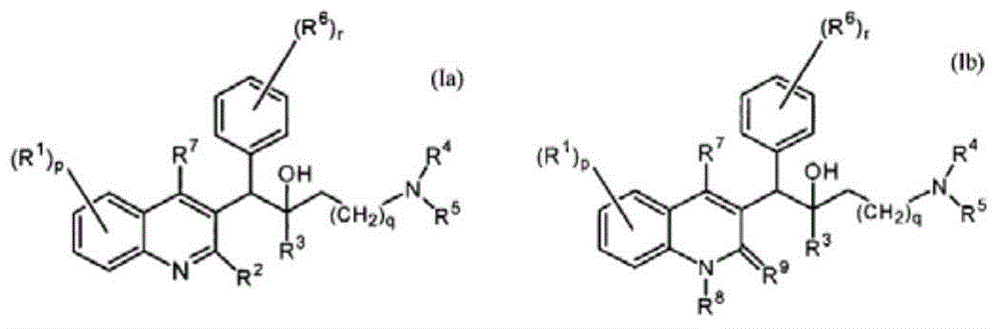
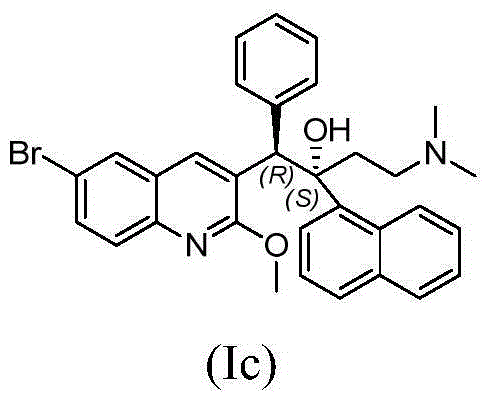
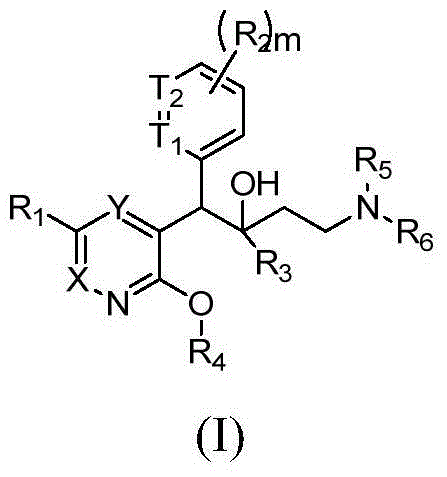
Pyridine derivative and application thereof to mycobacterium resistance
A compound and hydrate technology, applied in antibacterial drugs, organic chemistry, etc., can solve the problems of low efficacy, high price, toxicity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0265] Preparation of Intermediate A and Intermediate B
[0266]
[0267] step 1:
[0268] (5-Bromo-2-methoxypyridin-3-yl)(phenyl)methanol
[0269]
[0270] Under nitrogen protection, 3,5-dibromo-2-methoxypyridine (118g, 443mmol) was dissolved in 1.2L of anhydrous ether, and n-butyl lithium (2.5M n-hexane solution , 195mL, 487mmol), kept the temperature, stirred for 0.5 hours, dissolved benzaldehyde (47.0g, 443mmol) in 100mL of anhydrous ether, slowly added to the reaction system at -78°C, and slowly heated the mixture to 15- Stir for 1 hour at 25°C. Quench the reaction with 600mL of saturated ammonium chloride solution, extract 3 times with 200mL of ethyl acetate each time, combine the organic phases, wash with brine, dry over anhydrous sodium sulfate, concentrate in vacuo, and perform column chromatography (elution machine: petroleum ether / Ethyl acetate=50 / 1~10 / 1) isolated (5-bromo-2-methoxypyridin-3-yl)(phenyl)methanol (73.5 g, 56.0% yield) as a white solid. LC...
Embodiment 1
[0284] 2-(5-(4-(Dimethylamino)-2-hydroxy-2-(naphthalene-1-yl)-1-phenylbutyl)-3-(6-methoxypyridin-3-yl) ) benzonitrile
[0285]
[0286] Under nitrogen protection, intermediate A (1.00g, 1.98mmol), (2-cyanophenyl)boronic acid (349mg, 2.37mmol), potassium acetate (388mg, 3.96mmol) and Pd(dppf)Cl 2 (92mg, 0.1mmol) was added to the mixed solvent of dioxane / water (10mL / 2mL), and the reaction solution was heated to 80°C and stirred at this temperature for 5 hours under nitrogen protection. LCMS monitored that the reaction was complete. The reaction mixture was added to In water (30mL). Simultaneously extracted with ethyl acetate (10mL×3). The organic phases were combined, dried, and concentrated to obtain the crude compound, which was passed through preparative HPLC (GX-G; PhenomenexSynergiC18150*30mm*4um; acetonitrile15%-45%; water (0.225% fomicacid); 25mL / min) separated to obtain component A and component B. Component A was passed through chiral SFC (BergerMultiGramTMSFC, Me...
Embodiment 2
[0288] 1-(5-(2-bromophenyl)-2-methoxypyridin-3-yl)-4-(dimethylamino)-2-(naphthalene-1-yl)-1-phenylbutan-2 -alcohol
[0289]
[0290] Under nitrogen protection, intermediate B (2.00g, 3.62mmol), 1,2-dibromobenzene (1.02g, 4.34mmol), potassium acetate (710mg, 7.24mmol) and tetrakis (triphenylphosphine) palladium (209mg , 0.18mmol) was added to the mixed solution of dioxane / water (20mL / 4mL), the temperature was raised to 80°C, and the temperature was kept and stirred under nitrogen protection for 16 hours. LCMS monitored that the reaction was complete. The reaction mixture was added to water (40mL ), extracted with ethyl acetate (30mL×3). The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain the crude compound, which was passed through preparative HPLC (GX-G; PhenomenexSynergiC18150*30mm*4um; acetonitrile30%-60% water (0.225% fomicacid); 25mL / min) separated to obtain component A and component B. Component A was passed thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com