Recombinant bacillus calmette-guerin vaccine strain with over-expression mycobacterium tuberculosis Rv3586 and application of recombinant bacillus calmette-guerin vaccine strain
A technology of Mycobacterium tuberculosis and recombinant BCG, applied in the field of tuberculosis vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] Construction of Rv3586 Escherichia coli-Mycobacterium Shuttle Expression Vector
[0045] Primers were designed according to the genome Rv3586 gene sequence of the Mtb H37Rv strain:
[0046] P1: 5'-tttaagcttatgcacgctgtgactcgtccgacc-3' HindIII site
[0047] P2: 5'-gcgaagcttaaggcggataattattgatcgc-3' HindIII site
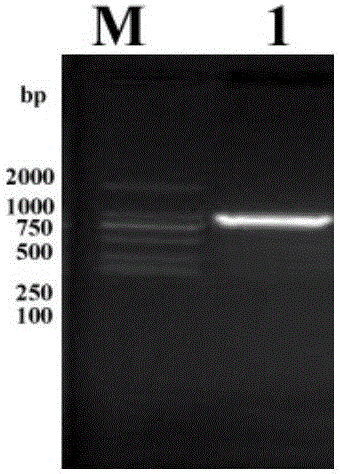
[0048] Primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. Use the genomic DNA of Mtb H37Rv strain as a template to amplify the target gene by PCR. Reaction parameters: pre-denaturation at 95°C for 5 minutes, denaturation at 95°C for 30 seconds, renaturation at 57°C for 1 minute, extension at 72°C for 100 seconds, 35 cycles, and extension at 72°C for 5 minutes after the last cycle . 1% agarose gel electrophoresis analysis showed that a 1 100bp target band was amplified ( figure 1 ). The target fragment was recovered by the gel recovery kit, digested with HindIII, and the digested product was recovered by 1% agarose gel electrophoresis. T4 DN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com