A kind of recombinant BCG and its application
A recombinant BCG vaccine and BCG vaccine technology, applied in the application, recombinant DNA technology, medical preparations containing active ingredients, etc., can solve the problems of uneven results, limited protection effect of adult tuberculosis, safety of BCG immunity, etc. The effect of strong protective effect, improved vaccine safety, and improved anti-infection protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
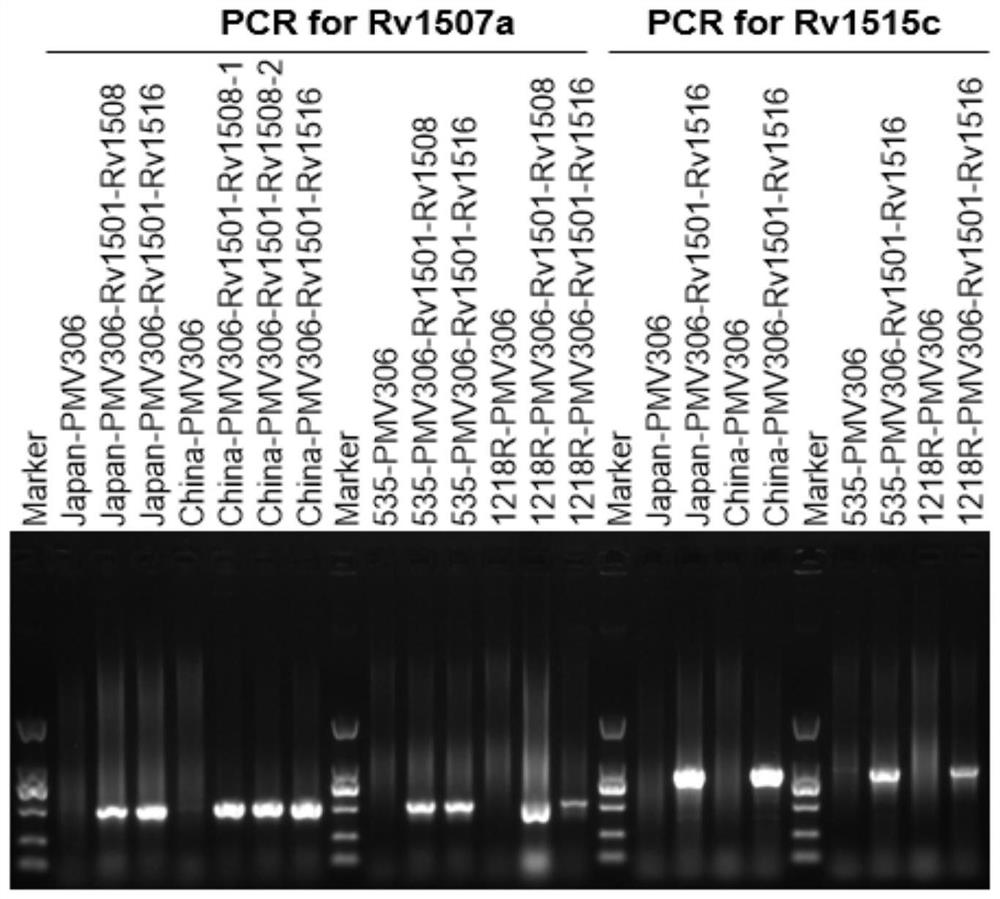
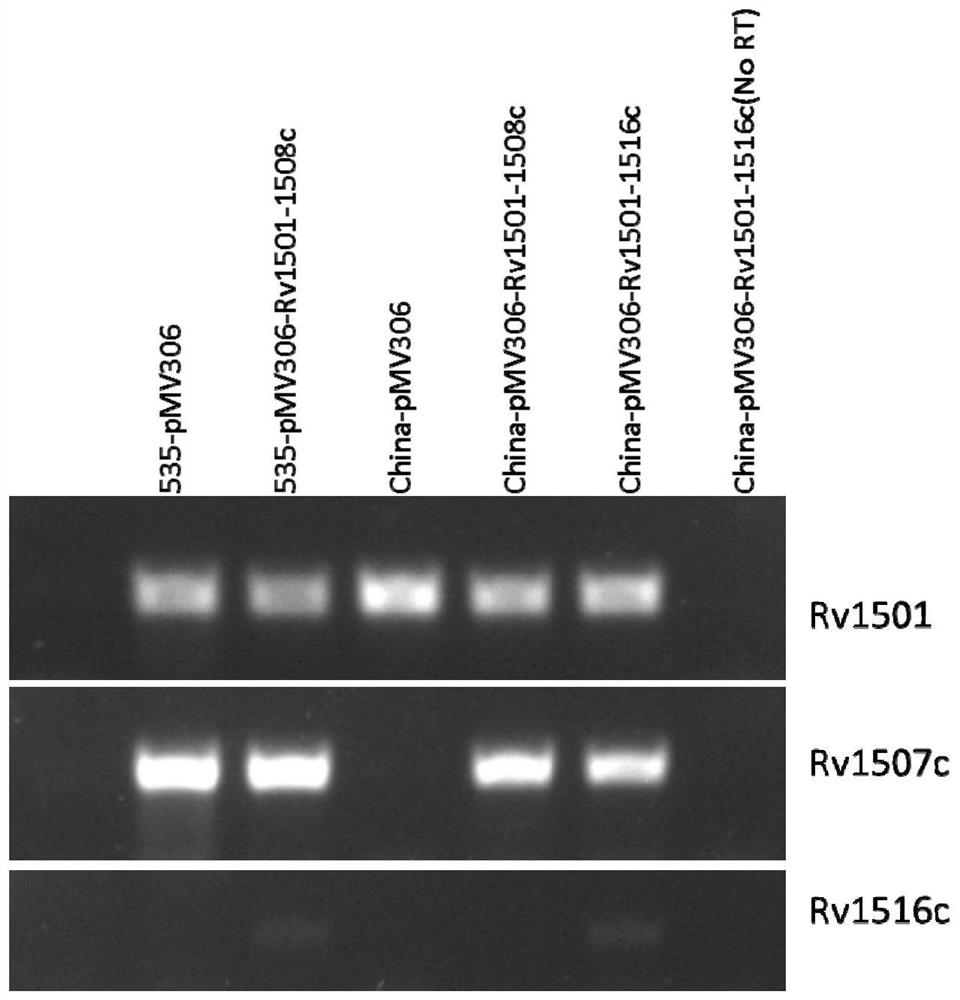
[0077] Example 1: Construction and identification of recombinant plasmids pMV306-Rv1501-Rv1508c and pMV306-Rv1501-Rv1516c
[0078] The integrative vector pMV306 was used for molecular cloning. Molecular biology techniques are routinely performed:
[0079] (1) First construct pMV306-Rv1501-Rv1502
[0080] The Rv1501-Rv1502 gene was amplified from the Mycobacterium tuberculosis H37Rv genome by PCR technology, (upstream primer 5'-CACTGGTCGACAATGTCACTTCATTTAGCAAC-3'(SEQ ID NO.3); downstream primer 5'-CATGAAAGCTTCGAATCATTGGAACAGCGG-3'(SEQ ID NO. 4)), the