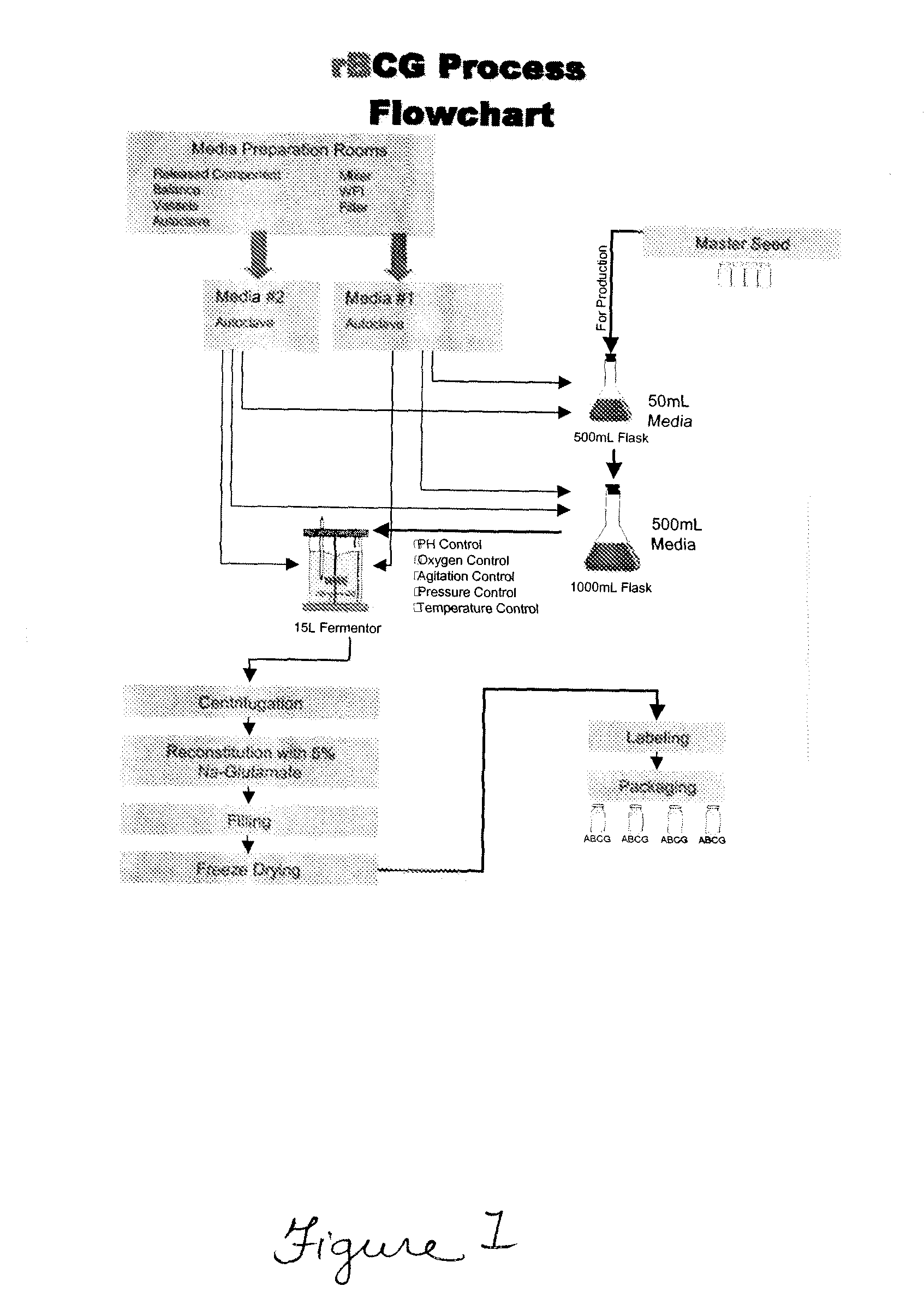
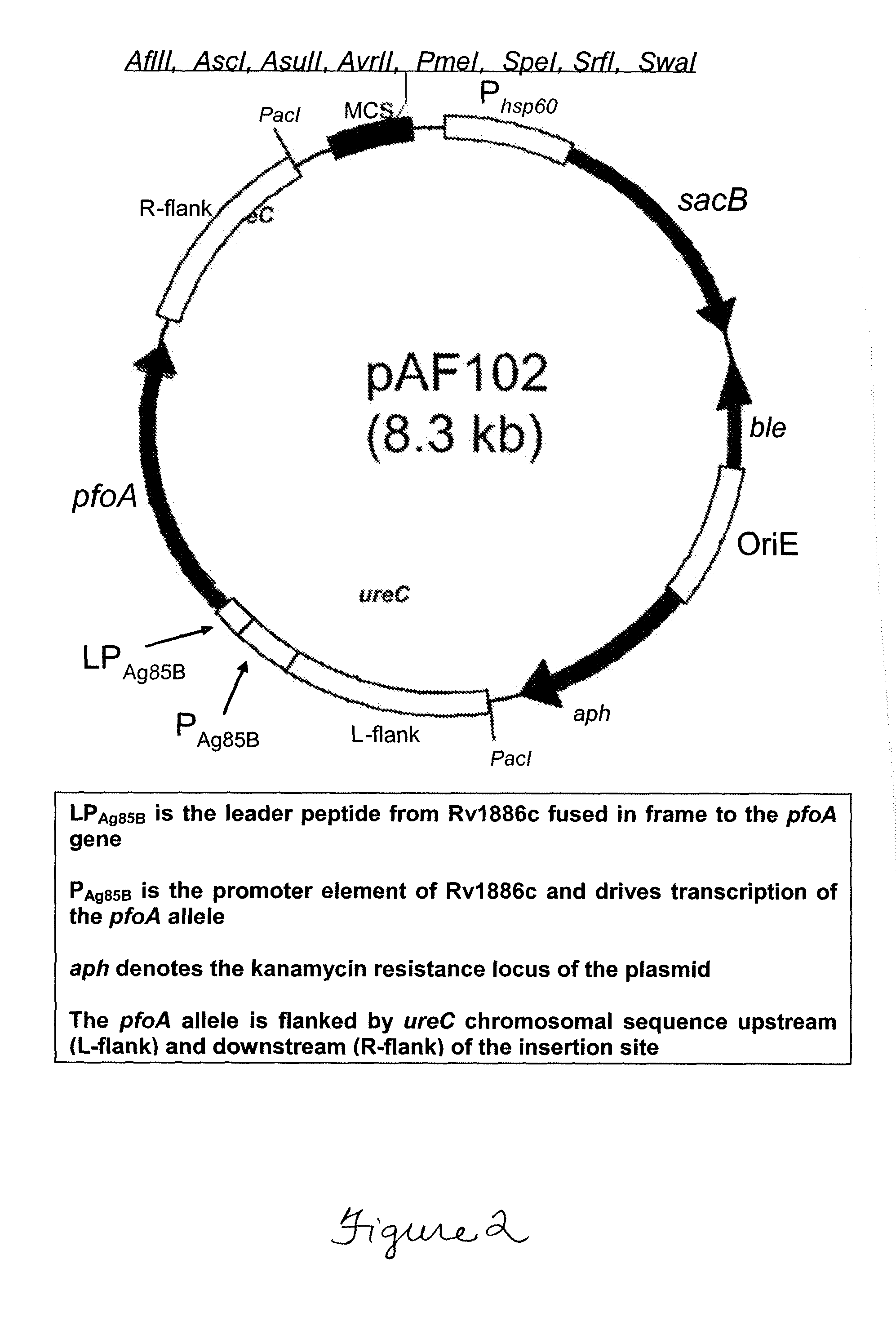
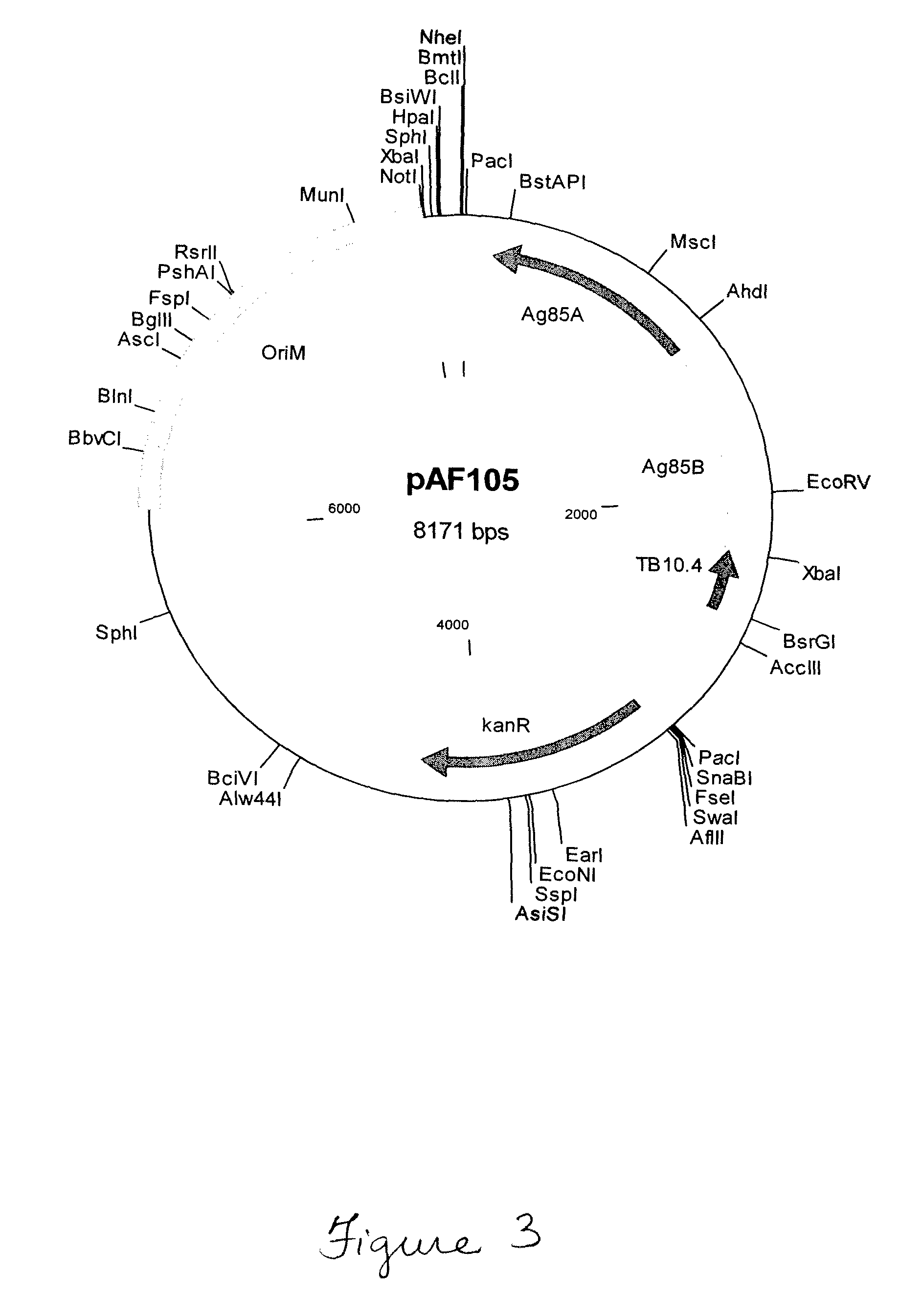
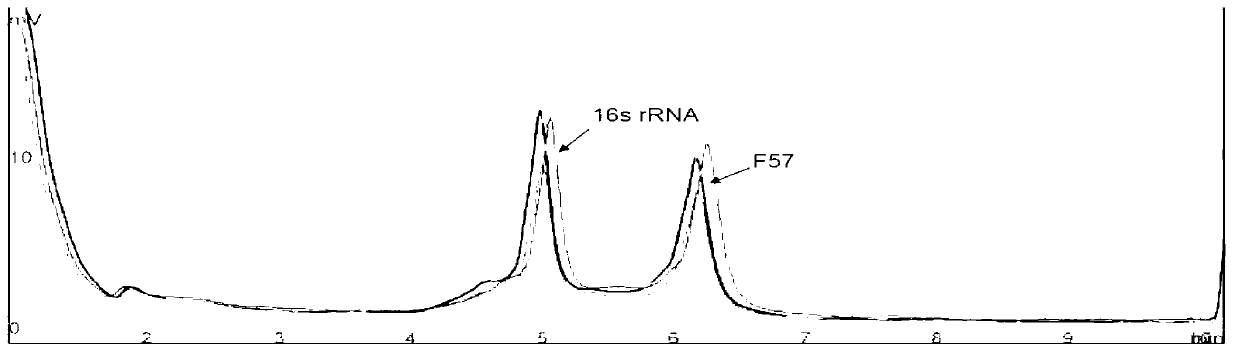
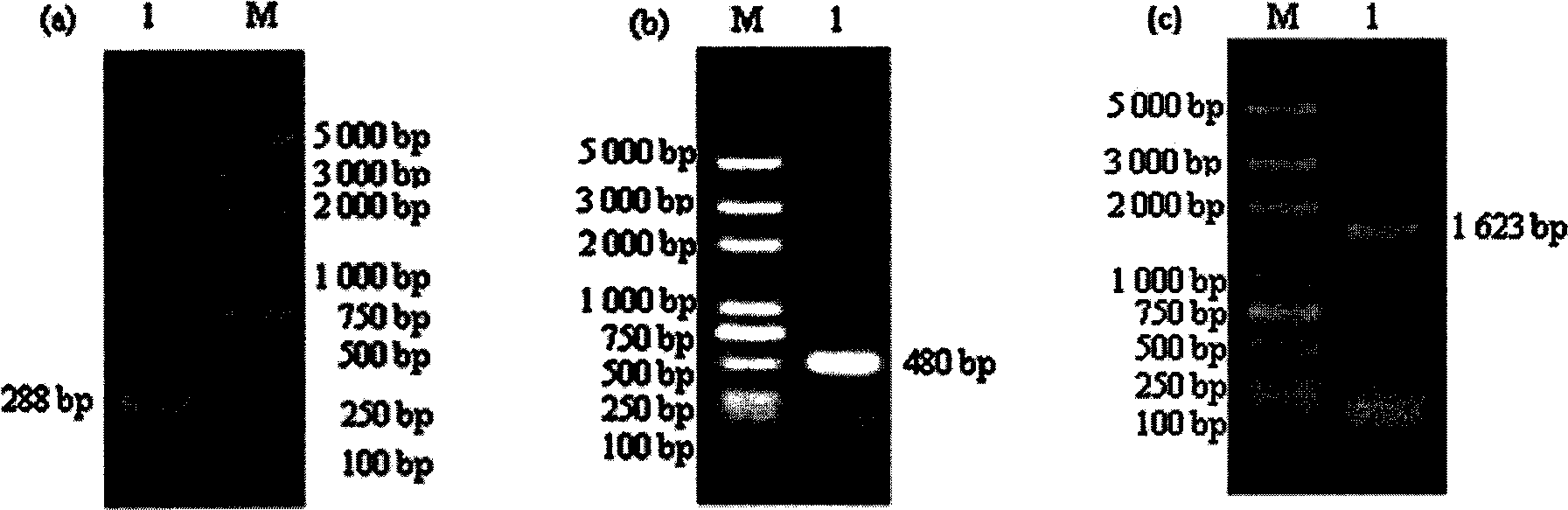
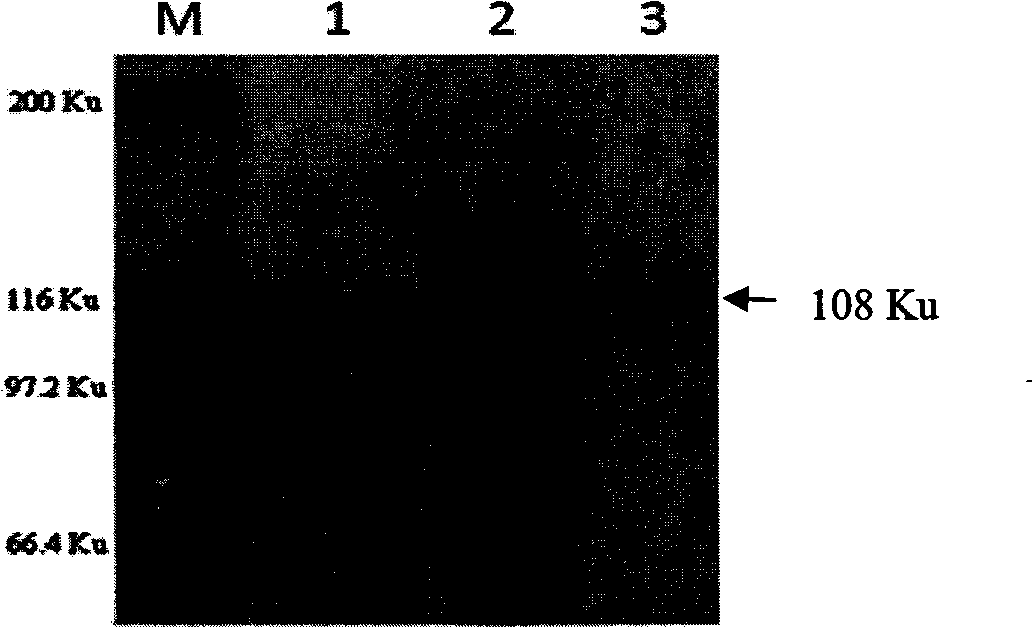
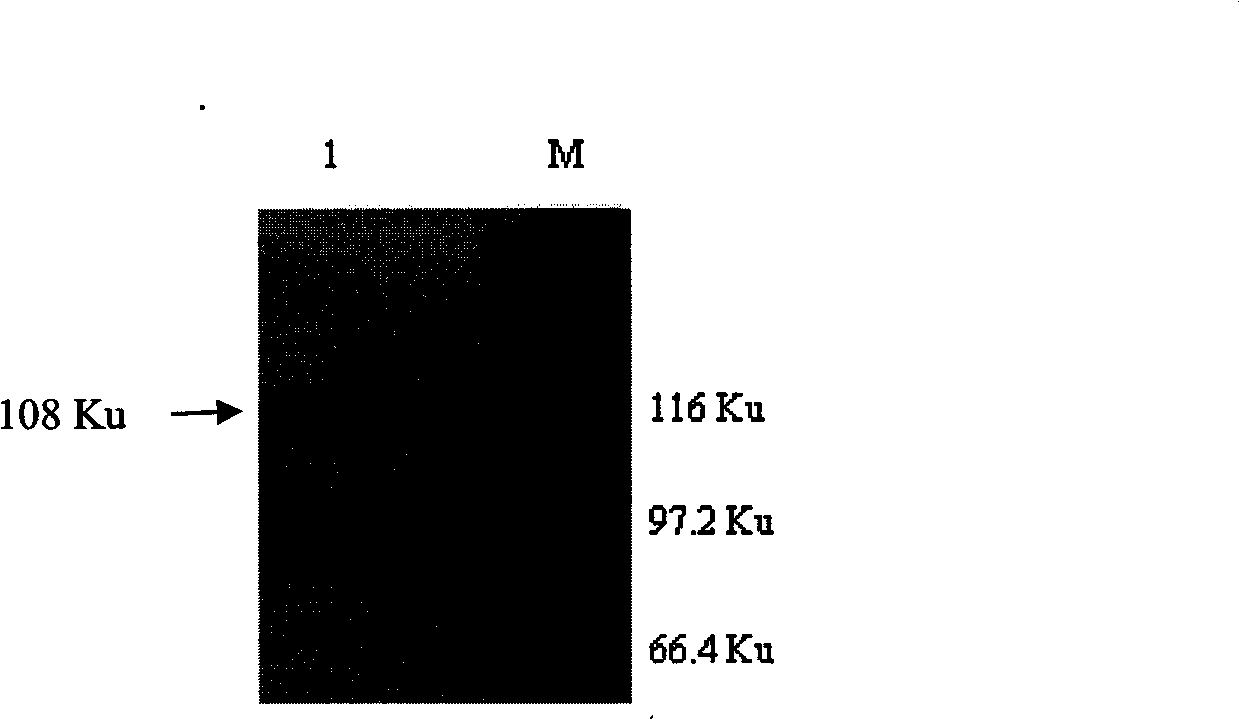
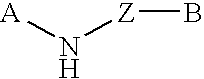Patents
Literature
46 results about "Pathogenic mycobacterium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
There are many other species under this genus are pathogenic. Obligate pathogens such as Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium leprea are highly pathogenic in animals. These species can cause tuberculosis and leprosy, debilitating diseases that may lead to death or disfigurement.
