Ambroxol hydrochloride solution for inhalation
A technology of ambroxol hydrochloride and solution, which is applied in the field of medicine and can solve problems such as low utilization rate and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
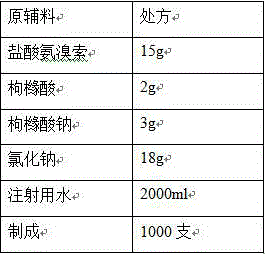
[0021]
[0022] Process:
[0023] (1) Add the prescribed amount of citric acid and sodium citrate into 80% of the prescribed amount of water for injection, stir to dissolve, add the prescribed amount of sodium chloride, and stir to dissolve completely;
[0024] (2) Add the prescribed amount of ambroxol hydrochloride, stir to dissolve, and add water for injection to the full amount;
[0025] (3) Add 0.03% (w / v) charcoal for needles, stir at 80°C for 10 minutes, filter through a 0.45 μm membrane to decarbonize, add water for injection to the full amount, stir and mix well, and prepare for filling;
[0026] (4) Measure the content and pH of the intermediate, and the pH of the intermediate is 4.93;
[0027] (5) Fine filtration with 0.22μm microporous membrane, each 2ml filled in ampoules, filled with nitrogen in the ampoules before filling and sealed;
[0028] (6) Sterilization: Sterilize at 121°C for 15 minutes (F 0 >12).
Embodiment 2
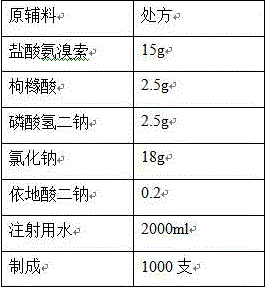
[0030]
[0031] Process:
[0032] (1) Add the prescribed amount of edetate disodium, citric acid, and disodium hydrogen phosphate into 80% of the prescribed amount of water for injection, stir to dissolve, add the prescribed amount of sodium chloride, and stir to dissolve completely;
[0033] (2) Add the prescribed amount of ambroxol hydrochloride, stir to dissolve, and add water for injection to the full amount;
[0034] (3) Add 0.05% (w / v) charcoal for needles, stir at 70°C for 15 minutes, filter through a 0.45 μm membrane to decarbonize, add water for injection to the full amount, stir and mix well, and prepare for filling;
[0035] (4) Measure the content and pH of the intermediate, and the pH of the intermediate is 5.20;
[0036] (5) Fine filter with 0.22μm microporous membrane, fill each 2ml bottle into an ampoule, fill the ampoule with nitrogen before filling, and seal it by melting. ;
[0037] (6) Sterilization: Sterilize at 121°C for 15 minutes (F 0 >12).
Embodiment 3
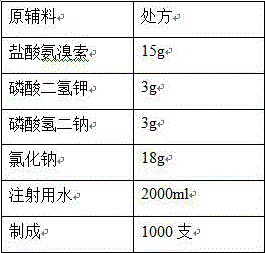
[0039]
[0040] Process:
[0041] (1) Add the prescribed amount of potassium dihydrogen phosphate and ambroxol hydrochloride into 80% of the prescribed amount of water for injection, stir to dissolve, add the prescribed amount of disodium hydrogen phosphate, and stir to dissolve completely;
[0042] (2) Add the prescribed amount of sodium chloride, stir to dissolve, and add water for injection to the full amount;
[0043] (3) Add 0.1% (w / v) charcoal for needles, stir at 60°C for 5 minutes, filter through a 0.45 μm membrane to decarbonize, add water for injection to the full amount, stir and mix well, and prepare for filling;
[0044] (4) Measure the content and pH of the intermediate, and the pH of the intermediate is 5.12;
[0045] (5) Fine filtration with 0.22μm microporous membrane, each 2ml filled in ampoules, filled with nitrogen in the ampoules before filling and sealed;
[0046] (6) Sterilization: Sterilize at 121°C for 15 minutes (F 0 >12).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com