amplification conditions are: 98°C for 5min, [98°C for 10s, (Tm-5)°C for 10s, 72°C for 1min / kbp] 30 cycles, and 72°C for 10min. PCR products were recovered using the AxyPrep PCR Product Recovery Kit (Axygen). The gene fragment and the pMV306 plasmid were digested with SalI and HindIII, and the digested fragment was recovered with the AxyPrep DNA Gel Extraction Kit and ligated to form the recombinant plasmid pMV306-Rv1501-Rv1502. ...
Embodiment 2
[0086] Example 2: Establishment and verification of recombinant BCG rBCG::RD4
[0087] (1) Preparation of BCG Competent Cells:
[0088] Get 1ml of BCG-China and BCG-Japan strains in logarithmic growth phase, aseptically inoculate in 50ml Middlebrook 7H9 liquid medium (Difco™) that has added 10% ADC (DIFCO, Bection-Dickinson), 0.2% glycerol and 0.05% Tween80 In 37 ° C static culture to OD600 = 0.8-1.0. 4 ° C centrifugation to collect bacteria. After resuspending with 10% glycerol, wash 3 times with 1 / 2, 1 / 10 and 1 / 50 of the original culture volume of glycerol, and finally resuspend with 1ml pre-cooled glycerol, aliquot into 100μL tubes, and store at -80°C spare.