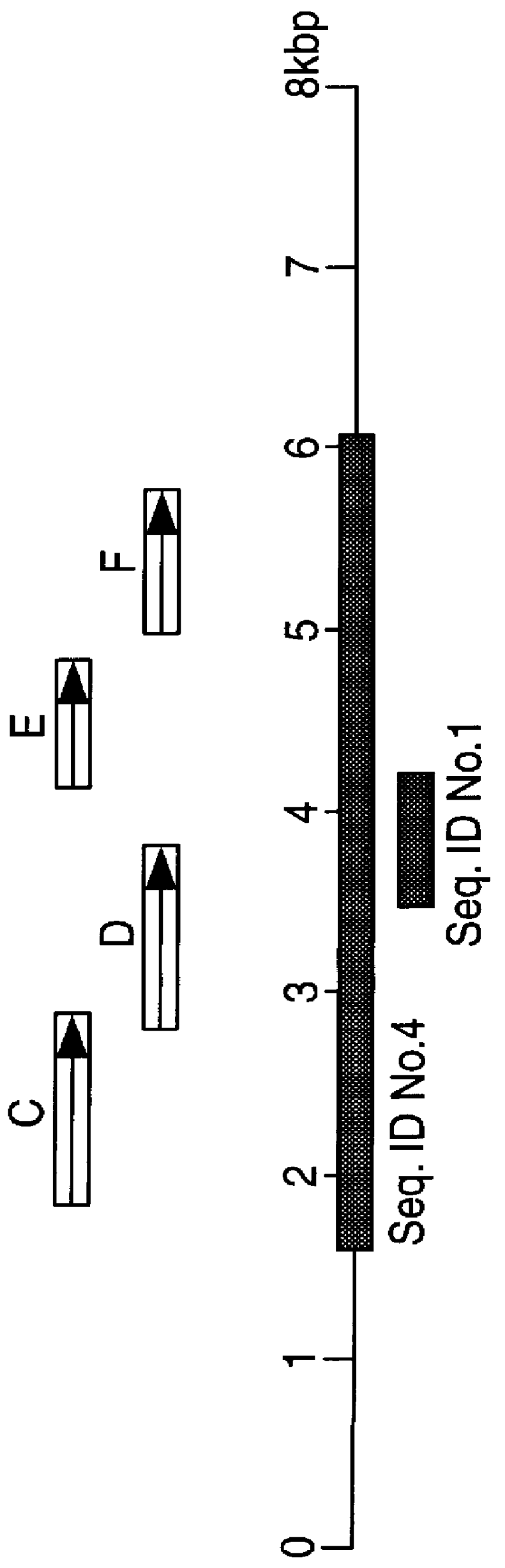
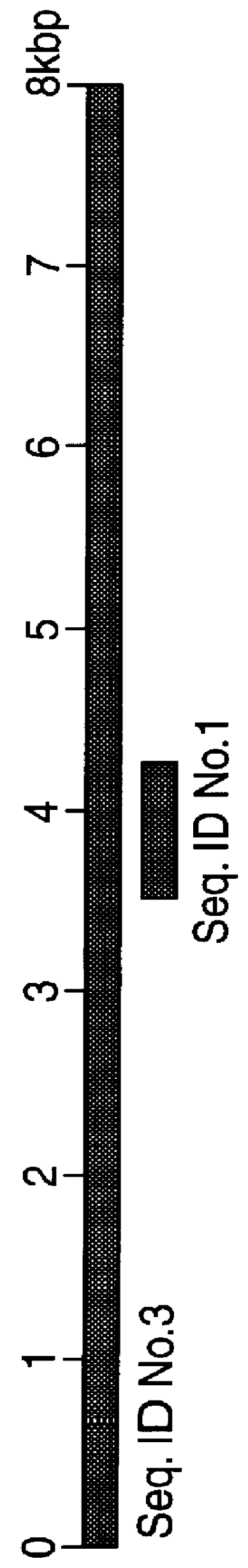
Polynucleotides and polypeptides in pathogenic mycobacteria and their use as diagnostics, vaccines and targets for chemotherapy
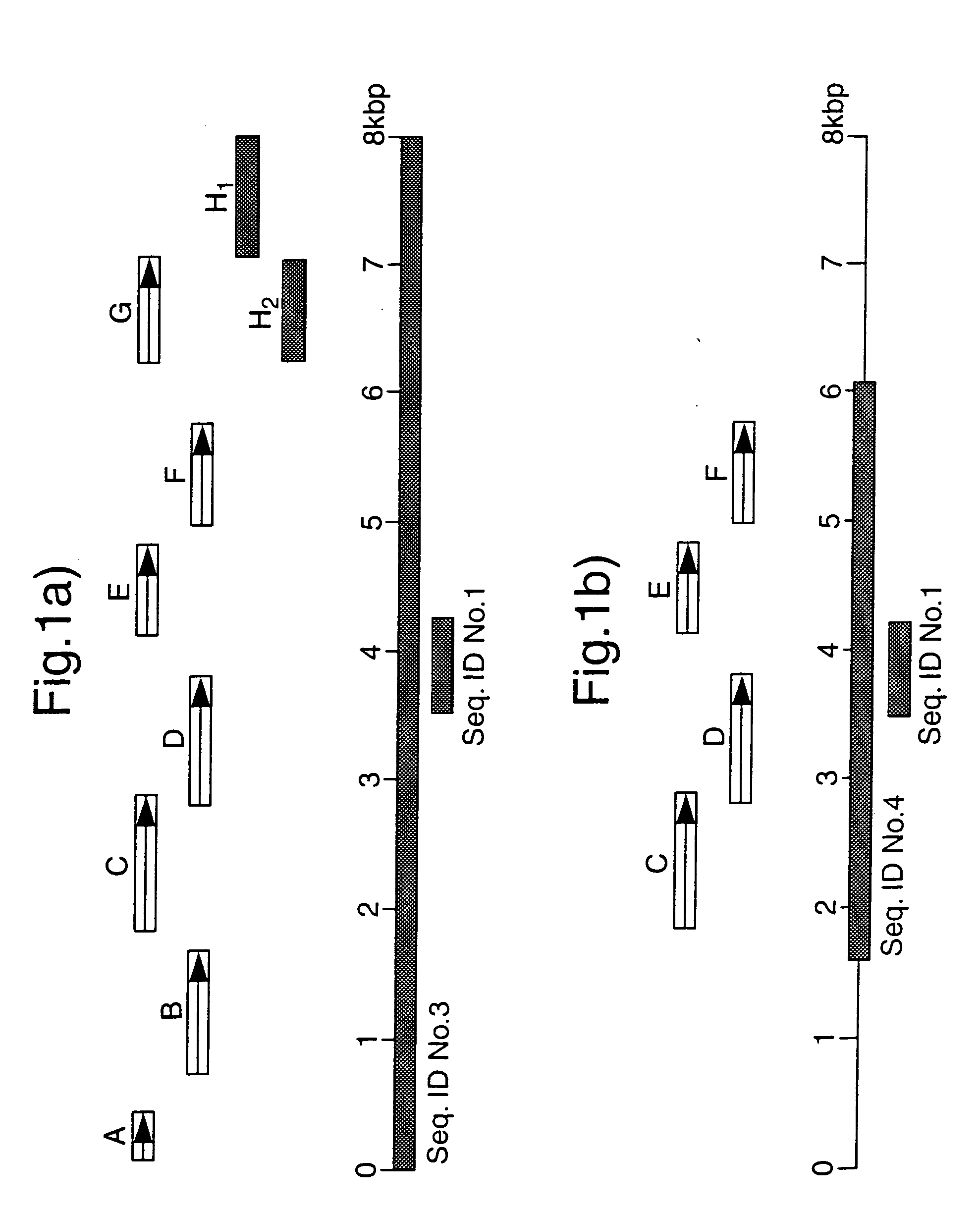
PCT No. PCT / GB96 / 03221 Sec. 371 Date Sep. 16, 1998 Sec. 102(e) Date Sep. 16, 1998 PCT Filed Dec. 23, 1996 PCT Pub. No. WO97 / 23624 PCT Pub. Date Jul. 3, 1997The invention provides a nucleotide sequence representing a pathogenicity island found in species of pathogenic mycobacteria. The islands are shown as SEQ ID NOs: 3 and 4 and comprises several open reading frames encoding polypeptides. These polypeptides and their use in diagnosis and therapy form a further aspect of the invention.
Owner:HAV VACCINES
Mycobacterial inhibitors
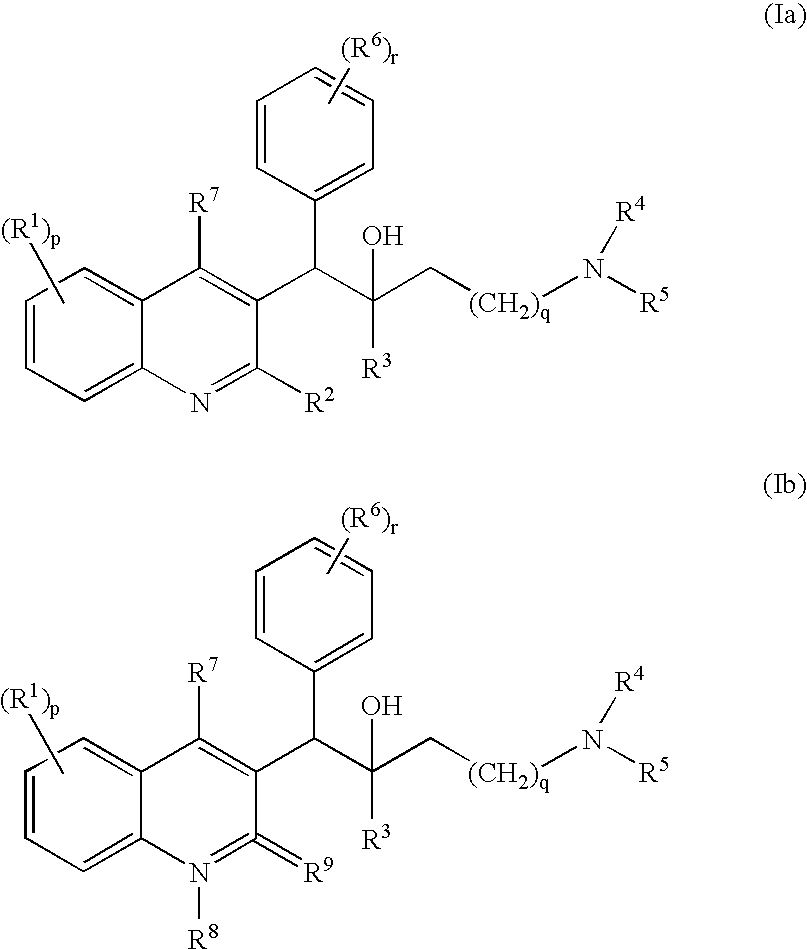
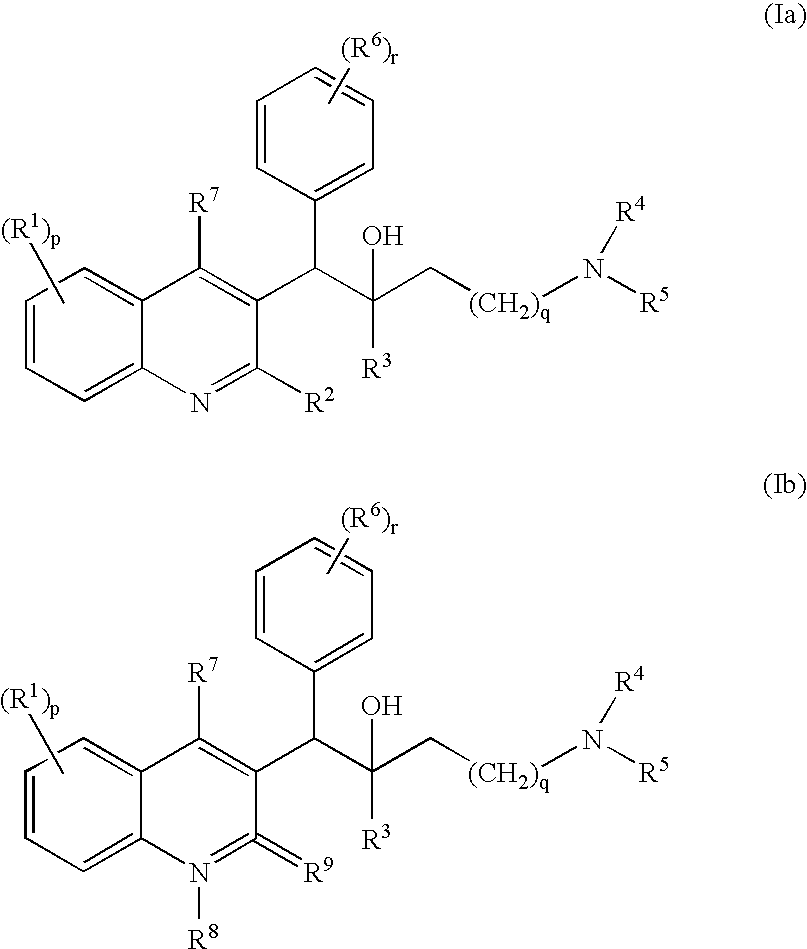
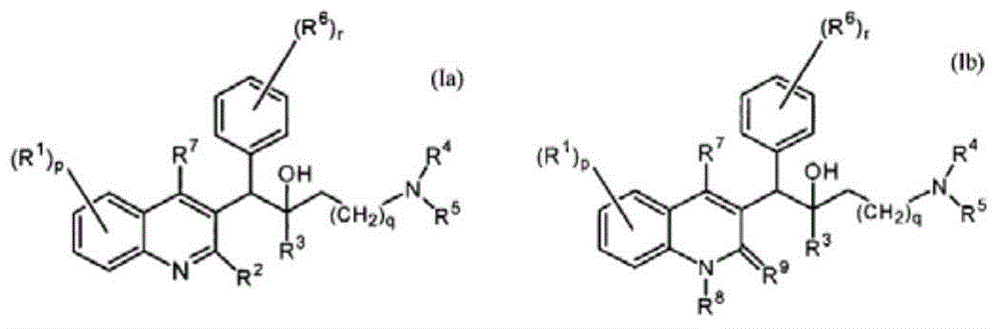
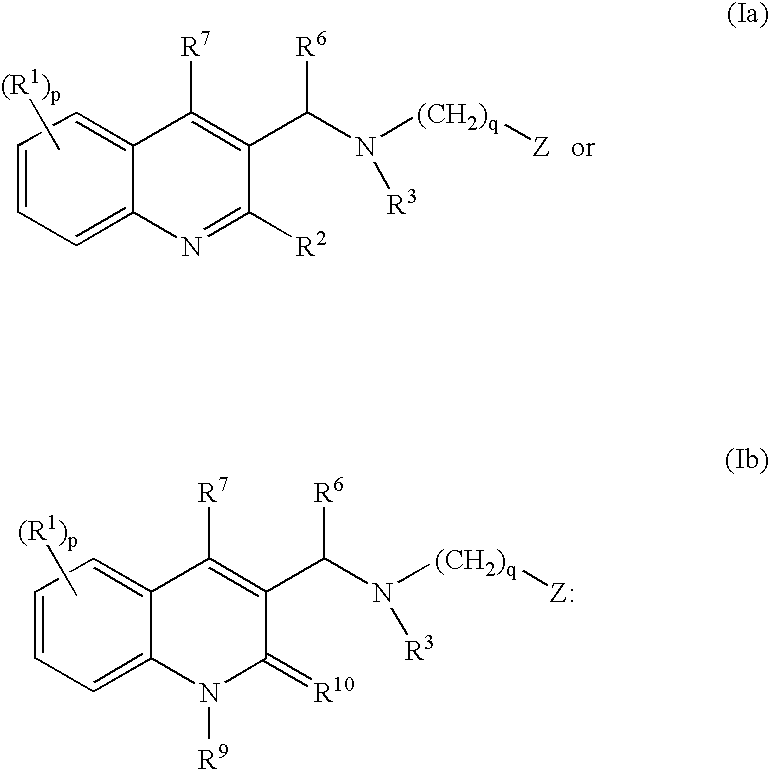
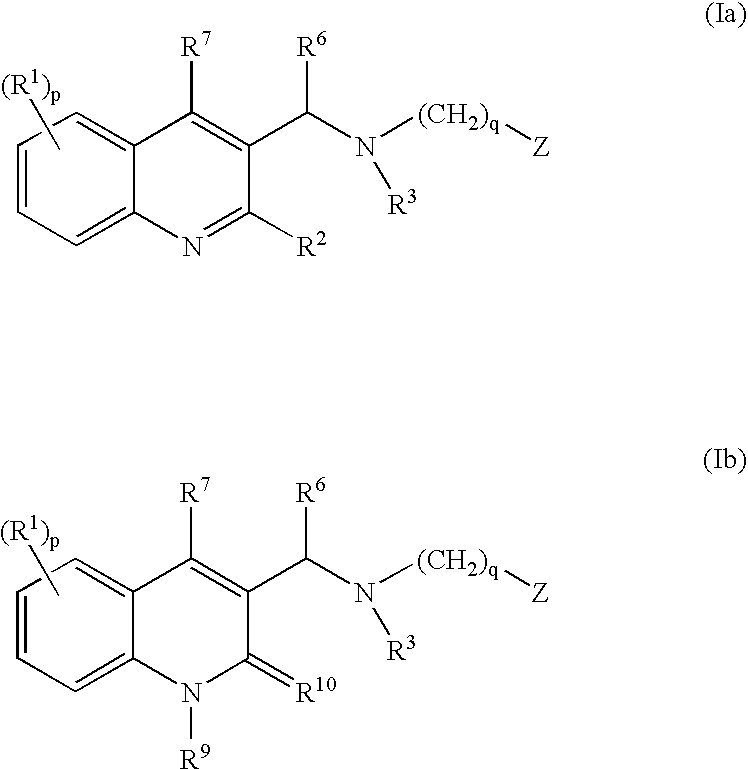
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or the general Formula (Ib)the pharmaceutically acceptable acid or base addition salts thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of mycobacterial diseases, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. In particular, compounds are claimed in which, independently from each other, R1 is bromo, p=1, R2 is alkyloxy, R3 is optionally substituted naphthyl or phenyl, q=1, R4 and R5 each independently are hydrogen, methyl or ethyl, R6 is hydrogen, r is equal to 0 or 1 and R7 is hydrogen. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of mycobacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Method for detecting pathogenic mycobacteria in clinical specimens
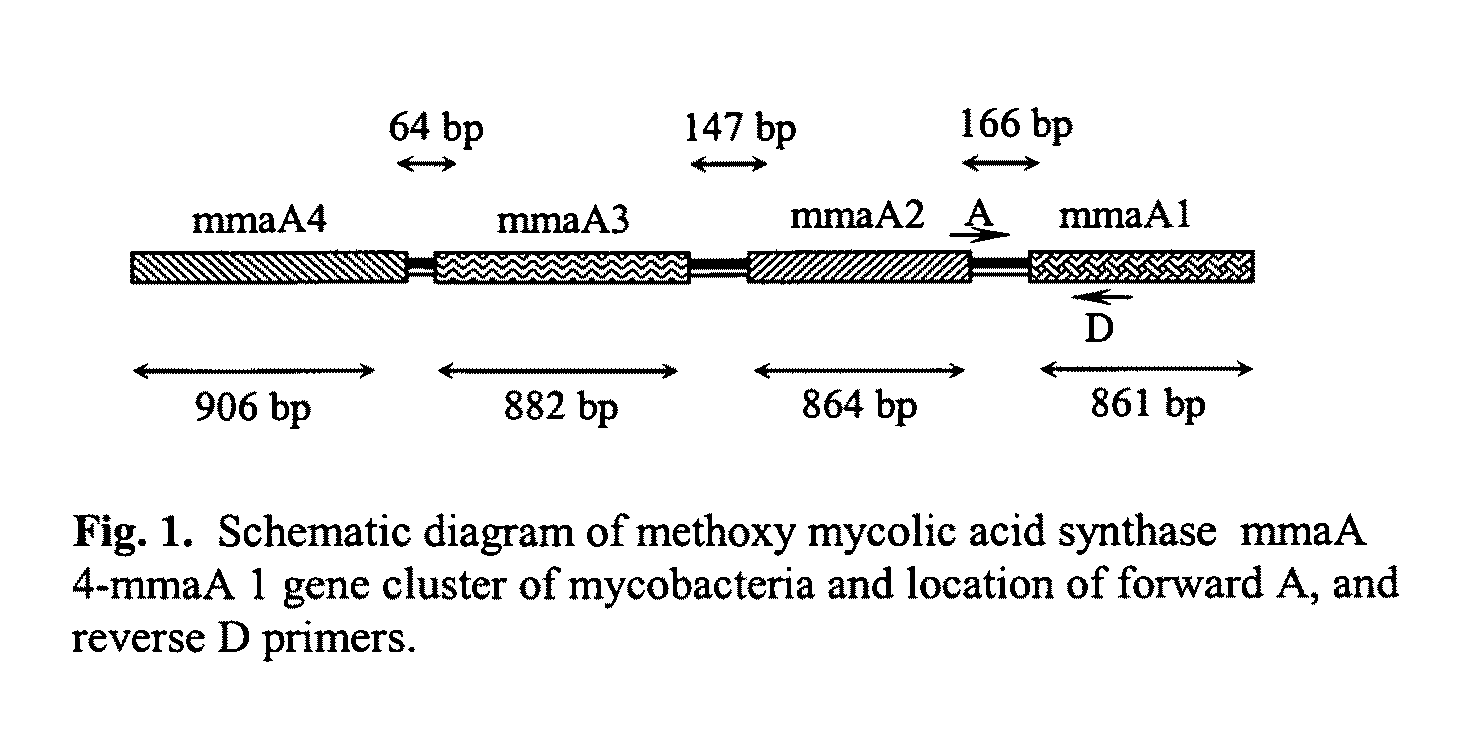
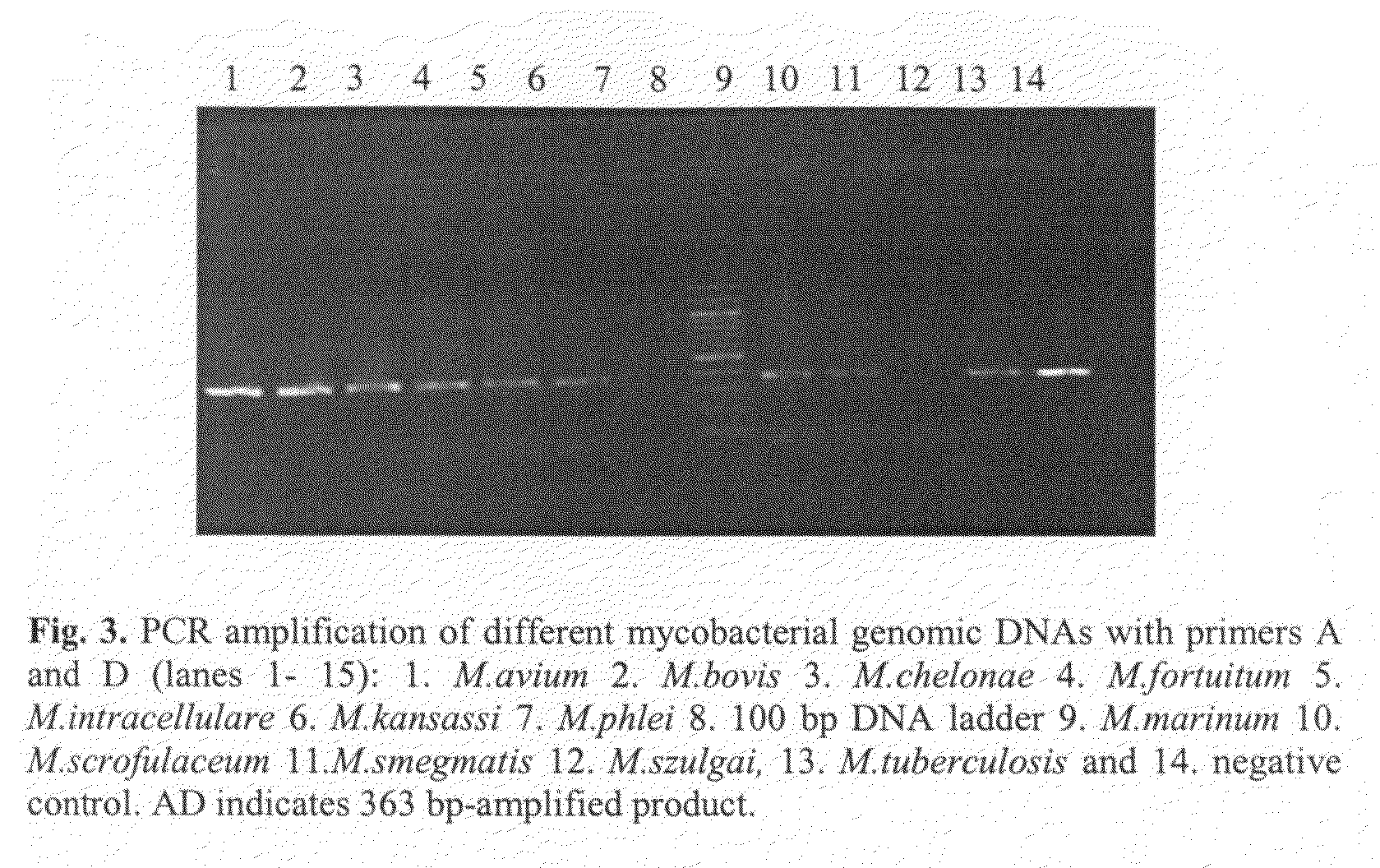
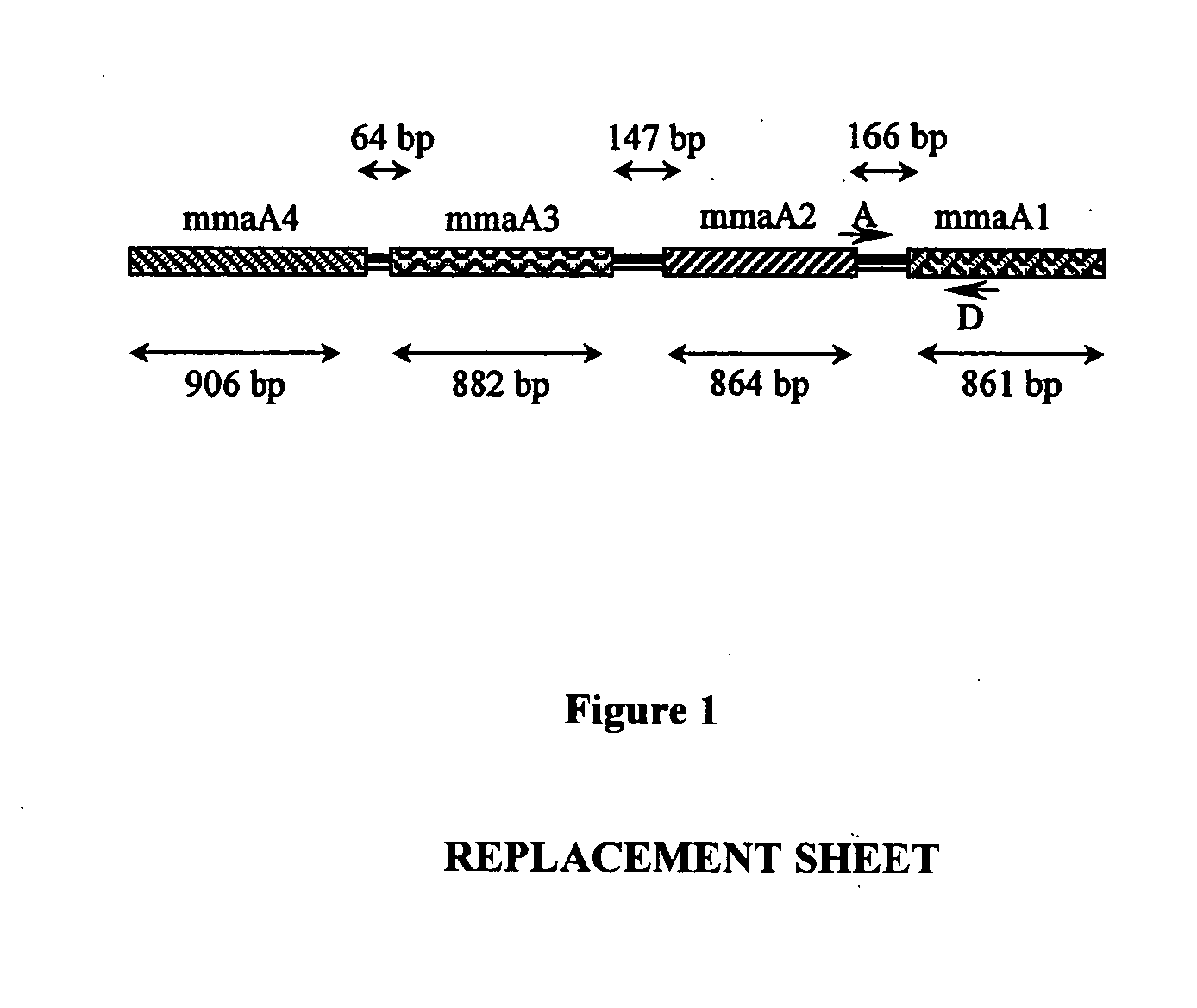
The present invention relates to detection of pathogenic mycobacteria in clinical specimens such as sputum, cerebrospinal fluid, gastric lavage and tissue biopsies etc., wherein the novel stretch of DNA that lies in the intergenic region between methyl mycolic acid synthase genes mmaA1 and mmaA2 and the flanking region in mmaA1 and mmaA2 genes and is the invention uses a pair of designed oligonucleotide primers that specifically amplifies the target DNA from the clinical specimens.
Owner:COUNCIL OF SCI & IND RES
Novel prime-boost combinations of attenuated mycobacterium
InactiveUS20090304750A1Effective protectionOrganic active ingredientsBacterial antigen ingredientsPrime boostMucosal vaccine
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:HONE DAVID +1
Detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and method thereof
ActiveCN101533018AStrong characteristicIncreased sensitivityHybrid peptidesMaterial analysisBCG immunizationMycobacterium Infections
