setup). When there was no more aerosol observed, the timer was stopped. The nebulizer was weighed again (W3), and the time of nebulization (t) was recorded. Total formulation nebulized was calculated as W2-W3 and total drug residue after nebulization was calculated as W3-W1. The nebulization rate of formulation was calculated according to the following equation:
NebulizationRate(gmin)=W2-W3t
[0195]Nebulization rates in g / min., as well as other relate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com