[0089] (2) Electrotransformation of BCG:
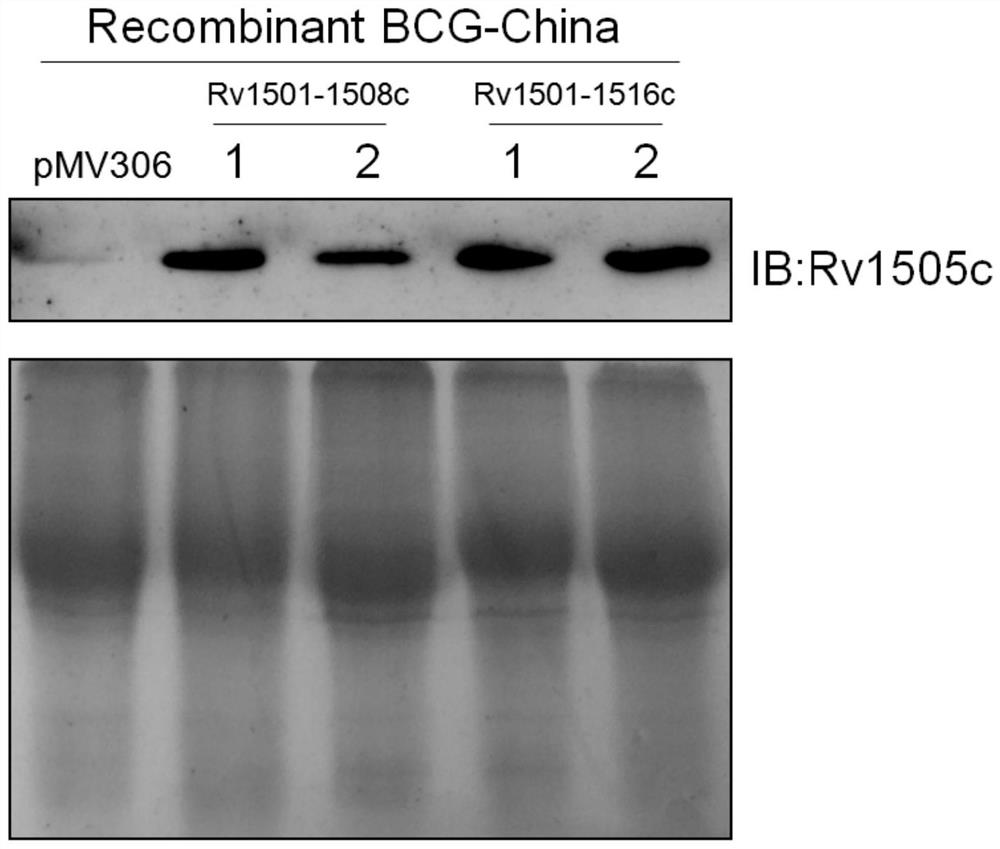
[0090] Add 5 μL of high-concentration plasmids (such as pMV306, pMV306-Rv1501-Rv1508c, pMV306-Rv1501-Rv1516c) and 200 μL of BCG competent cells to the labeled 0.2 cm Bio-Rad electroporation cuvette, gently blow and mix, and use Bio -rad GenePulser electroporator electropo...
Embodiment 3
[0112] Example 3: Safety Evaluation of Recombinant BCG rBCG::RD4
[0113] Six-week-old female SCID mice were randomly divided into 20 groups. Each mouse was injected with 100 μL (1×107 CFU) of recombinant BCG rBCG::RD4 and rBCG::306 through the tail vein, and PBS was used as a negative control. On the second day after infection, 2 mice in each group were sacrificed, the lungs and spleens were aseptically isolated and the bacterial load was counted to determine the infection dose. The remaining mice were observed for a long time, their body weight changes and death conditions were recorded, and the survival curve of the mice was drawn.
[0114] Statistics found that the half-life time of wild-type BCG (i.e. BCG::pMV306) group, BCG::Rv1501-1508c group and BCG::Rv1501-1516c group were 63, 77 and 67.5 days respectively (attached image 3). Log-rank statistical analysis showed that the survival period of the mice in the BCG::Rv1501-1508c group was significantly longer than that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com