The invention belongs to the field of immunodetection and relates to a detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and a method thereof. The detection reagent comprises combined fusion protein rE6-M63-H70 used as a specific stimulation origin, the combined fusion protein can effectively stimulate sensitized peripheral blood lymphocyte cultured in vitro to release Gamma-interferon (IFN-Gamma) at a high level. The cow IFN-Gamma release test established by using the detection reagent rE6-M63-H70 combined fusion protein as the stimulation origin overcomes the insufficiencies of serology detection method and the IFN-Gamma release test with PPD as the stimulation origin, thus enjoying very high sensitivity and specificity and distinguishing cow pathogenic mycobacteria ( such as mycobacterium bovis) infection from non-pathogenic mycobacteria (such as mycobacterium avium or non-pathogenic mycobacteria) infection and even distinguishing the cow pathogenic mycobacteria infection from BGG immunity; therefore, the detection kit and the method of the invention can be effectively used to detect the clinical cow pathogenic mycobacteria infection.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Multiplex polymerase chain reaction (mPCR)-denaturing high-performance liquid chromatography (DHPLC) primers and method for detecting and identifying mycobacterium
InactiveCN102808031ARapid identificationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlMycobacterium Infections
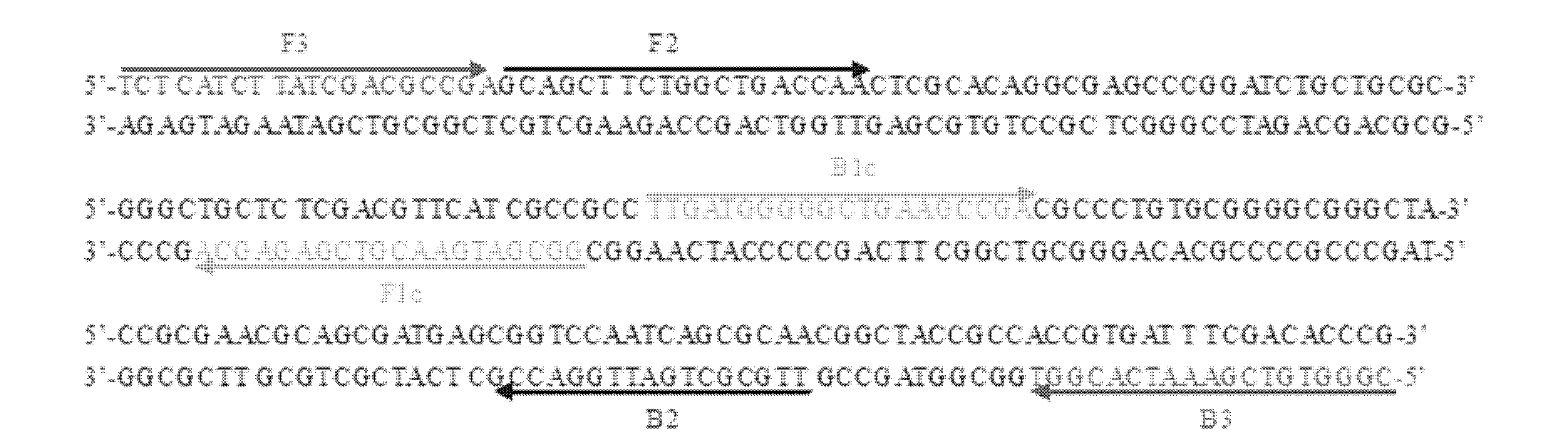
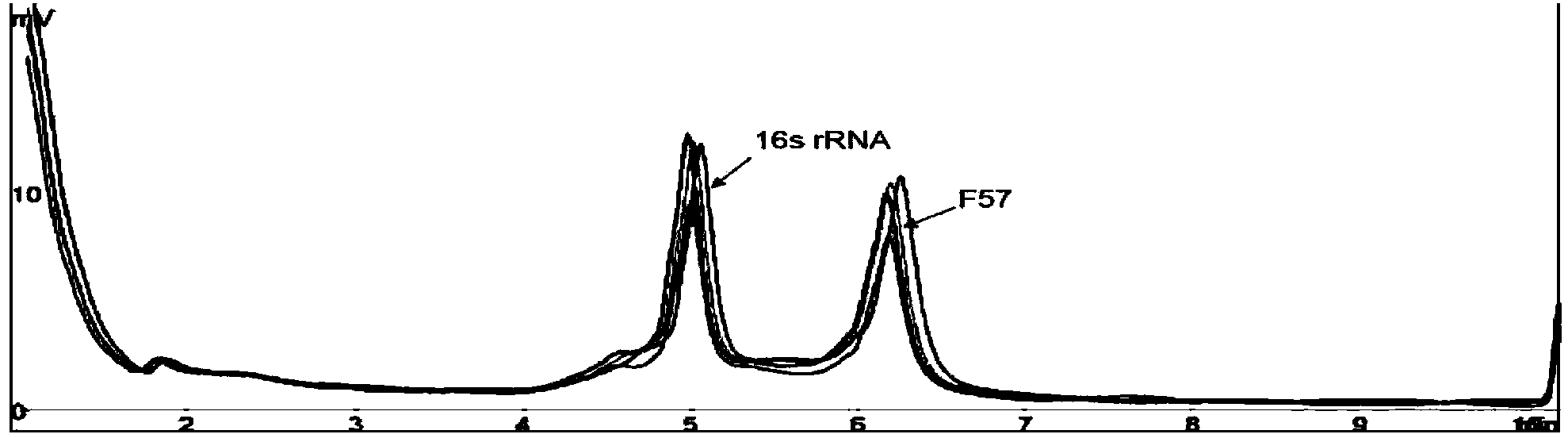
The invention provides a group of nucleic acids used in a quintuple multiplex polymerase chain reaction (mPCR)-denaturing high-performance liquid chromatography (DHPLC) method for detecting mycobacterium and identifying pathogenic mycobacterium. The nucleic acids comprise five pairs of primers of which the nucleic acid sequences are shown as SEQ ID No.1 and SEQ ID No.2, SEQ ID No.4 and SEQ ID No.5, SEQ ID No.7 and SEQ ID No.8, SEQ ID No.10 and SEQ ID No.11, and SEQ ID No.13 and SEQ ID No.14, and PCR amplification products which are used as positive control and of which the nucleic acid sequences are shown as SEQ ID No.3, SEQ ID No.5, SEQ ID No.9, SEQ ID No.12 and SEQ ID No.15. The invention also provides a kit using the nucleic acids and a detection method; the method is high in specificity and flexibility and easy to operate, and high flux can be achieved; and the method has important practical significance to clinical identification on the mycobacterium infection and infectious agents of the mycobacterium infection.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Immuno-fluorescent staining method for detecting mycobacterium tuberculosis in leukocytes and kit
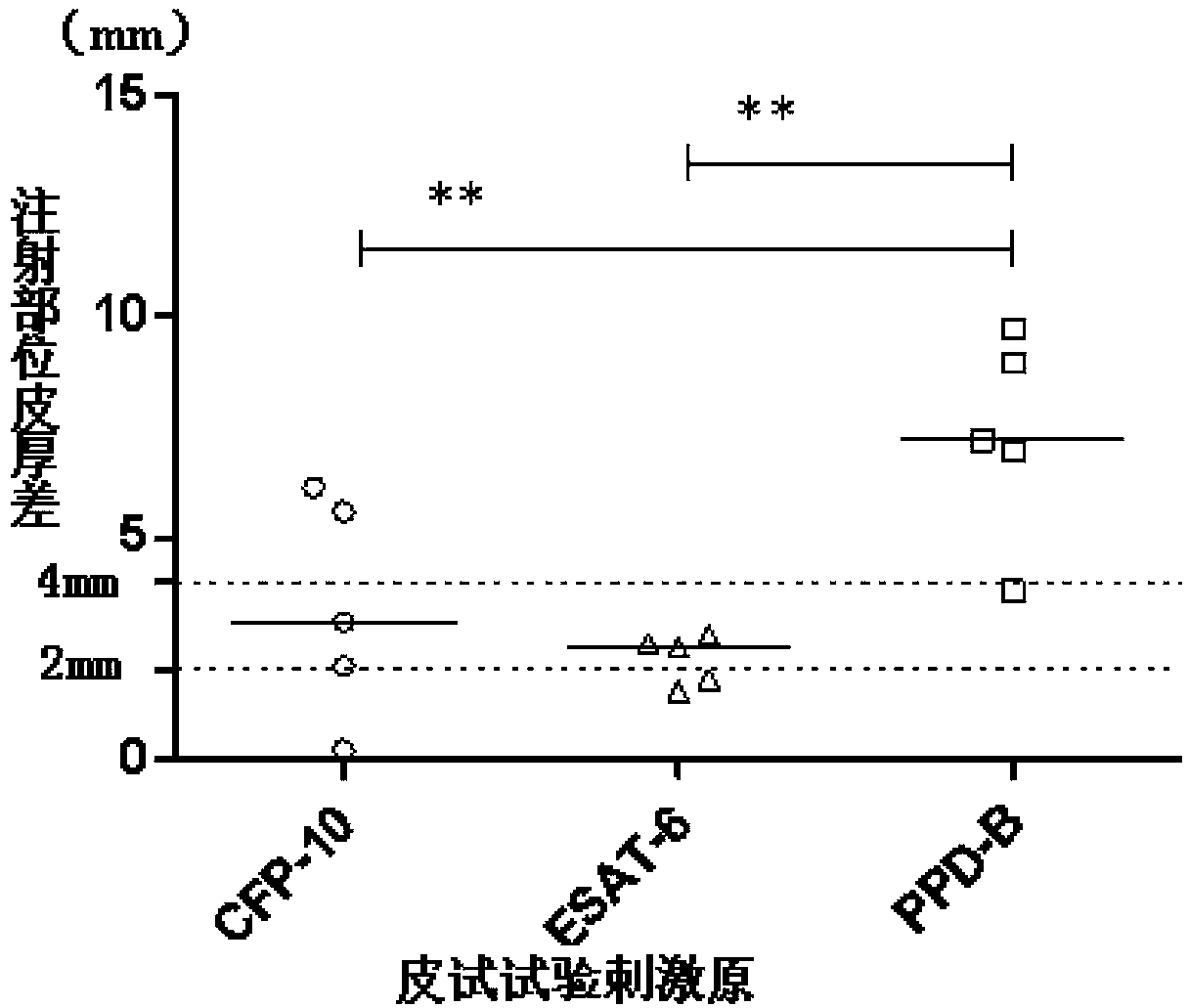
The invention discloses a method for rapidly detecting mycobacterium tuberculosis in blood (blood plasma and blood cells) through fluorescent staining and a kit.ESAT-6 and CFP-10 antigens of the mycobacterium tuberculosis in the blood are specifically detected by using monoclonal antibodies of RD-1 zone ESAT-6 and CFP-10 antigens of the mycobacterium and are used for etiologic detection and early-stage and rapid clinical diagnosis of tuberculosis diseases.According to the method, the blood is divided into the blood plasma and the blood cells, the mycobacterium tuberculosis enriched in the blood plasma is separated by using a micro-fluid device based on a membrane, meanwhile a slide is coated with hemocytes (leukocytes), and the mycobacterium tuberculosis in the blood plasma and the leukocytes are detected by using the monoclonal antibodies of the ESAT-6 and CFP-10 antigens and adopting direct and indirect fluorescent staining methods.The method is simple in operation, high in sensitivity and strong in specificity and is a definite tuberculosis etiology diagnosis method, and the kit for detecting the mycobacterium tuberculosis in the blood is developed.In addition, by means of the method, pathogenic mycobacterium tuberculosis infection can be detected, and theoretically non-pathogenic mycobacterium tuberculosis produced in blood due to bacillus calmette guerin vaccine inoculation can be also distinguished.
Owner:肖乐义
Novel polynucleotides and polypeptides in pathogenic mycobacteria and their use as diagnostics, vaccines and targets for chemotherapy
InactiveUS20040260078A1Sugar derivativesPeptide/protein ingredientsReading Frames (Nucleotide Sequence)Nucleotide
The invention provides a nucleotide sequence representing a pathogenicity island found in species of pathogenic mycobacteria. The islands are shown as SEQ ID NOS: 3 and 4 and comprises several open reading frames encoding polypeptides. These polypeptides and their use in diagnosis and therapy form a further aspect of the invention.
Owner:ST GEORGES HOSPITAL MEDICAL SCHOOL
Use of bacterial beta-lactamase for in vitro diagnostics and in vivo imaging, diagnostics and therapeutics
ActiveUS20140127712A1Decrease in gamma emissionEffective conditioningBioreactor/fermenter combinationsBiological substance pretreatmentsBiologyPathogenic mycobacterium
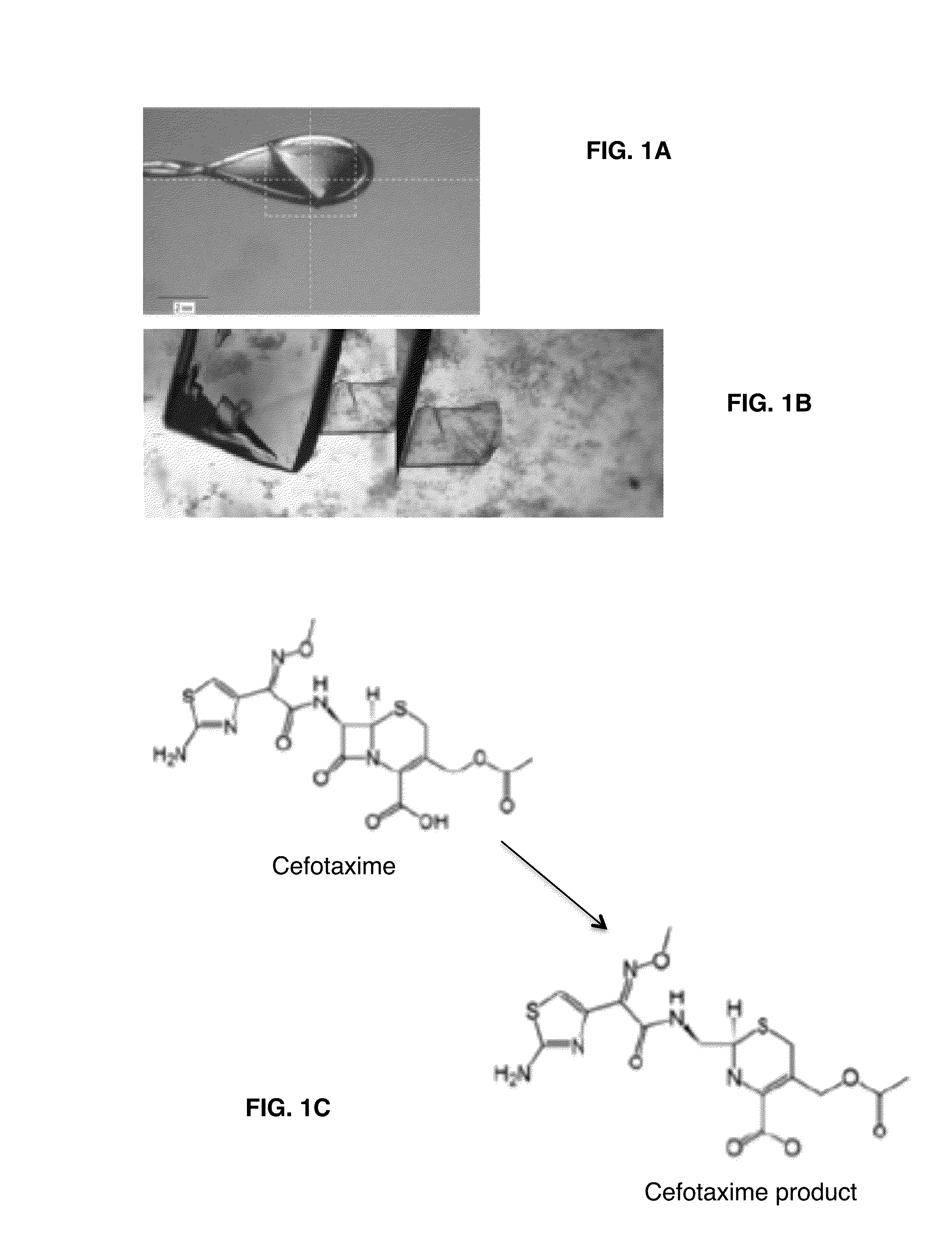
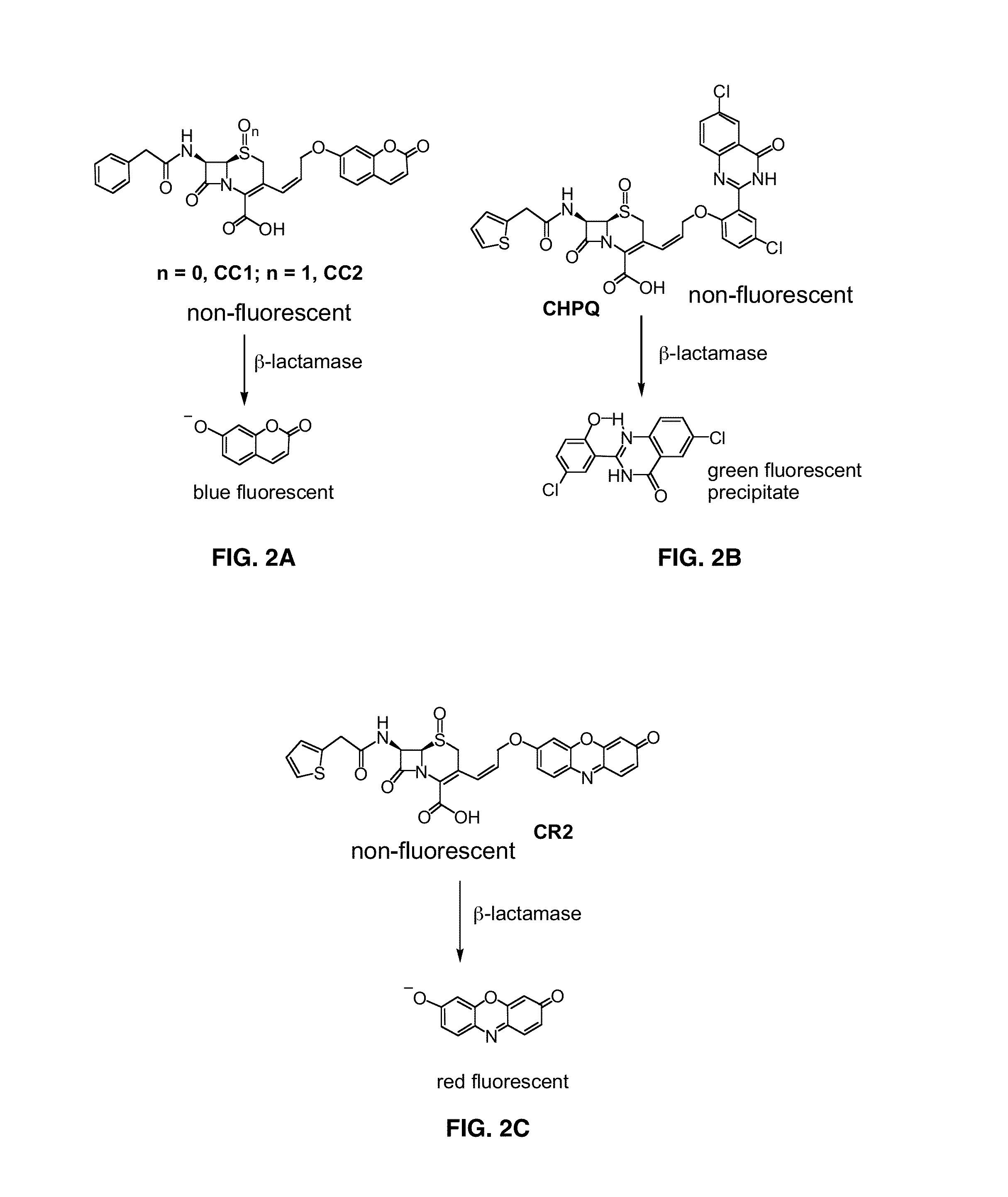
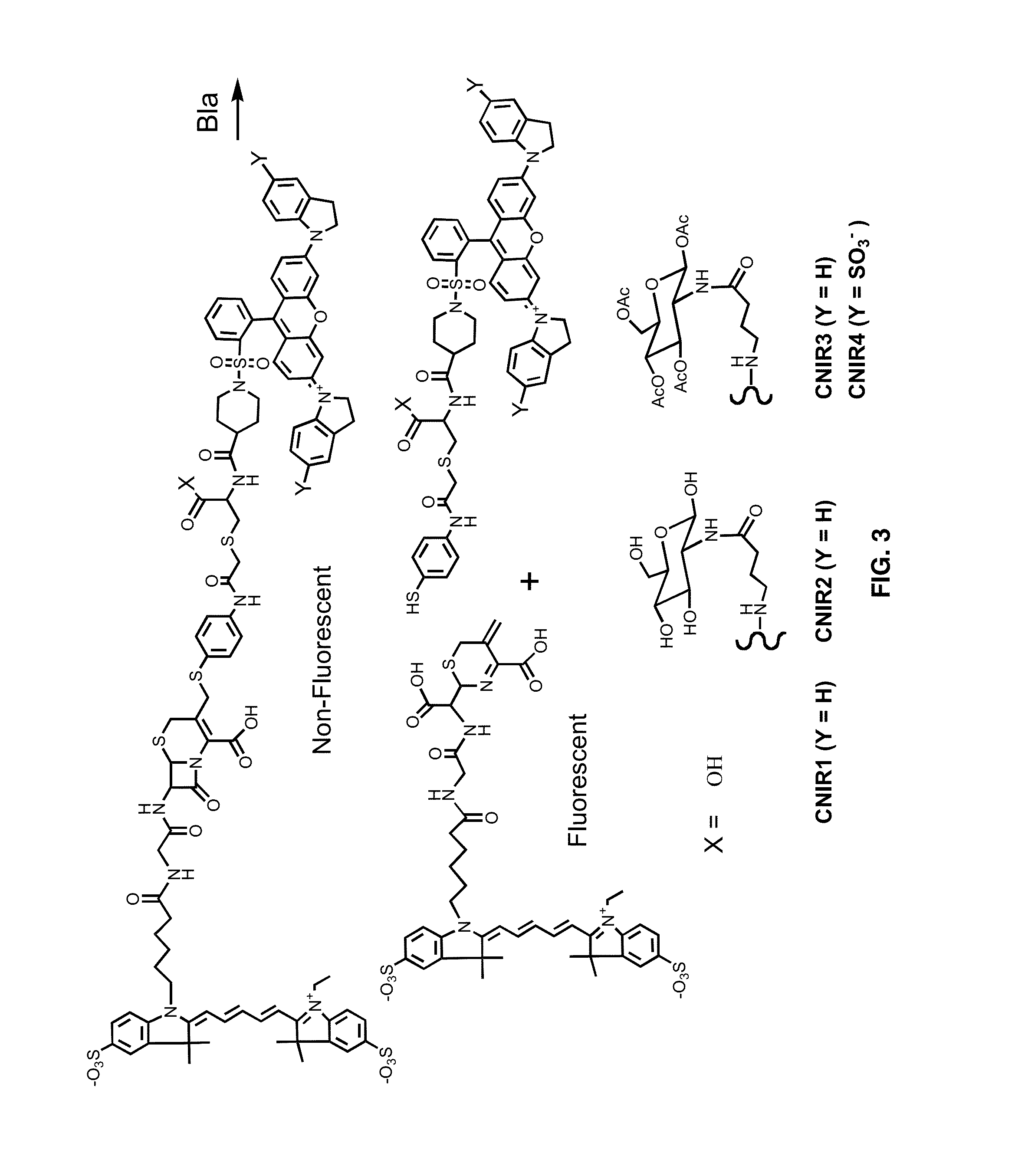
Provided herein are assays and in vitro methods to determine susceptibility to a drug effective against a pathogenic bacteria, for example, a pathogenic Mycobacteria, that has a beta-lactamase activity. An excitation wavelength is delivered to a biological sample obtained from a subject having an infection from the pathogenic bacteria in the presence of a beta-lactamase substrate. The intensity of a signal, such as a fluorescent, luminescent or colorimetric signal, at an emission wavelength of a product of the beta-lactamase on the subject is correlated to drug susceptibility. Also provided is an assay system for monitoring drug susceptibility of a pathogenic bacteria comprising color-producing substrates for a beta-lactamase of the pathogenic bacteria, an assay device for visibly detecting a product of the beta-lactamase on the substrate thereof and a reader configured to quantify the visibly detected product.
Owner:TEXAS A&M UNIVERSITY
Pyridine derivative and application thereof to mycobacterium resistance
ActiveCN105330595AGrowth inhibitionAntibacterial agentsOrganic chemistryMycobacterium marinumMycobacterium species
The invention discloses a preparation method and application of a series of novel pyridine derivatives. The derivatives can be used for the treatment of related diseases caused by mycobacterium species, especially for diseases caused by pathogenic mycobacterium, such as Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium avium and Mycobacterium marinum.
Owner:SHANGHAI JIA TAN PHARMATECH CO LTD +1
Method for detecting pathogenic mycobacteria in clinical specimens
InactiveUS20050123928A1Sugar derivativesMicrobiological testing/measurementTissue biopsyGastric lavage
The present invention relates to detection of pathogenic mycobacteria in clinical specimens such as sputum, cerebrospinal fluid, gastric lavage and tissue biopsies etc., wherein the novel stretch of DNA that lies in the intergenic region between methyl mycolic acid synthase genes mmaA1 and mmaA2 and the flanking region in mmaA1 and mmaA2 genes and is the invention uses a pair of designed oligonucleotide primers that specifically amplifies the target DNA from the clinical specimens
Owner:COUNCIL OF SCI & IND RES
Novel prime-boost combinations of attenuated mycobacterium
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:AERAS GLOBAL TB VACCINE FOUND
Mycobacterium bovis infection detection kit meditated by recombined fusion protein and method thereof
ActiveCN101533017AInfection Effectively DistinguishesStrong characteristicMaterial analysisBCG immunizationImmuno detection
The invention belongs to the field of immunodetection and relates to a mycobacterium bovis infection detection kit meditated by recombined fusion protein and a method thereof. The detection reagent comprises combined fusion protein rE6-M63-H65 used as a specific stimulation origin; the combined fusion protein can effectively stimulate sensitized peripheral blood lymphocyte cultured in vitro to release Gamma interferon (IFN-Gamma) at a high level. The cow IFN-Gamma release test established by using the detection reagent rE6-M63-H65 combined fusion protein as the stimulation origin overcomes the insufficiencies of serology detection method and the IFN-Gamma release test with PPD as the stimulation origin, thus enjoying very high sensitivity and specificity and distinguishing mycobacterium bovis infection from mycobacterium avium or non-pathogenic mycobacteria infection and even distinguishing the mycobacterium bovis infection from BGG immunity; therefore, the detection kit and the method of the invention can be effectively used to detect the mycobacterium bovis infection.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
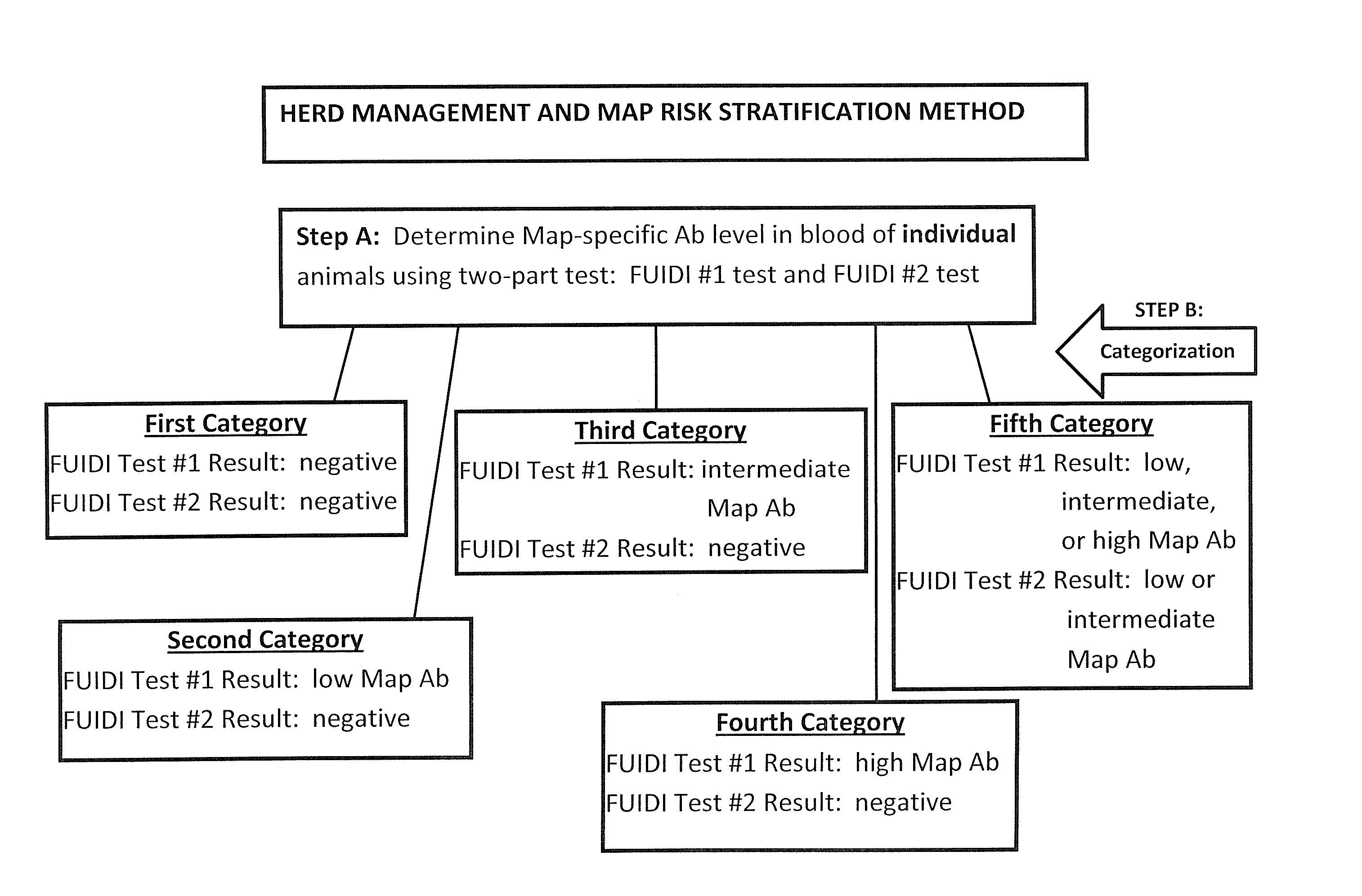
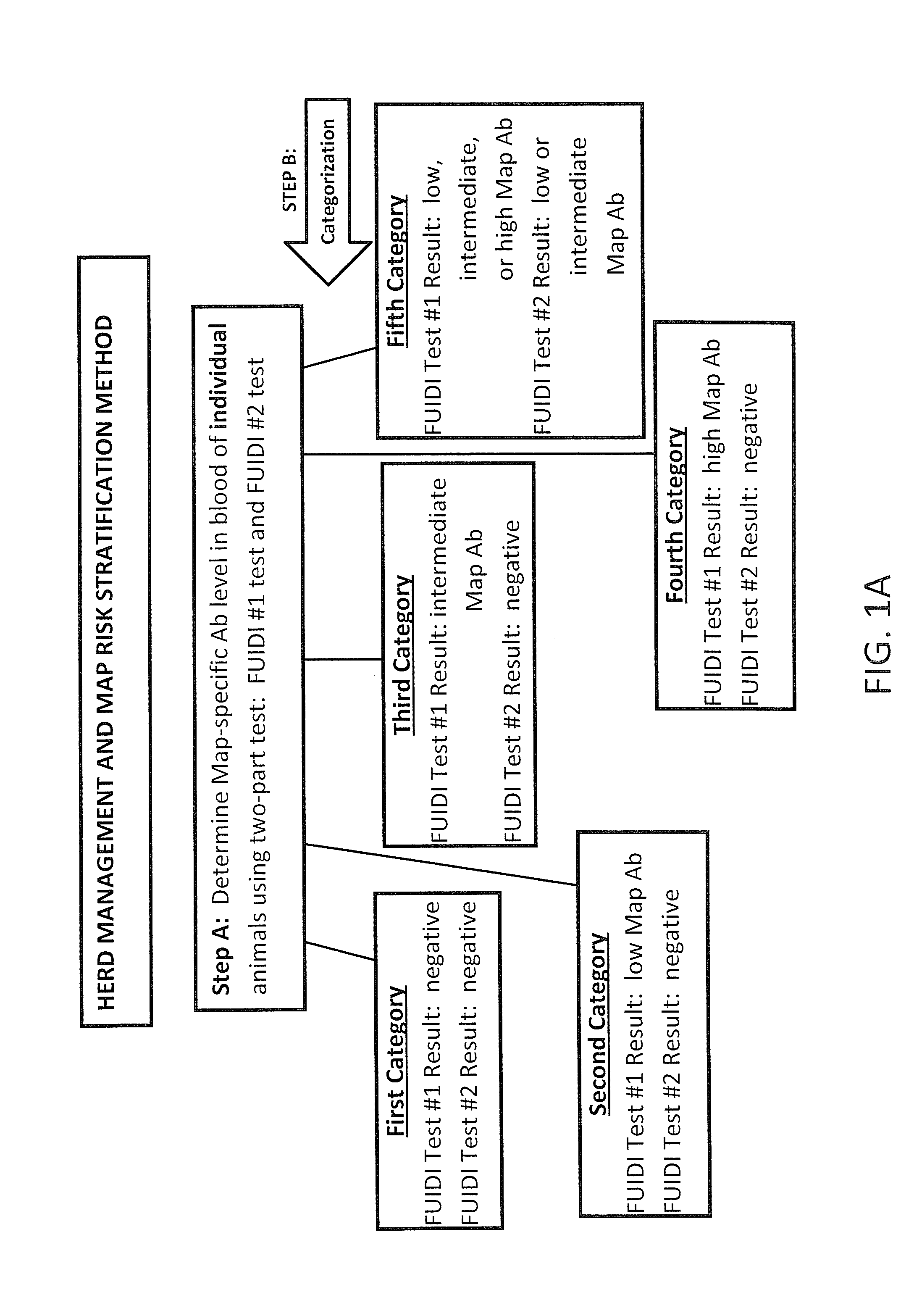
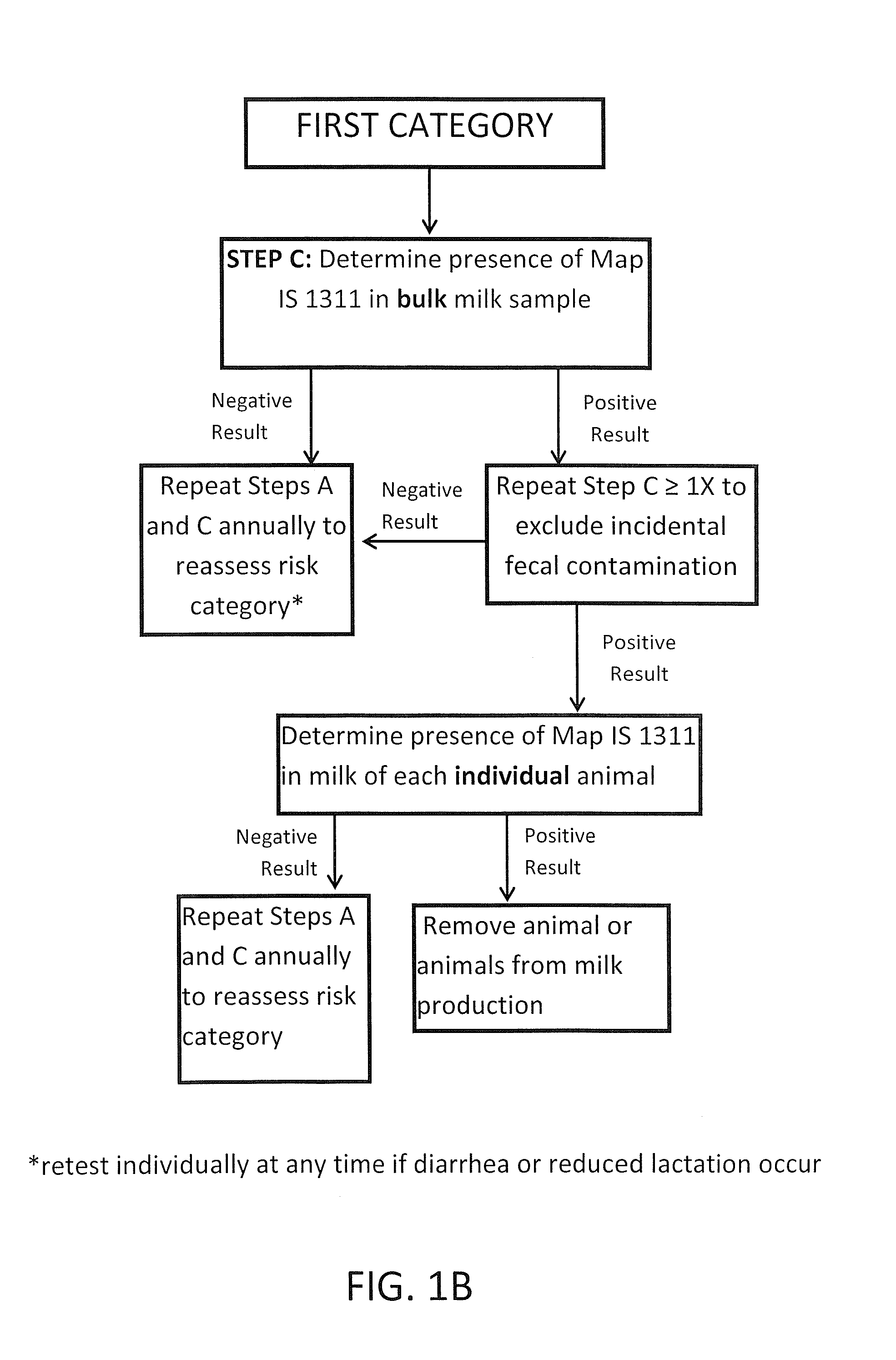
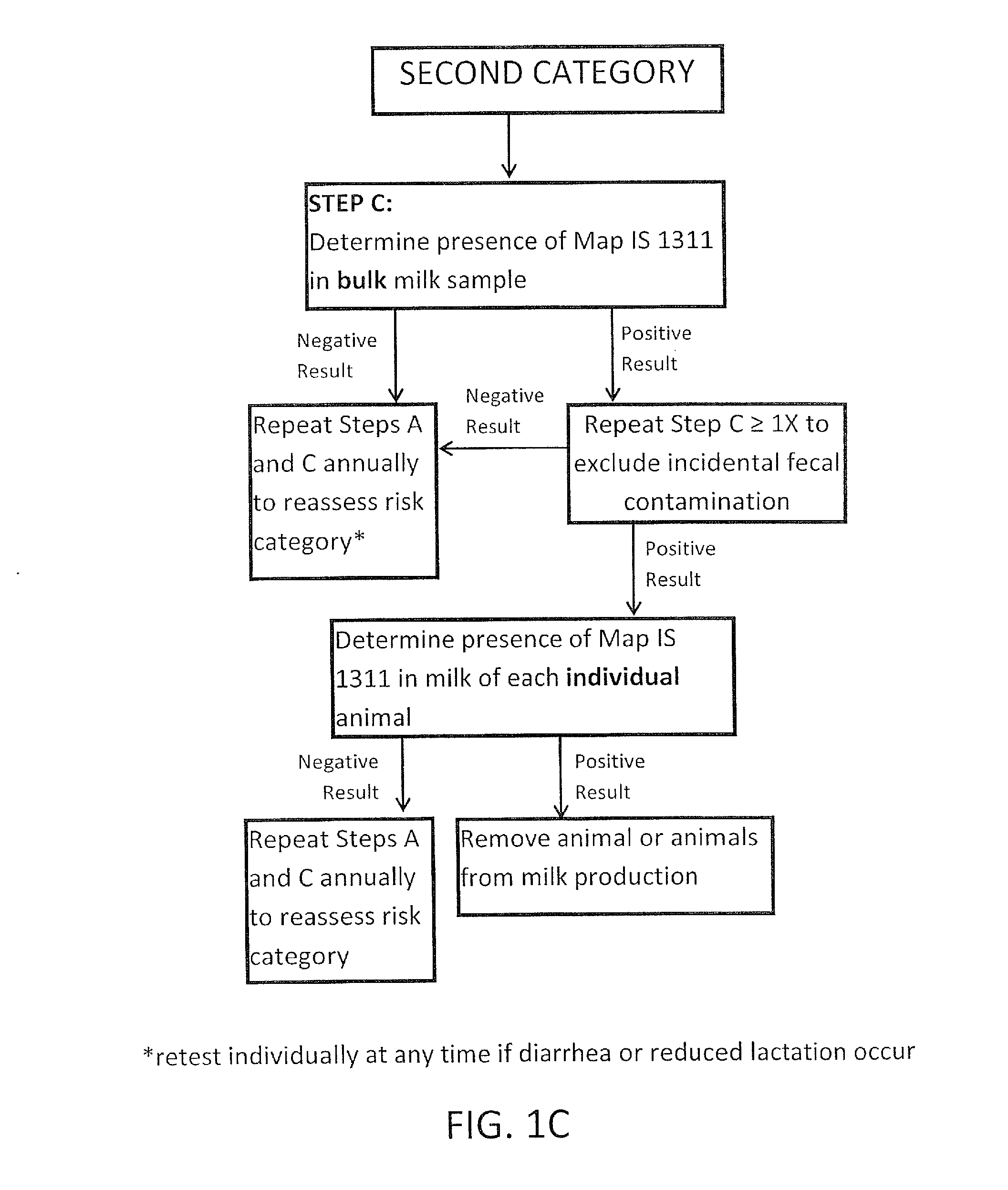
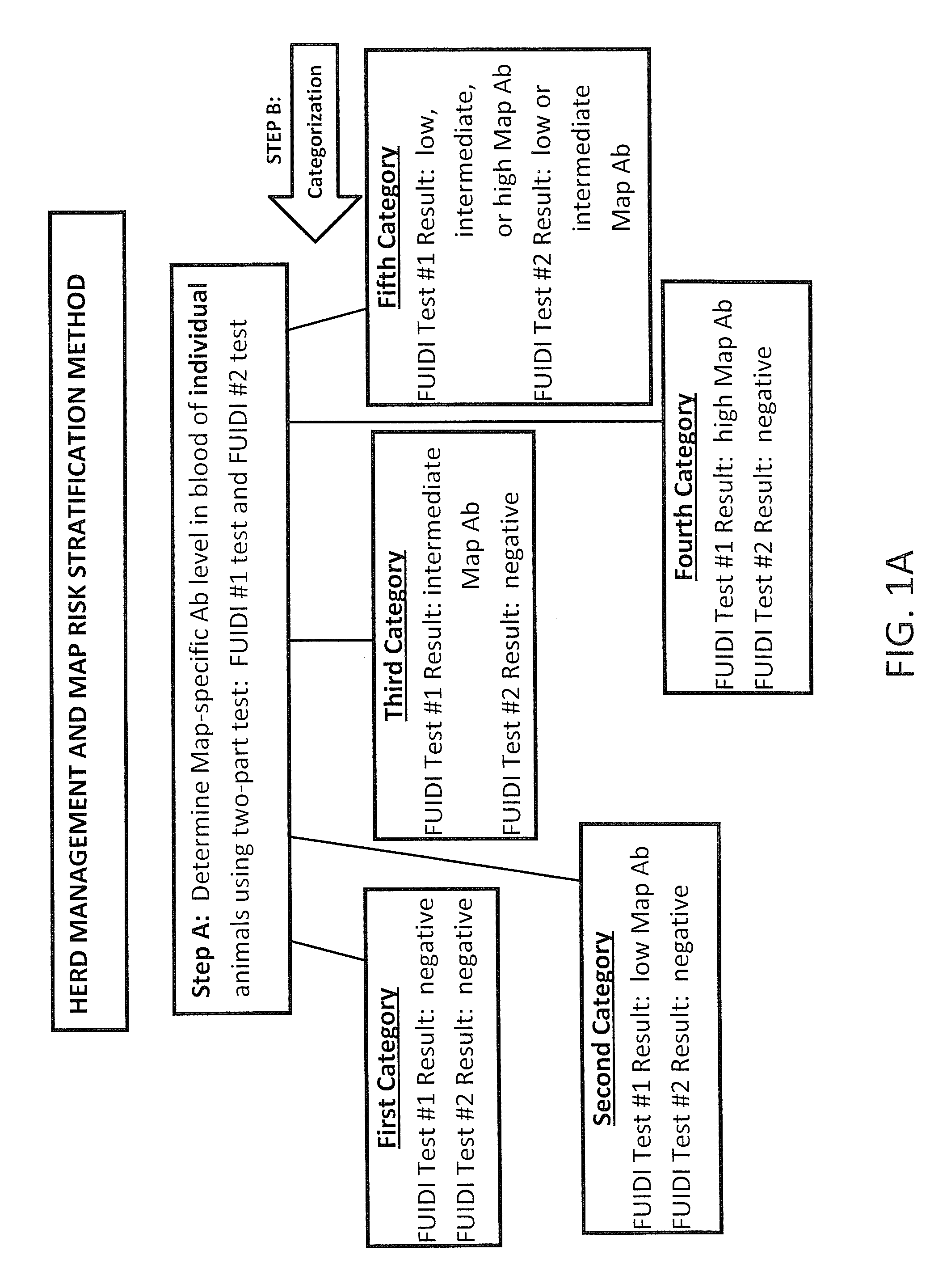
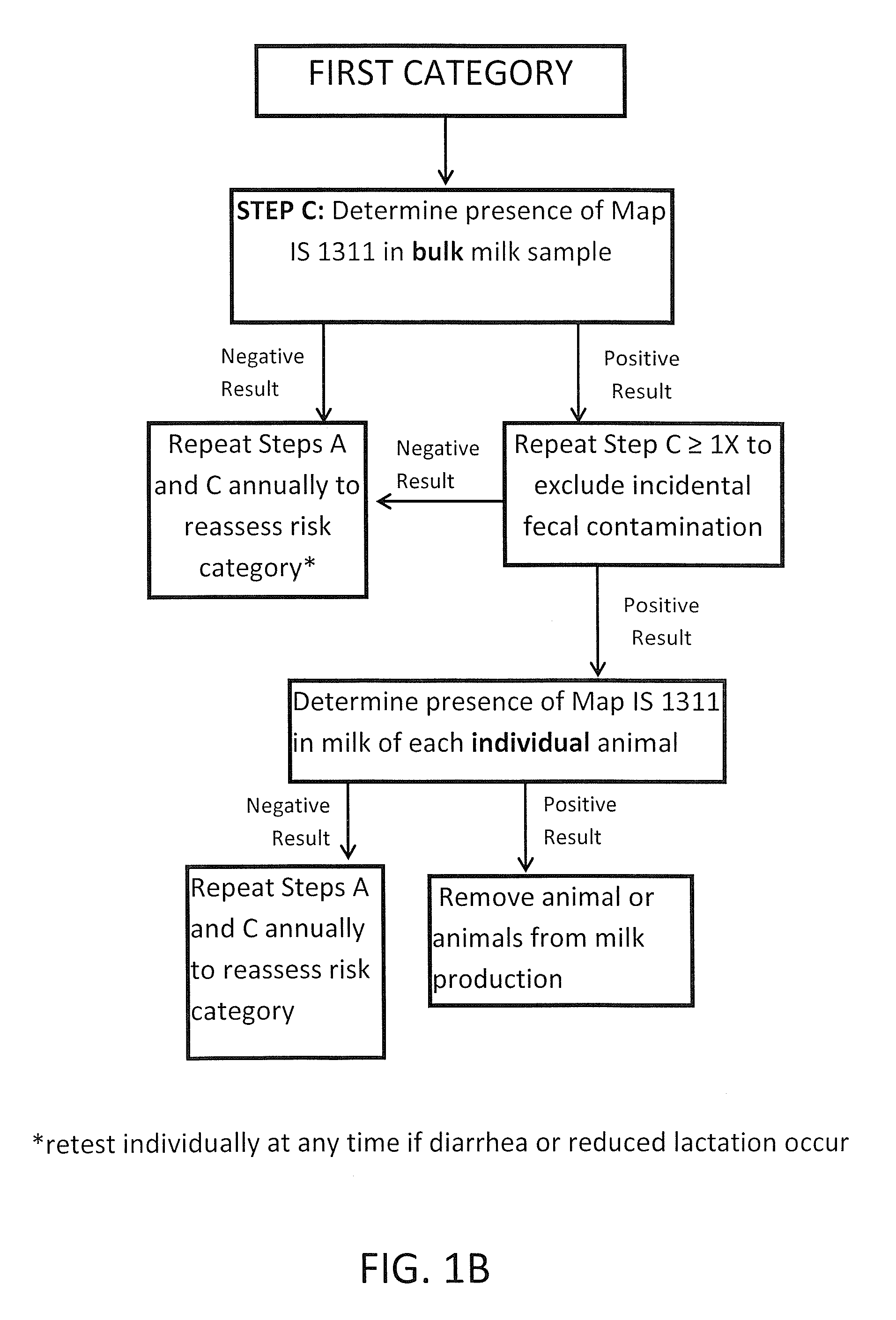
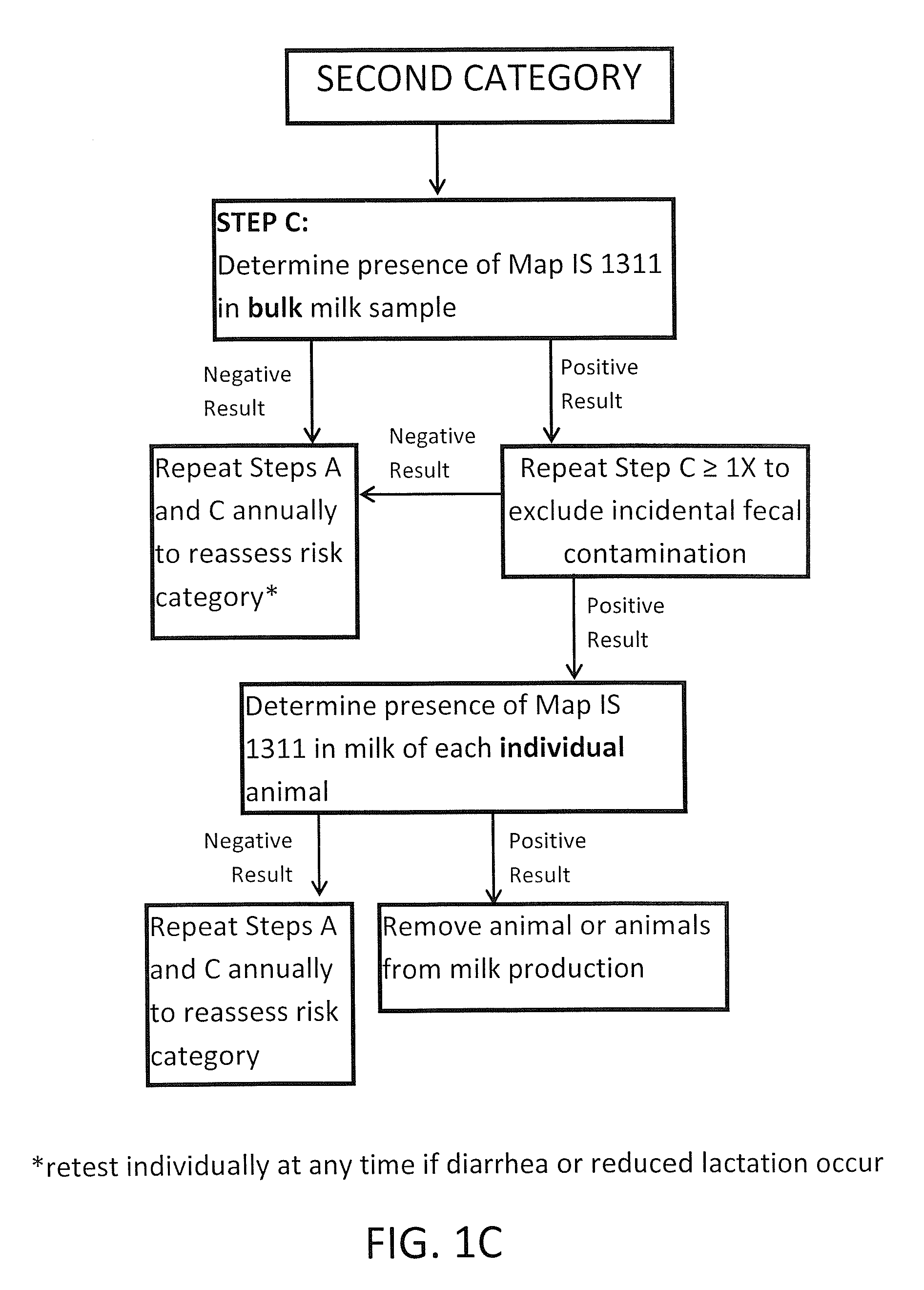
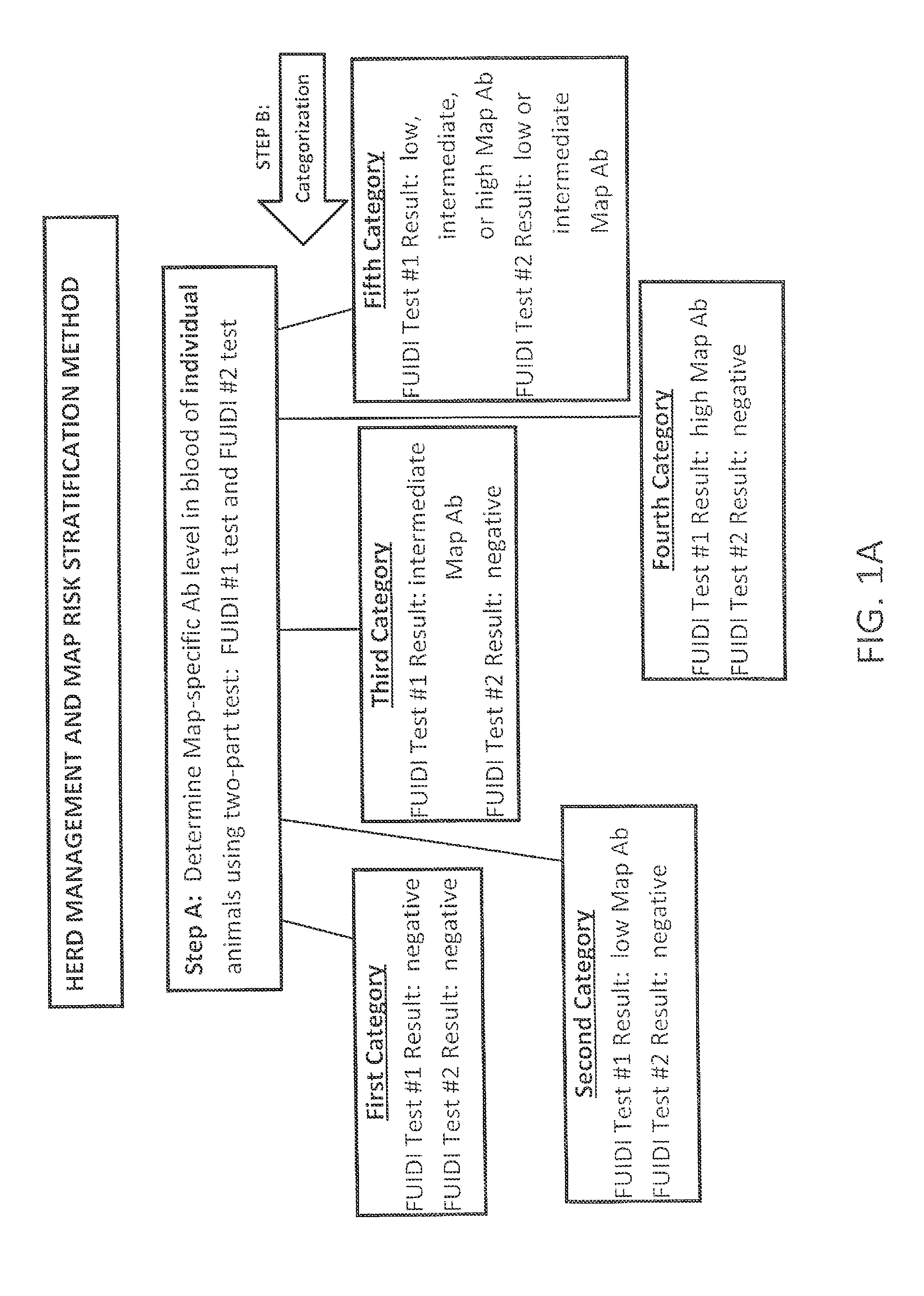
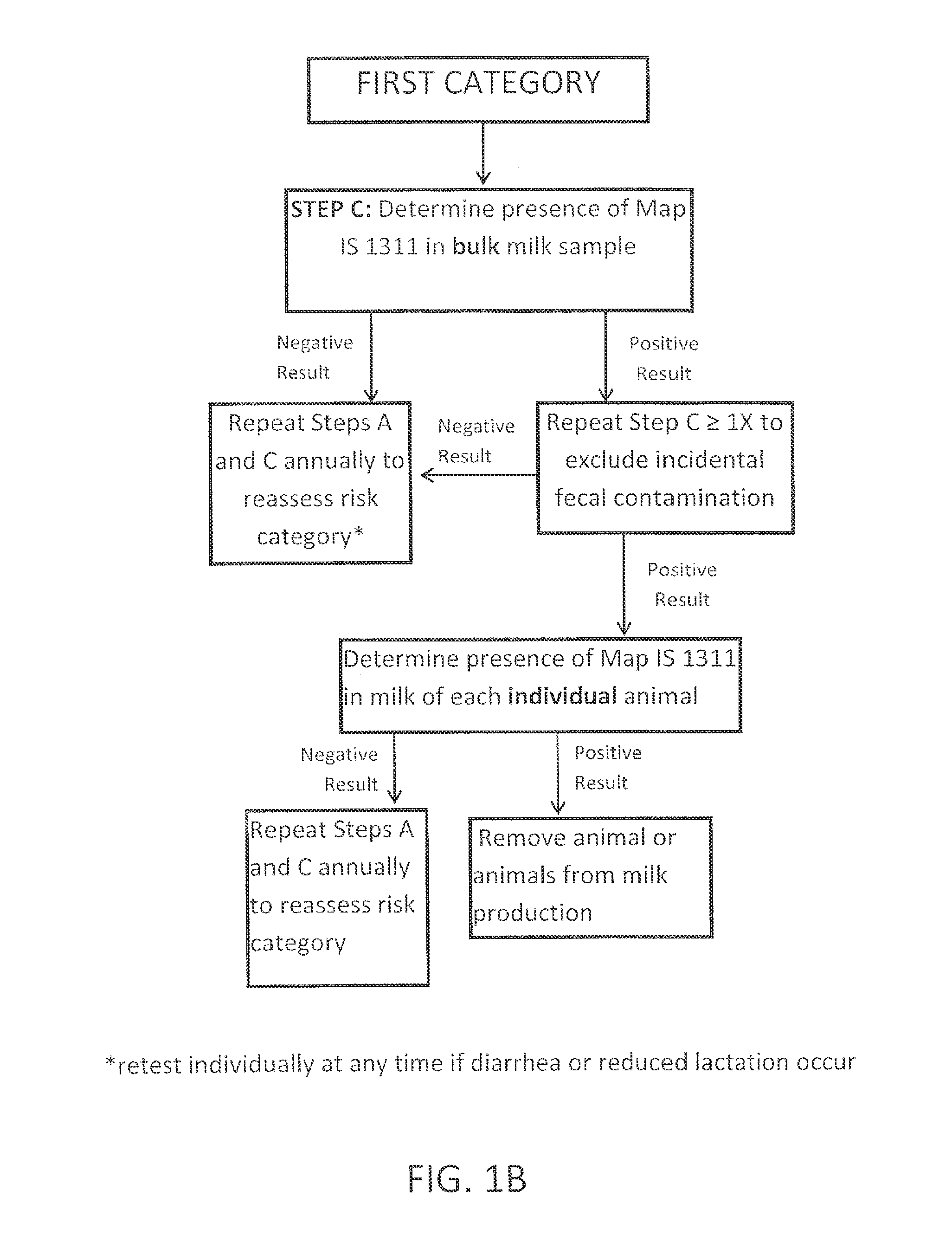
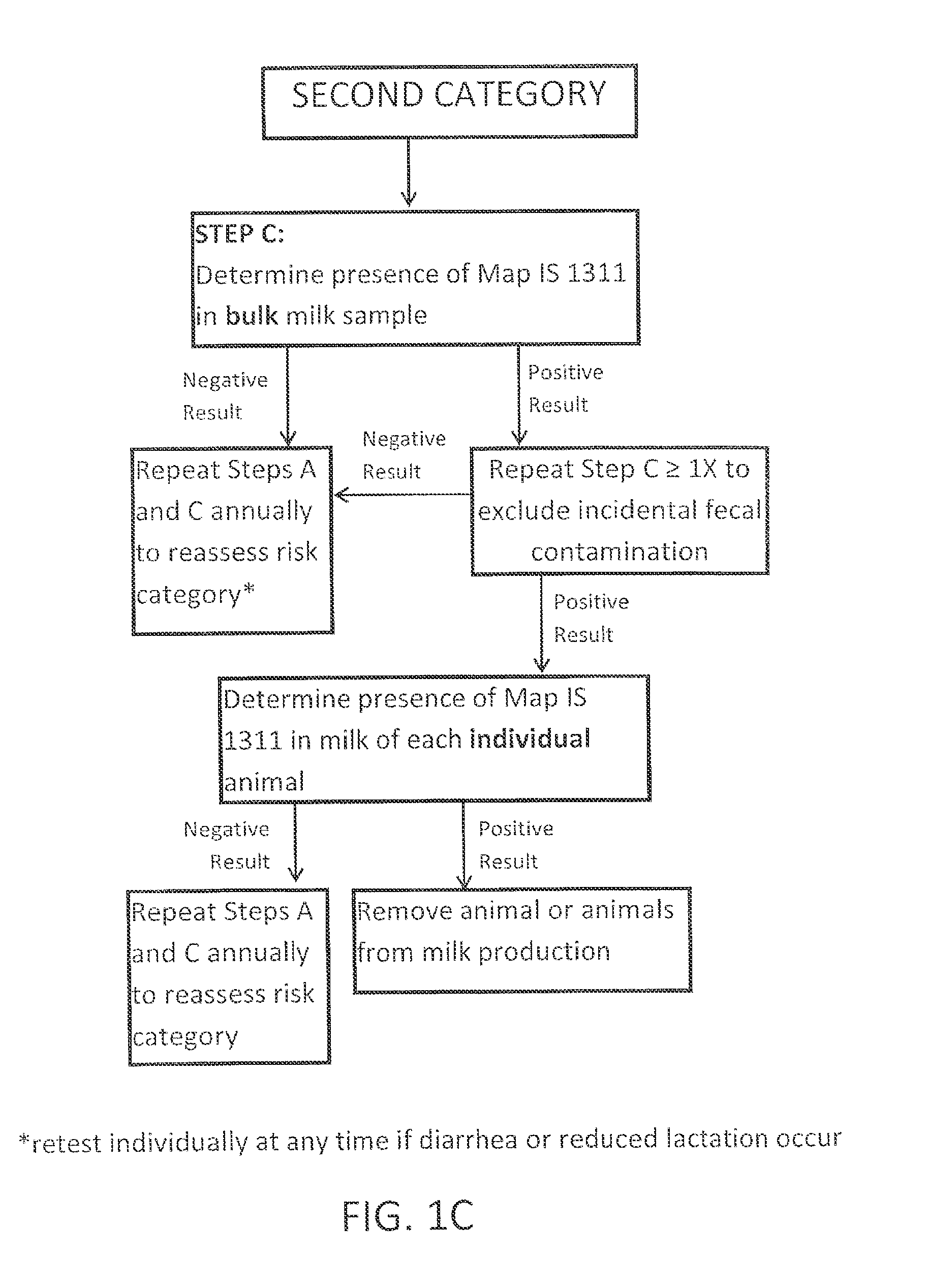
Fuidi herd management and risk stratification methods
InactiveUS20140116352A1Enhanced genetic abilityMicrobiological testing/measurementBiological testingCvd riskBiology
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp.paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
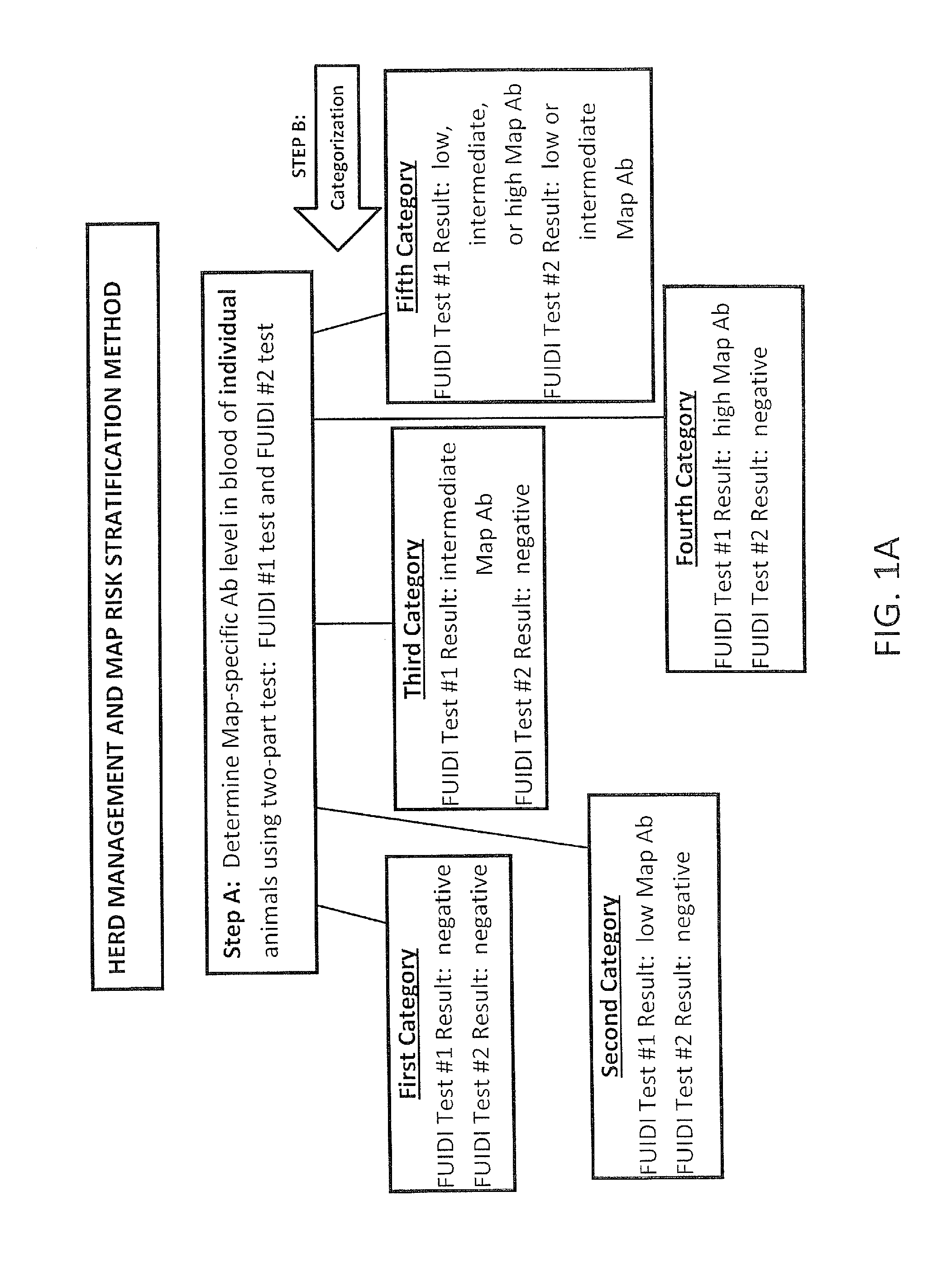
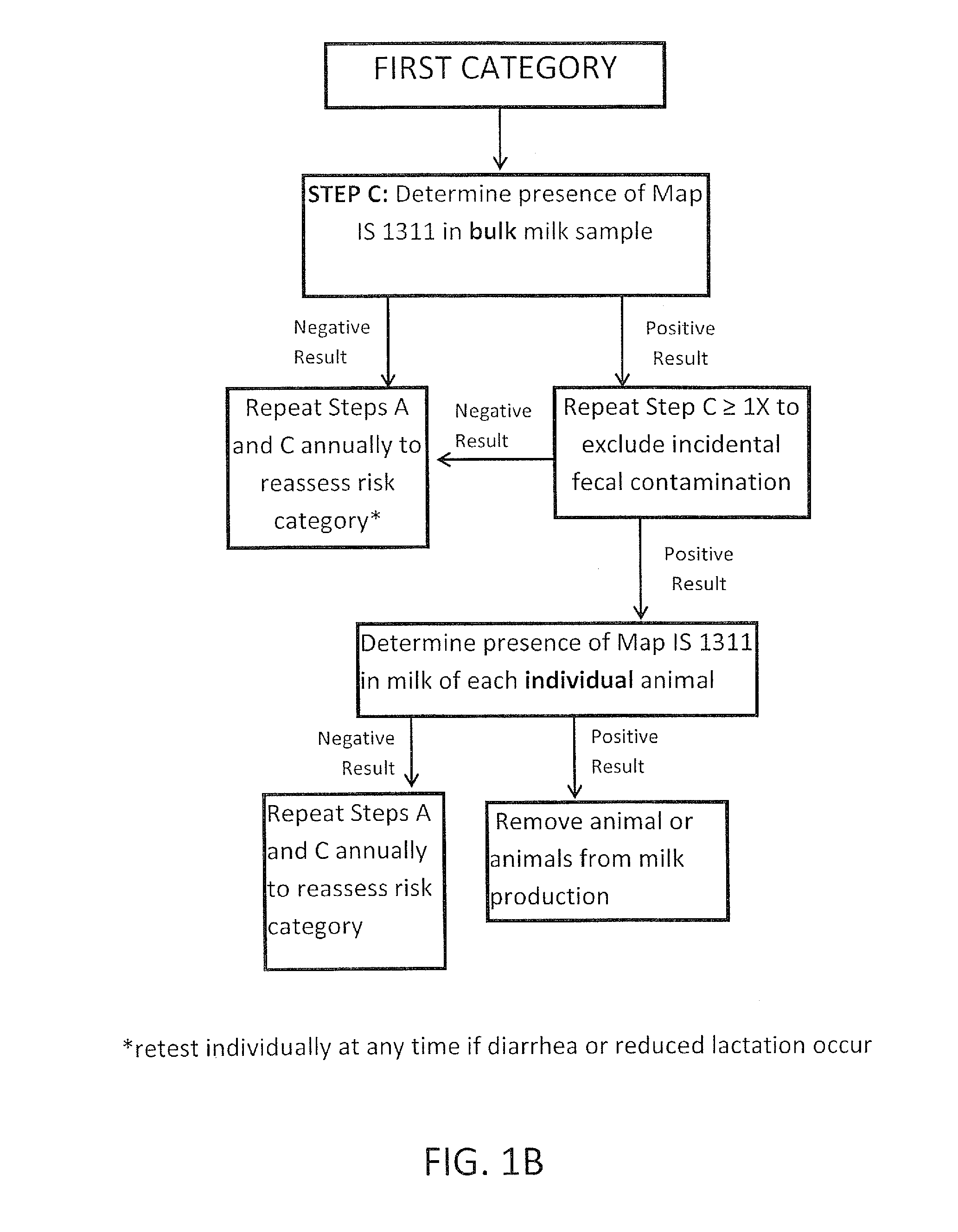
Fuidi herd management and risk stratification methods
InactiveUS20140116353A1Reduce the amount requiredEnhanced genetic abilityMicrobiological testing/measurementBiological testingCvd riskHerd management
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp. paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
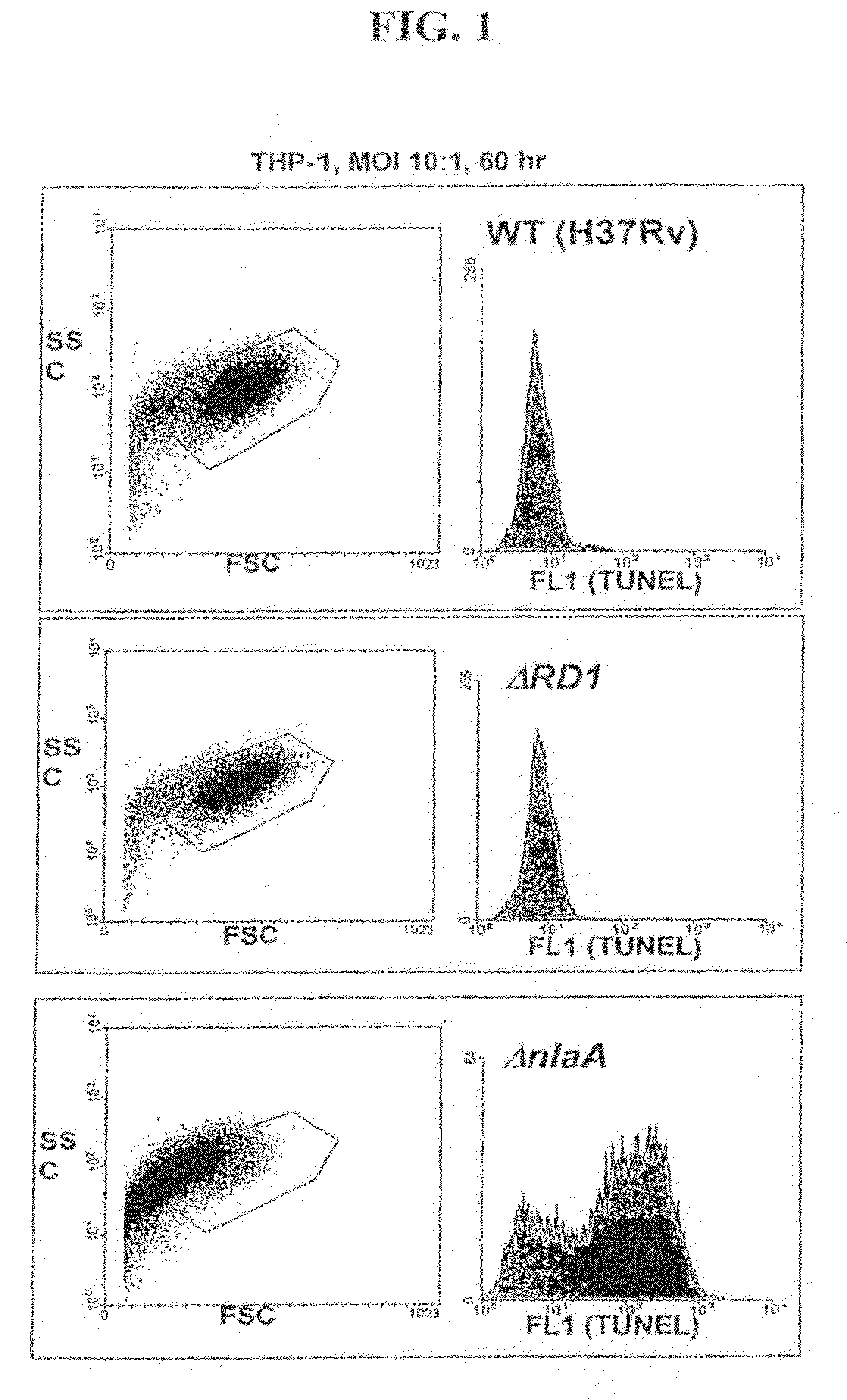
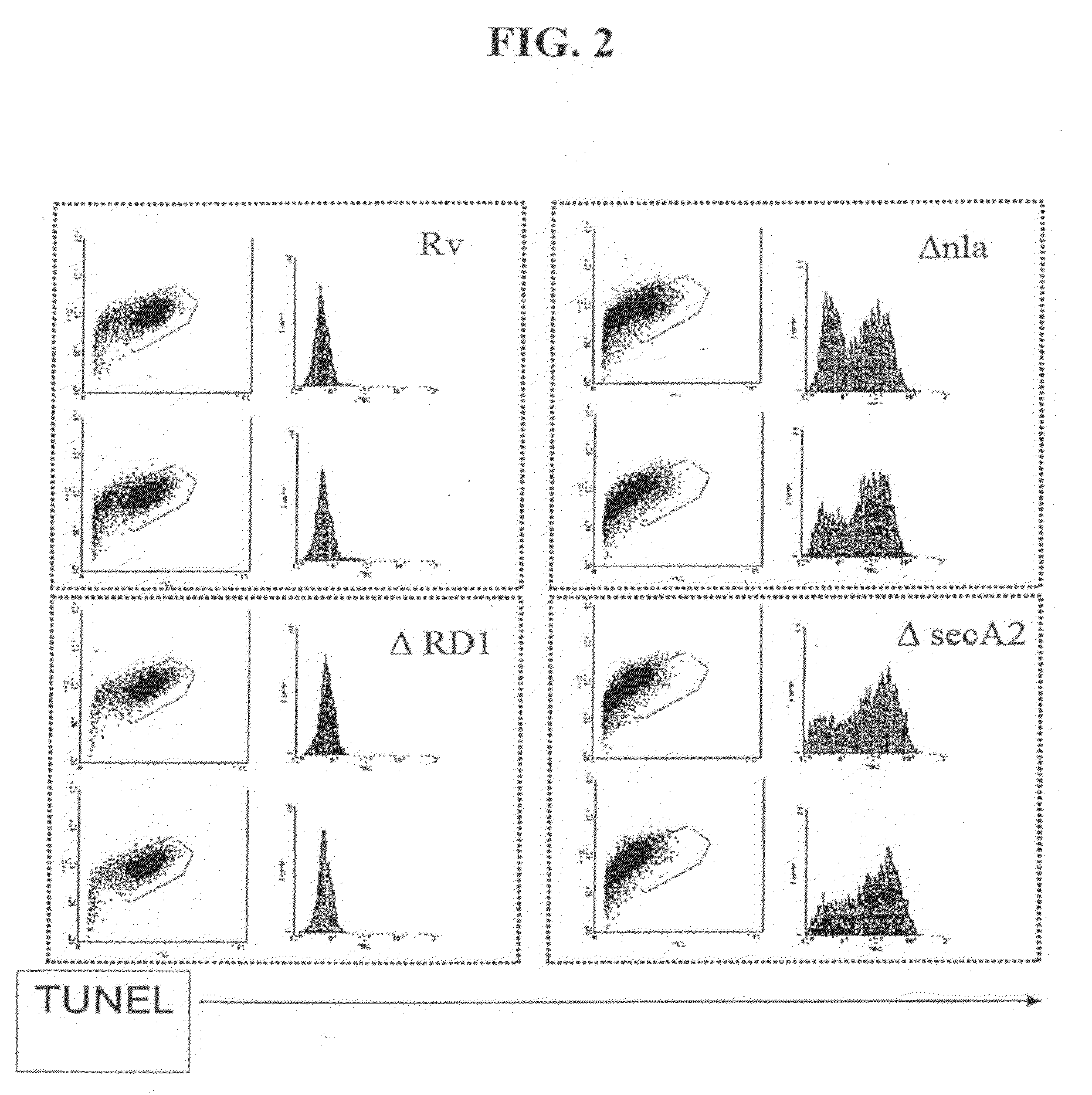
Mycobacterial SecA2 Mutants
ActiveUS20090110696A1Inhibit apoptosisImprove abilitiesAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsMammal
Provided are mycobacteria comprising (a) a mutation that is not in a SecA2 gene that attenuates the virulence of the mycobacteria in a mammalian host, and (b) a mutation in a SecA2 gene that eliminates SecA2 activity. Also provided are mycobacteria that comprise a mutation in a SecA2 gene that eliminates SecA2 activity, where the mycobacteria are not Mycobacterium tuberculosis or Mycobacterium smegmatis. Additionally provided are methods of inducing an immune response in a mammal and methods of inducing an immune response to a pathogenic mycobacterium in a human using the above mycobacterial mutants.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +2
Immunoassay method and kit for assaying mycobacterium tuberculosis from biological samples
The invention discloses a method and a kit for quickly assaying mycobacterium tuberculosis from blood or cerebrospinal fluid by means of fluorescence staining.Particularly, anti-mycobacterium tuberculosis antibodies, especially monoclonal or polyclonal antibodies of ESAT-6 and CFP-10 antigens in RD-1 (region of difference-1) are used for assaying the mycobacterium tuberculosis from biological samples enriched by a microporous membrane-based enrichment device through an indirect or direct immunization method or an SPA method.The method has the advantages of simplicity in operation, high sensitivity, high specificity and capability of distinguishing characteristics of pathogenic mycobacterium tuberculosis and non-pathogenic mycobacterium tuberculosis caused by Bacillus Calmette-Guerin vaccination, can be used for pathogenic detection of tuberculosis diseases and early rapid clinical diagnosis and is a specific tuberculosis etiology diagnostic method.
Owner:肖乐义
Isothermal nucleic acid amplification technique based kit for diagnosis of tuberculosis
InactiveCN102732601AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid amplification techniqueAgricultural science
The invention discloses an isothermal nucleic acid amplification technique based kit for diagnosis of tuberculosis. The invention provides a special primer for assisting identification of pathogenic mycobacteria; and the special primer comprises DNA shown in the sequence 1 in the sequence table, DNA shown in the sequence 2 in the sequence table, DNA shown in the sequence 3 in the sequence table and DNA shown in the sequence 4 in the sequence table. The invention also protects a kit containing the special primer. The kit provided by the invention has the advantages of high sensitivity, strong specificity, easy operation, simple equipment and low cost, can be used for the detection of pathogenic mycobacteria to further assist diagnosis of tuberculosis, and has significant application prospect.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
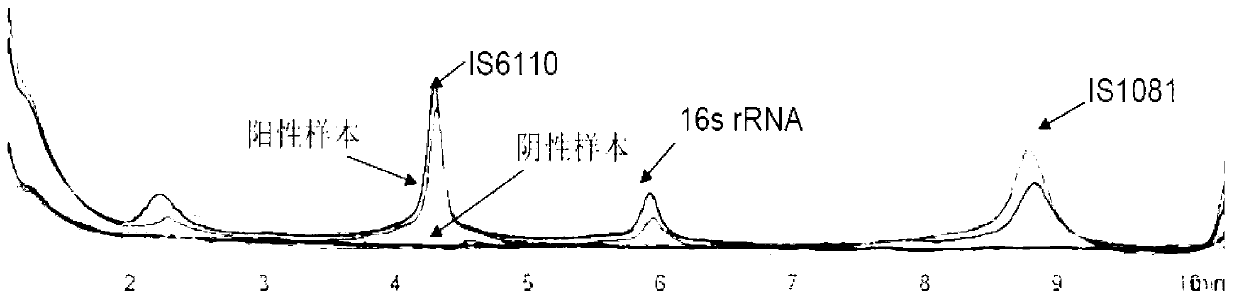
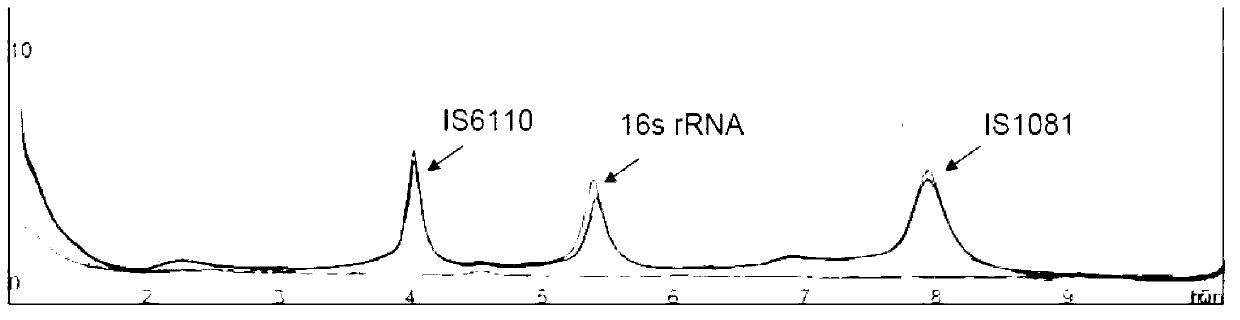
Reagent kit for detecting pathogenic mycobacterium tuberculosis through uniting multiplex PCR, nested-PCR and touch-down PCR
ActiveCN110438205AStrong specificityImprove efficiencyMicrobiological testing/measurementAgainst vector-borne diseasesMicrobiologyMultiplex pcrs
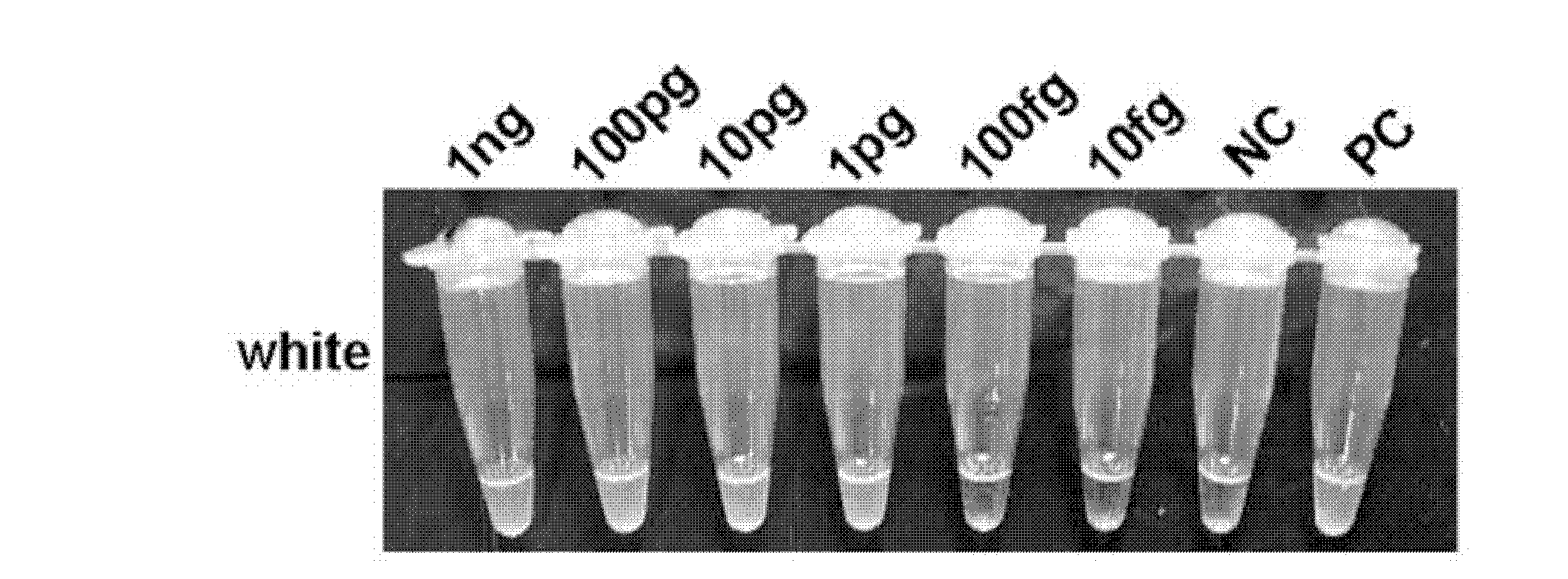
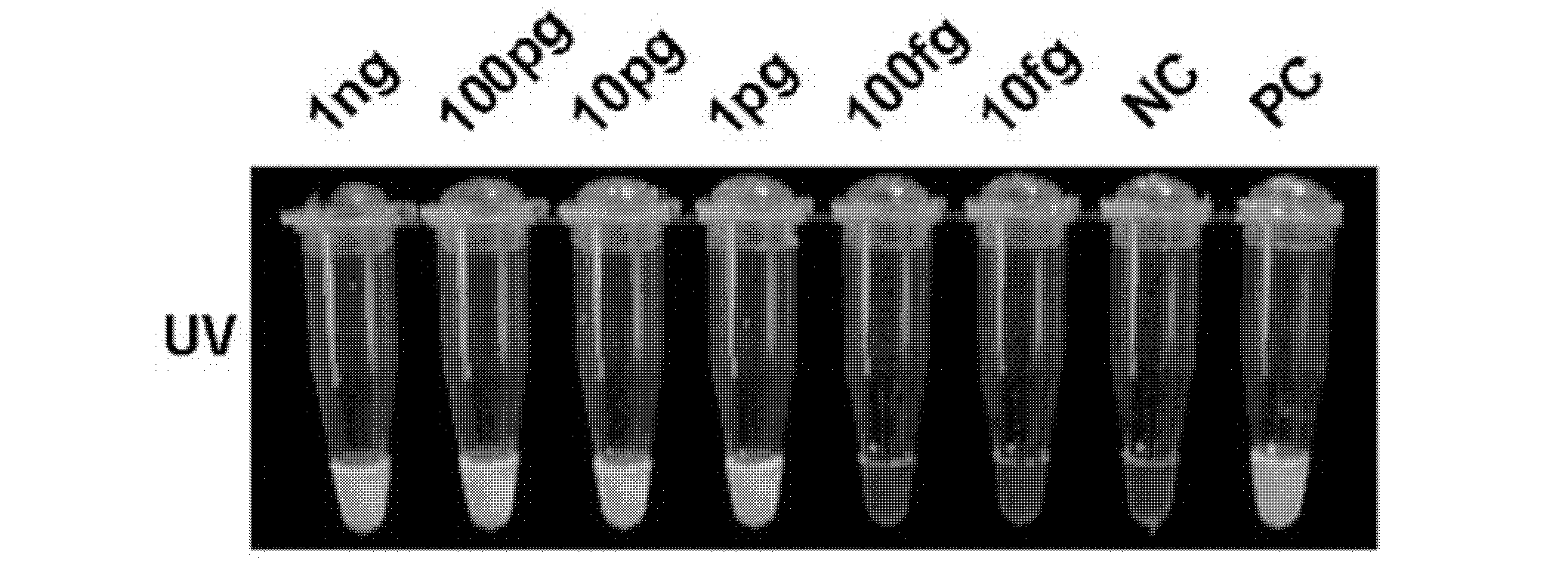
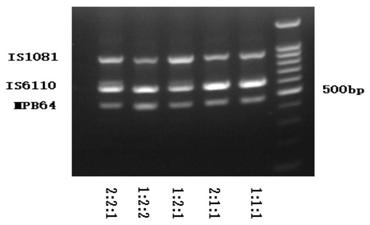
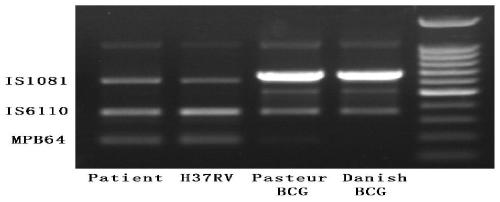
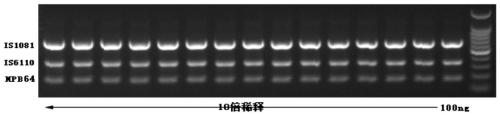
The invention relates to a reagent kit for detecting pathogenic mycobacterium tuberculosis through uniting multiplex PCR, nested-PCR and touch-down PCR. The reagent kit adopts a technique uniting themultiplex PCR, the nested-PCR and the touch-down PCR, and through 3 groups of primers of IS6110, IS1081 and MPB64, 3 molecule markers of IS6110, IS1081 and MPB64 are detected in a multiplex, efficientand specific manner, so that the pathogenic mycobacterium tuberculosis can be rapidly diagnosed, and determination can be more precise. The reagent kit can detect conservative molecule markers including IS6110 and IS1081 of the pathogenic mycobacterium tuberculosis, besides, can detect MPB64 molecule markers in a wild strain of the pathogenic mycobacterium tuberculosis, and further has accurate judgment significance in the pathogenic mycobacterium tuberculosis, and the detection right rate of the pathogenic mycobacterium tuberculosis can be substantially increased.
Owner:天津市泌尿外科研究所
Kit for identifying nucleic acid of mycobacterium pathogeny through multiple PCR (polymerase chain reaction)
InactiveCN104388575AEasy to identifyFast and accurate identificationMicrobiological testing/measurementMicroorganism based processesMicrobiologyMultiplex pcrs
The invention provides a kit for identifying nucleic acid of mycobacterium pathogeny through multiple PCR (polymerase chain reaction). The kit comprises primer composition as follows: 5'-acggtgggtactaggtgtgggtttc-3', 5'-tctgcgattagcgactaagacttca-3', 5'-gcgttgaccgagatggattat-3', 5'-gctcatctcacccagttggc-3', 5'-ttccgaatcccttgtga-3', 5'-ggagagcgccgttgta-3' or 5'-agtcgccgtggcttctctttta-3'. The kit has high sensitivity and high specificity, is simple to operate and can realize rapid and large-flux detection for virulent and avirulent mycobacterium.
Owner:YANGZHOU UNIV
Antibacterial Quinoline Derivatives
ActiveUS20080182855A1Ease of administrationImprove uniformityAntibacterial agentsBiocideMycobacterium marinumQuinoline
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or Formula (Ib):the pharmaceutically acceptable acid or base addition salts thereof, the quaternary amines thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of a bacterial disease including a mycobacterial disease, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of bacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Composition of mycobacterium polysaccharide and its preparation
InactiveCN100358535CPromote safe productionShorten the production cycleOrganic active ingredientsBacteriaDiseaseWhite blood cell
Owner:北京首医科技有限公司 +1
Gene engineering preparation for detecting bovine mycobacterium infection
ActiveCN102690884BStrong specificityClear compositionMicrobiological testing/measurementMicroorganism based processesMycobacterium InfectionsMycobacterium austroafricanum
The invention belongs to the field of biological detection. The invention provides a gene engineering preparation for detecting bovine mycobacterium infection. The gene engineering preparation comprises a mixture of three recombinant bovine mycobacterium proteins; the protein mixture can stimulate bovine mycobacterium infecting animals to produce a DTH (delayed type hypersensitivity) reaction. The gene engineering preparation provided by the invention has the advantages of high specificity, bio-security, component accuracy, content stability, low cost, standardized production and so on. Allergic hypodermic tests established with the detection reagents can increase experimental sensitivity and specificity, and can distinguish bovine mycobacterium infection and avian mycobacterium or non-pathogenic mycobacterium infection, so that the gene engineering preparation can be effectively used for clinic detection of bovine tuberculosis. A primer for preparing the gene engineering preparation further is provided by the invention.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Long-acting formulations
This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the anti-TB compound bedaquiline, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of a pathogenic mycobacterial infection.
Owner:JANSSEN PHARMA NV
Fuidi herd management and risk stratification methods
InactiveUS9128098B2Enhanced genetic abilityMicrobiological testing/measurementBiological testingCvd riskBiology
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp. paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
Tuberculosis and medicament-resistant tubercular personalized yelk polyclone antibody and method of preparing the same and applications
InactiveCN101249264AAvoid infectionImprove accuracyAntibacterial agentsEgg immunoglobulinsHigh risk populationsYolk
The invention discloses a yolk polycolonal antibody formulation of personalized tuberculosis and drug-resistance tuberculosis, a preparation method, and application thereof. Pathogenic mycobacterium tuberculosis or dru-resistance tuberculosis is cultivated after being separated from the serum or sputum of tuberculosis patients, and then produced into personalized vaccine through the inactivating process of formaldehyde which can be used for the immune injection of healthy hen birds; specific polycolonal IgY antibody aimed at the patients and the pathogenic mycobacterium tuberculosis can be obtained from the yolk produced by immunized hen birds; after degermation, extraction, purification, liposome microencapsulation, etc., the specific polycolonal IgY antibody formulation can be obtained, which can be used for the passive immunotherapy of the patients with tuberculosis or drug-resistance tuberculosis and for preventing tuberculosis for high-risk population. The formulation is a safe, high-effect, and no-drug-resistance personalized biological formulation, which can also be applied to all the detecting methods on the basis of tuberculosis antigen-antibody immunoreaction, and can quickly and individually detect the infection of tuberculosis or drug-resistance mycobacterium tuberculosis.
Owner:沈星灿
A recombinant plasmid, its construction method and its application to precise genome transformation of mycobacteria
ActiveCN107236748BUnderstand the pathogenic mechanismIncrease productionHydrolasesFermentationGenome editingHormone drug
The invention relates to a genome transformation technology and discloses a recombinant plasmid, a construction method and accurate genome transformation for mycobacterium. The technical scheme relates to plasmid construction capable of carrying out accurate genome editing in mycobacterium and an application thereof in continuously accurate gene knockout in the mycobacterium. By using the method, the length of a target DNA fragment for genome editing of mycobacterium can be at the bp-Mb level in theory, and no scar is left on a genome after transformation. Through construction of the method, vitally important genome editing means can be provided for the research on pathogenesis of high pathogenic mycobacterium and genome transformation of an industrial strain of important mycobacterium for producing an important steroid hormone prodrug.
Owner:南通汇成生物科技有限公司 +1
Multiplex polymerase chain reaction (mPCR)-denaturing high-performance liquid chromatography (DHPLC) primers and method for detecting and identifying mycobacterium
InactiveCN102808031BRapid identificationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlMycobacterium Infections
The invention provides a group of nucleic acids used in a quintuple multiplex polymerase chain reaction (mPCR)-denaturing high-performance liquid chromatography (DHPLC) method for detecting mycobacterium and identifying pathogenic mycobacterium. The nucleic acids comprise five pairs of primers of which the nucleic acid sequences are shown as SEQ ID No.1 and SEQ ID No.2, SEQ ID No.4 and SEQ ID No.5, SEQ ID No.7 and SEQ ID No.8, SEQ ID No.10 and SEQ ID No.11, and SEQ ID No.13 and SEQ ID No.14, and PCR amplification products which are used as positive control and of which the nucleic acid sequences are shown as SEQ ID No.3, SEQ ID No.5, SEQ ID No.9, SEQ ID No.12 and SEQ ID No.15. The invention also provides a kit using the nucleic acids and a detection method; the method is high in specificity and flexibility and easy to operate, and high flux can be achieved; and the method has important practical significance to clinical identification on the mycobacterium infection and infectious agents of the mycobacterium infection.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Mycobacterial sulfation pathway proteins and methods of use thereof
Novel mycobacterial sulfation pathway proteins and polypeptides related thereto, as well as nucleic acid compositions encoding the same, are provided. The subject polypeptide and nucleic acid compositions find use in a variety of applications, including research, diagnostic, and therapeutic agent screening applications. Also provided are methods of inhibiting growth and / or virulence of a pathogenic mycobacterium, and methods of treating disease conditions associated with a pathogenic mycobacterium, particularly by administering an inhibitor of a mycobacterial sulfation pathway protein. The present invention further provides genetically modified mycobacteria having a defect in a sulfation pathway enzyme gene; and immunogenic compositions that include such genetically modified mycobacteria.
Owner:RGT UNIV OF CALIFORNIA
Fuidi herd management and risk stratification methods
InactiveUS20150216144A1Enhanced genetic abilityReduce the amount requiredSugar derivativesMicrobiological testing/measurementBiologyHerd management
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp. paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com